Abstract
Autism spectrum disorders are associated with atypically excessive early brain growth. Recent studies suggest that later cortical development, specifically cortical thickness, during adolescence and young adulthood is also aberrant. Nevertheless, previous studies of other surface-based metrics (e.g. surface area and gyrification) at high-resolution in autism spectrum disorders are limited. Forty-one males with autism spectrum disorders and 39 typically developing males matched on age (mean ∼17; range = 12–24 years) and IQ (mean ∼113; range = 85–143) provided high-resolution 3 T anatomical magnetic resonance imaging scans. The FreeSurfer image analysis suite quantified vertex-level surface area and gyrification. There were gyrification increases in the autism spectrum disorders group (relative to typically developing subjects) localized to bilateral posterior cortices (cluster corrected P < 0.01). Furthermore, the association between vocabulary knowledge and gyrification in left inferior parietal cortex (typically developing group: positive correlation; autism spectrum disorders group: no association) differed between groups. Finally, there were no group differences in surface area, and there was no interaction between age and group for either surface area or gyrification (both groups showed decreasing gyrification with increasing age). The present study complements and extends previous work by providing the first evidence of increased gyrification (though no differences in surface area) at high resolution among adolescents and young adults with autism spectrum disorders and by showing a dissociation in the relationship between vocabulary and gyrification in autism spectrum disorders versus typically developing subjects. In contrast with previous findings of age-related cortical thinning in this same autism spectrum disorders sample, here we find that increases in gyrification are maintained across adolescence and young adulthood, implicating developmentally dissociable cortical atypicalities in autism spectrum disorders.
Keywords: autism, MRI, gyrification, cortical folding, surface area
Introduction
Autism spectrum disorders (ASD) are associated with atypical patterns of brain growth and development based on various metrics including head circumference, brain weight, and brain volume using MRI (for review, see Courchesne et al., 2007). This atypical growth pattern may contribute to the increasingly recognized aberrant cortical connectivity in ASD (Booth et al., 2011; Müller et al., 2011; Vissers et al., 2012). However, two aspects of brain structure that may be crucial to functional connectivity (White and Hilgetag, 2011) but have been relatively understudied in ASD are cortical gyrification (brain folding patterns) and surface area.
Cortical gyrification in autism spectrum disorders
Cortical gyrification is an early developing process (Clouchoux et al., 2012) that may go awry in disorders such as ASD, with early developmental origin. Indeed, several post-mortem studies have indicated the presence of gyral abnormalities, such as polymicrogyria, in a significant minority of ASD cases examined (Ritvo et al., 1986; Bailey et al., 1998). The first study to assess gyrification in ASD using MRI identified the presence of polymicrogyria in 5 of 13 primarily adult subjects with high functioning ASD whereas two others had macrogyria (Piven et al., 1990) based on expert ratings (i.e. qualitative examination) of scans. More recently, the primary metric used in various studies is the gyrification index, which is the ratio of the inner contour (including sulcal crevices) to the perimeter of the outer or superficially exposed surface, as first described and quantified by Zilles et al. (1988). At least two studies have used 2D methods to assess gyrification within limited cortical regions in ASD. Hardan et al. (2004) assessed the gyrification ratio in a single coronal slice of prefrontal cortex (the first slice anterior to the corpus callosum) among 30 males with ASD versus 32 matched typically developing male control subjects. No group differences emerged in the total sample, but when the sample was divided into those ≥18 years versus those <18 years of age, diagnostic group differences emerged in the younger group. Children and adolescents with ASD had greater prefrontal gyrification than controls, whereas no differences were found among adults. Investigating a wide age range (8–38 years), Casanova et al. (2009) found no ASD–typically developing group differences in gyrification using 40 randomly selected coronal slices among 14 males with ASD compared with 28 typically developing males matched on age.
Although other studies have been completed on related metrics of sulcal morphometry (Levitt et al., 2003), sulcal depth (Nordahl et al., 2007), and gyral complexity (Williams et al., 2012) in ASD, only two studies to date have assessed gyrification specifically across the entire cortex in ASD. Kates et al. (2009), using lateralized lobar-based averages, found greater gyrification in right parietal cortex among 14 lower functioning children with ASD compared with 14 typically developing control subjects. Meguid et al. (2010), comparing lower functioning children with ASD (n = 10) to those with fragile X syndrome (n = 7), found no differences in a single 3D metric of gyrification across the entire cortex. This latter study did not include a typically developing control group, thus the atypicality of gyrification from normative expectations was not assessed.
Taken together, these studies generally implicate increased gyrification in ASD children and adolescents when diagnostic group differences are found; however, they have been limited in several important ways. Initial studies focused on gyrification using one (frontal cortex) coronal slice, rather than taking a whole brain approach. Studies examining gyrification across the entire cortex in ASD have included predominantly lower functioning individuals, complicating interpretations of the source of group differences (i.e. comorbid intellectual disability versus ASD alone). Sample sizes in the whole brain studies have been relatively small (<15 per group), limiting power and the capability to detect group differences. The whole brain studies have focused on the developmental window of childhood, thus the periods of adolescence and young adulthood, when cortical atypicalities in ASD have been demonstrated (Wallace et al., 2010) and when whole-brain gyrification continues to decrease during typical development (White et al., 2010; Raznahan et al., 2011), have been largely unexplored. Finally and perhaps most importantly, 3D methods that provide high-resolution metrics of local gyrification have not been used in a sample of individuals with ASD. Collapsing across the entire cortex or lobar regions may obscure more regionally specific gyrification differences in ASD that these more powerful techniques can detect.
To this point, recent advances in neuroimaging analysis methodologies have allowed the examination of local gyrification at ever increasing resolutions (e.g. the vertex level). Schaer et al. (2008) have developed a well validated and reliable methodology within the FreeSurfer software package that has been successfully applied to several neurodevelopmental disorders, including 22q deletion syndrome (Schaer et al., 2009), intellectual disability (Zhang et al., 2010), schizophrenia (Palaniyappan et al., 2011) and conduct disorder (Hyatt et al., 2012).
Gyrification and intelligence
Grey matter volume is the product of cortical thickness and cortical surface area. Interestingly, as compared to other mammals, humans have disproportionately large cortical surface area in relation to their associated grey matter volume. For example, although the human brain has a ∼10-fold increase in surface area compared with that of macaques, the cortex is only 2-fold thicker (Barondes et al., 1997). This discrepancy becomes greater when descending the mammalian evolutionary chain. To accommodate this oversized cortical sheet [approximately three times the size of the inner surface of the skull in humans (Van Essen, 1997)], the brain folds in upon itself resulting in high levels of gyrification. This increased gyrification in humans versus other mammalian species may contribute to our considerable cognitive capacities (White et al., 2010). Accordingly, individual differences in intelligence have been shown to positively correlate with global (Kates et al., 2009) and local (Luders et al., 2008) measures of gyrification in typically developing populations. Furthermore, individuals with intellectual impairments have reduced gyrification compared to their typically developing peers (Bonnici et al., 2007; Zhang et al., 2010). In contrast, there is evidence to suggest that this relationship between gyrification and IQ does not hold in ASD. Kates et al. (2009) found that although there was a significant positive association between IQ and gyrification index (particularly in temporal and parietal lobes) in 14 typically developing children, no such correlation was found in a group of 14 children with ASD. However, it should be noted that in this study the IQ scores were considerably different between groups. The ASD group’s average IQ fell in the range of intellectual impairment (mean = 66.6, SD = 18.27), whereas the control group’s IQ (mean = 120.9, SD = 11.18) was well above the standardized average of 100. This discrepancy in IQ scores and ranges could have contributed to a positive association in one group and no association in the other. We therefore sought to examine the association between IQ (and component subtest performance) and gyrification in ASD and typically developing individuals matched on IQ, which also fell in the average or higher range of scores.
Surface area in autism spectrum disorders
Given that previous studies have demonstrated exaggerated brain growth in ASD during the preschool years (Courchesne et al., 2007), it is somewhat surprising that only three studies to date have used surface-based MRI methods to directly quantify and compare surface area in samples of individuals with ASD versus typically developing control subjects. For 41 individuals (mean age = 34; range = 12–64 years) with ASD, Raznahan et al. (2009) found no difference in total surface area compared with 30 typically developing control subjects. In a subsequent study including additional subjects, Raznahan et al. (2010) found that there was neither a main effect of group nor a group × age interaction in lobar-level surface area when comparing 76 individuals with ASD to 51 neurotypical control subjects ranging from 10–60 years of age. However, these studies were limited, such as failing to match groups on IQ and providing clinical characterization on a majority, but not all, of the ASD subjects. Among 25 children with ASD compared with 63 typically developing control subjects (ages 6–15 years), Mak-Fan et al. (2012) found no group differences in surface area, but there was a significant group × age interaction in occipital lobe surface area; children with ASD exhibited greater occipital lobe surface area than typically developing controls, but only at the older end of their age range (centred at age 14.5 years). Taken together, the univariate studies (see Ecker et al., 2010 for a multivariate approach that used high-resolution surface area, among other cortical metrics) suggest comparable surface area in ASD and typically developing groups of school-age children, adolescents and adults, though findings on group by age interactions for surface area are mixed. However, none of the aforementioned univariate studies assessed surface area at high-resolution (e.g. vertex-level), thus whether surface area group differences and interactions with age might be found from more fine grain assessment remains an open question.
Current study
The present study is the first to utilize 3D methods to examine both high-resolution local gyrification and surface area in age and IQ matched adolescent and young adult males with ASD versus typically developing males. We assess not only ASD–typically developing group differences for each of these metrics, but also the comparability of age-related changes in local gyrification and surface area during adolescence and young adulthood in each of these groups. Consistent with the extant literature, we expect to find greater gyrification in the ASD group than in the typically developing subjects, particularly in posterior cortical regions; however, we expect age-related changes in gyrification to be comparable between the two groups. We also explore other elements of cortical structure (i.e. pial surface area and sulcal depth/gyral height) that may contribute to any observed gyrification differences in ASD. Finally, we examine possible ASD-typically developing group discrepancies in the association between IQ (and component subtest performance) and local gyrification and any potential links between autistic traits and local gyrification in both groups. While IQ and its subcomponent processes are expected to be correlated with local gyrification in the typically developing group, this relationship will be diminished or absent in the ASD subjects. In the ASD group, we expect to find gyrification associated with autistic traits in regions where group differences are documented. For typically developing subjects, we expect that gyrification will be associated with autistic traits in regions where cortical structure has been previously linked with autistic traits in other typically developing samples (e.g. right superior temporal sulcus; Wallace et al., 2012).
Materials and methods
Participants
Thirty-nine typically developing males between 12 and 23 years of age and 41 high-functioning males with an ASD between 12 and 24 years of age recruited from the Washington, DC metropolitan area participated in the study. All 41 participants with ASD met Diagnostic and Statistical Manual of Mental Disorders-IV diagnostic criteria as assessed by an experienced clinician (26 Asperger’s syndrome, 11 high-functioning autism, three pervasive developmental disorder-not otherwise specified and one with either Asperger’s syndrome or high-functioning autism, which could not be distinguished because of missing early language development data). Forty ASD participants received both the Autism Diagnostic Interview or Autism Diagnostic Interview-Revised (Le Couteur et al., 1989; Lord et al., 1994) and the Autism Diagnostic Observation Schedule (Lord et al., 2000) administered by a trained, research-reliable clinician (one ASD participant received neither of these instruments). ASD participants received either Module 3 (n = 13) or 4 (n = 27) of the Autism Diagnostic Observation Schedule. All ASD participants’ scores met cut-off for the category designated as ‘Broad ASD’ according to criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (CPEA; see Lainhart et al., 2006). Because the Autism Diagnostic Interview and Autism Diagnostic Observation Schedule do not provide an algorithm for Asperger's syndrome, Lainhart et al. (2006) developed criteria that include an individual on the broad autism spectrum if s/he meets the Autism Diagnostic Interview cut-off for ‘autism' in the social domain and at least one other domain or meets the Autism Diagnostic Observation Schedule cut-off for the combined social and communication score. Exclusion criteria for the ASD group included an IQ < 85 or any known comorbid medical conditions, such as fragile X syndrome or other genetic disorders, and brain trauma/injury. In the ASD group, 22 individuals were taking one or more psychotropic medications: 12 were taking stimulants, 14 were taking selective serotonin reuptake inhibitors, four were taking atypical antipsychotics, two were taking anxiolytics, one was taking a mood stabilizer and one was taking a norepinephrine agonist.
Parents of typically developing children and adults underwent telephone screenings. Typically developing individuals were excluded from participation if they had ever received mental health treatment for anxiety, depression, or any other psychiatric condition, taken psychiatric medications, required special services in school, been diagnosed with a genetic disorder or neurological disorder, or had brain trauma/injury that could potentially affect brain development and/or cognitive functioning. IQ scores were obtained from all participants. All Full Scale IQ scores were 85 or above, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI; ASD: n = 34, typically developing: n = 39), Wechsler Adult Intelligence Scale-III (WAIS-III; ASD: n = 3), Wechsler Intelligence Scale for Children-III (WISC-III; ASD: n = 2), or Wechsler Intelligence Scale for Children-IV (WISC-IV; ASD: n = 2). Finally, parent ratings of autistic (primarily social-communication) traits, were obtained for both groups using the total score from the Social Responsiveness Scale (Constantino and Gruber, 2005), as it is the most psychometrically sound index based on factor analytical, reliability, and validity studies (Constantino et al., 2004). Participant groups did not differ (all P-values > 0.05) in terms of Full Scale IQ, age, or handedness (Table 1). Informed assent and consent were obtained from all participants and/or their parent/guardian when appropriate in accordance with an NIH IRB approved protocol.
Table 1.
Demographic characteristics
| ASD (n = 41) | Typically developing (n = 39) | |
|---|---|---|
| Age | 16.75 (2.84) | 16.95 (2.71) |
| Full Scale IQ | 113.02 (14.88) | 114.23 (10.80) |
| Verbal IQ | 112.00 (16.78) | 112.79 (12.26) |
| Performance IQ | 110.95 (13.79) | 112.08 (10.78) |
| Handedness (R:L) | 37:4 | 35:4 |
| Social Responsive Scale total‡ | 88.73 (27.28) | 19.88 (12.66) |
| Autism Diagnostic Interview, Social* | 18.95 (5.21) | — |
| Autism Diagnostic Interview, Verbal Communication* | 14.95 (4.61) | — |
| Autism Diagnostic Interview, Restricted and Repetitive Behaviour* | 5.65 (2.82) | — |
| Autism Diagnostic Observation Schedule, Social* | 8.18 (3.28) | — |
| Autism Diagnostic Observation Schedule, Communication* | 4.08 (1.72) | — |
| Autism Diagnostic Observation Schedule, Stereotyped behaviour* | 1.30 (1.59) | — |
Mean (SD).
‡ASD n = 37, typically developing n = 36.
*n = 40.
Imaging parameters
One high-resolution (1.07 × 1.07 × 1.2 mm) T1-weighted structural image was obtained axially from each subject with a MPRAGE sequence (124 slices, 1.2 mm slice thickness, 224 × 224 acquisition matrix, flip angle = 6°, field of view = 24 cm, inversion time = 725 ms) on a 3 T General Electric Signa scanner using an 8-channel head coil.
Surface reconstruction and local gyrification index calculation
Version 5.1 of the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) was used to generate a cortical surface (composed of a mesh of triangles) allowing for the measurement of surface area and local gyrification index at each vertex. The technical details of the surface extraction procedures are described in previous publications (Dale et al., 1999; Fischl et al., 1999, 2004). Initial steps in this surface-based, multi-step, and semi-automated morphometric pipeline include visual inspection of data for motion artifacts, transformation to Talairach space, intensity normalization for correction of magnetic field inhomogeneities, and removal of non-brain tissues (e.g. skull stripping). The resulting surface models generated were reviewed for accuracy and manually edited in all cases due to over-inclusion of white matter around the optic nerve.
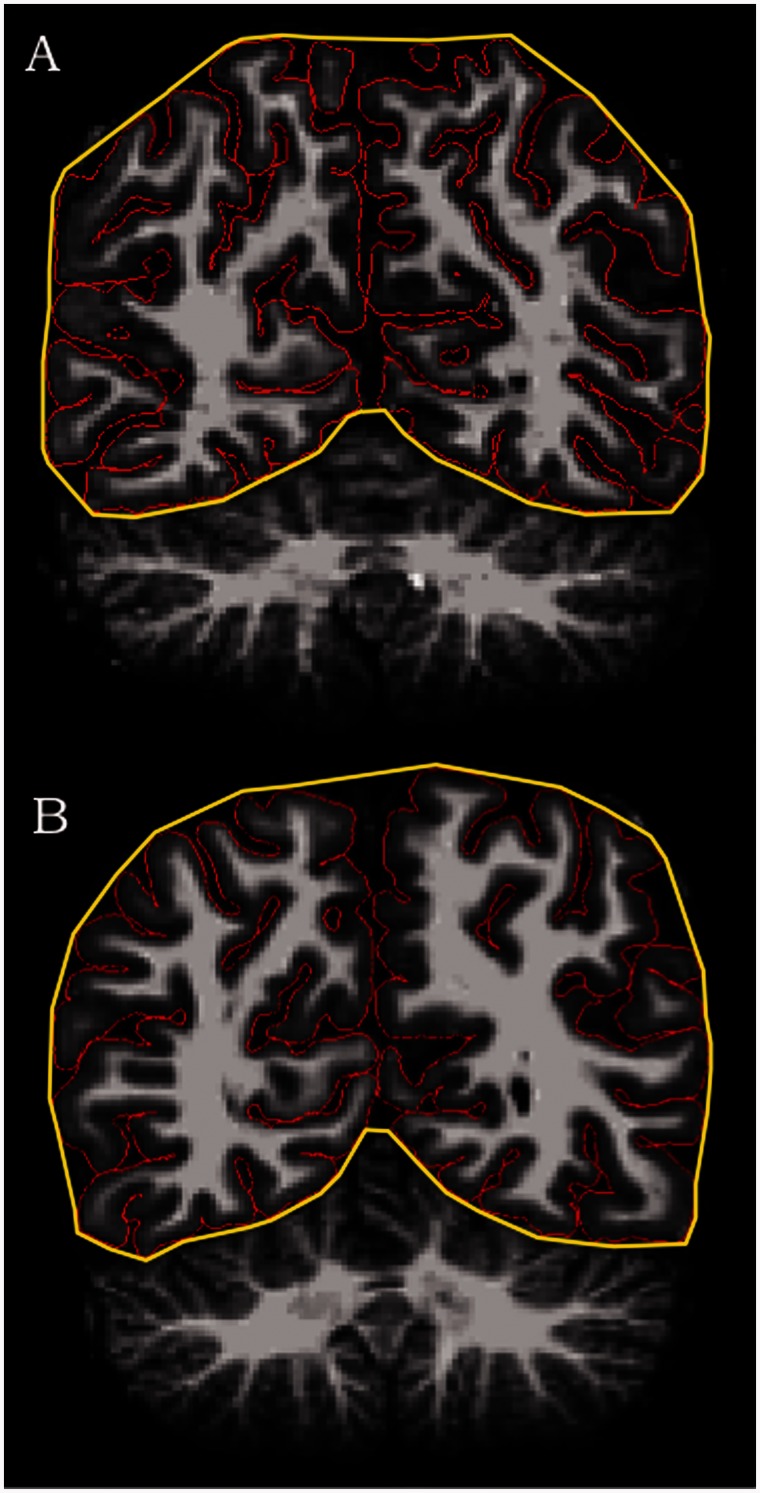
As previously mentioned, a measure of local gyrification index developed by Schaer et al. (2008) can be utilized in the context of the FreeSurfer analysis suite. This tool provides a 3D surface-based measure of gyrification, building upon the original 2D method applied to each coronal slice, as described by Zilles et al. (1988) and used by many others. The original gyrification index was defined as the ratio of the inner cortical contour to the perimeter of the outer surface (see Fig. 1 for a depiction in the coronal view). In FreeSurfer, tessellated outer (the hull surface) and inner (the pial surface) contours provide vertex-based surfaces for calculating 3D local gyrification index. Using each vertex as the centre point of a spherical 25 mm region of interest, the ratio of the convoluted inner pial surface to the closely fitting outer perimeter provides the local gyrification index metric at high resolution across the entire cortex. Detailed methodological information on surface area computation can be found in a recent study by Winkler et al. (2012). In short, surface area is quantified by assigning an area to each vertex equal to the average of its surrounding triangles. When the vertex areas are summed over all vertices, the total is equal to the sum of the areas of the triangles.
Figure 1.
Coronal slice of MRI scans showing greater gyrification in left precuneus for (A) a male with an autism spectrum disorder versus (B) a typically developing male.
Vertex-level surface area and local gyrification index values were obtained and mapped onto a normalized cortical surface. Resulting surface area and local gyrification index maps were smoothed with a 5 mm full-width at half-maximum kernel.
Statistical analysis
Group differences in surface area and local gyrification index were examined on the surface maps vertex by vertex using a least squares general linear model. To control for multiple comparisons, cluster correction was completed using Monte Carlo simulation with 10 000 iterations (vertex-wise threshold of P < 0.01). Because previous work demonstrated interactions between age and other metrics of brain structure in adolescents and adults with ASD (Raznahan et al., 2010; Wallace et al., 2010), we examined age group (using a median split; ≤17 years versus >17 years) by diagnosis (ASD versus typically developing) interactions on the vertex-level surface area and local gyrification index data. Younger and older participants did not differ (P-values > 0.05) in terms of IQ (for both ASD and typically developing groups) or symptomatology scores (for the ASD group only). We also explored the contribution of other elements of cortical structure to any observed group differences in gyrification by extracting values for each of these metrics (i.e. pial surface area and sulcal depth/gyral height) only within the clusters where the ASD group differed significantly from typically developing controls. Next, we evaluated ASD-typically developing group discrepancies in the association between local gyrification index (both vertex-level and in regions where group differences were found) and not only full-scale IQ (all subjects), but also performance on two subtests (ASD: n = 38, typically developing: n = 39 for both), Vocabulary (ASD: mean = 57.94, SD = 10.85; typically developing: mean = 58.21, SD = 7.67; t = 0.12, P = 0.90) and Matrix Reasoning (ASD: mean = 56.32, SD = 7.17; typically developing: mean = 56.08, SD = 7.40; t = 0.14, P = 0.89) using the different offset, different slope (DODS) design matrix provided by Qdec in FreeSurfer. Lastly, we investigated links between autistic traits (using the total score from the Social Responsiveness Scale) and gyrification in both groups. For the ASD group, Pearson correlations were used to evaluate this relationship only within regions of group difference surviving cluster correction, while for the typically developing group, this correlation was examined across the whole brain in FreeSurfer’s Qdec application.
Results
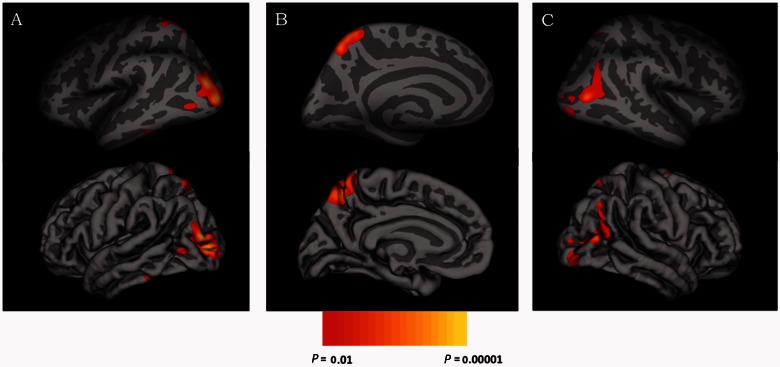
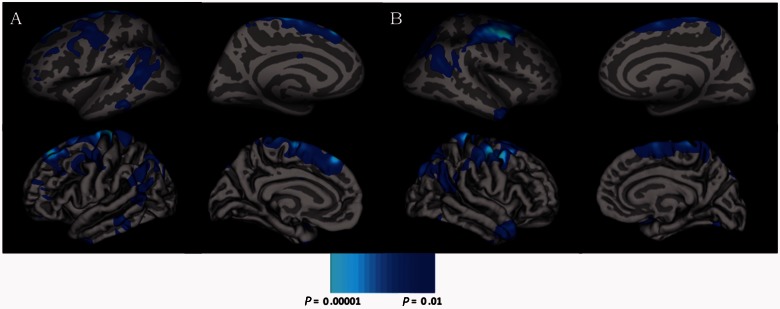
Vertex × vertex analyses conducted on the cortical surface revealed greater gyrification (cluster corrected P < 0.01) among ASD individuals as compared to typically developing individuals, but no differences in surface area. Greater gyrification was found in three clusters of posterior cortices, which were located bilaterally in lateral occipital regions and in left precuneus (Table 2 and Fig. 2). These regional gyrification increases were not due to differences in global brain size, as groups did not differ in intracranial volume. Interactions between age and diagnostic group were not significant for either surface area or local gyrification index (P-values > 0.05). However, there was a significant (cluster corrected P < 0.01) negative correlation between age and gyrification in large swaths of bilateral frontal, posterior temporal, and parietal cortices in the combined group of ASD and typically developing individuals (Table 3 and Fig. 3).
Table 2.
Cluster corrected (P < 0.01) regions differing in gyrification between the autism spectrum disorders group and the group of typically developing controls
| Region | Size (mm2) | x | y | z | Clusterwise P-value | Number of vertices |
|---|---|---|---|---|---|---|
| Left lateral occipital | 1307 | −23.6 | −90.0 | 5.2 | 0.001 | 1910 |
| Left precuneus | 1160 | −9.2 | −58.6 | 52.8 | 0.003 | 2639 |
| Right temporal-occipital | 790 | 48.4 | −70.9 | 13.0 | 0.04 | 1347 |
Figure 2.
Inflated and pial surface maps (dark grey = sulci; light grey = gyri) of both the (A) lateral and (B) medial surfaces of the left hemisphere and the (C) lateral surface of the right hemisphere showing greater cortical gyrification in the autism spectrum disorders group as compared to the group of typically developing control subjects.
Table 3.
Cluster corrected (P < 0.01) regions demonstrating age-related decreases in gyrification in the combined sample of individuals with autism spectrum disorders and typically developing controls
| Region | Size (mm2) | x | y | z | Clusterwise P-value | Number of vertices |
|---|---|---|---|---|---|---|
| Left superior frontal | 3918 | −16.0 | 38.2 | 41.6 | 0.0001 | 7947 |
| Left inferior parietal | 2237 | −49.6 | −47.9 | 17.8 | 0.0001 | 4713 |
| Left precentral | 2035 | −28.2 | −7.9 | 42.8 | 0.0001 | 4372 |
| Right caudal middle frontal | 6873 | 39.5 | 4.8 | 45.7 | 0.0001 | 15018 |
| Right inferior parietal | 3964 | 33.7 | −67.3 | 27.4 | 0.0001 | 7810 |
| Right middle temporal | 877 | 47.9 | 2.8 | −29.4 | 0.02 | 1403 |
Figure 3.
Inflated and pial surface maps (dark grey = sulci; light grey = gyri) of the lateral and medial views of the (A) left and (B) right hemispheres showing decreasing cortical gyrification with increasing age in the combined group of individuals with autism spectrum disorders and typically developing control subjects.
Follow-up analyses were completed in order to evaluate whether psychotropic medication usage may have had an effect on the local gyrification index group differences. The mean local gyrification index extracted from two of the three posterior cortical clusters did not differ between unmedicated (n = 16) and medicated (n = 25) subjects with ASD (P-values > 0.18), whereas local gyrification index in the left lateral occipital cluster was greater in the medicated group (P = 0.04). Furthermore, we found that unmedicated subjects with ASD demonstrated greater gyrification in these three posterior regions than did typically developing individuals, though the local gyrification index difference in only two of the three regions (the left precuneus and right posterior cortices) reached statistical significance (P < 0.05). Overall, these analyses (with diminished statistical power) corroborated the whole-brain analyses presented above, suggesting that increased gyrification in ASD is largely independent of psychotropic medication usage.
When exploring further within the three clusters where the ASD group had significantly increased local gyrification index compared with controls, we found that pial surface area was larger in the precuneus region of interest in the ASD group (ASD mean = 1924, SD = 271; typically developing mean = 1792, SD = 236), and sulcal depth/gyral height was greater in the ASD group in the left lateral occipital cortex (ASD mean = 0.07, SD = 0.09; typically developing mean = −0.002, SD = 0.08). Moreover, across the two groups, pial surface area was significantly correlated with local gyrification index in each of the three regions of interest (all r > 0.22, P-values < 0.05), while sulcal depth/gyral height was significantly associated with local gyrification index in the left lateral occipital cortex (r = 0.43, P < 0.001).
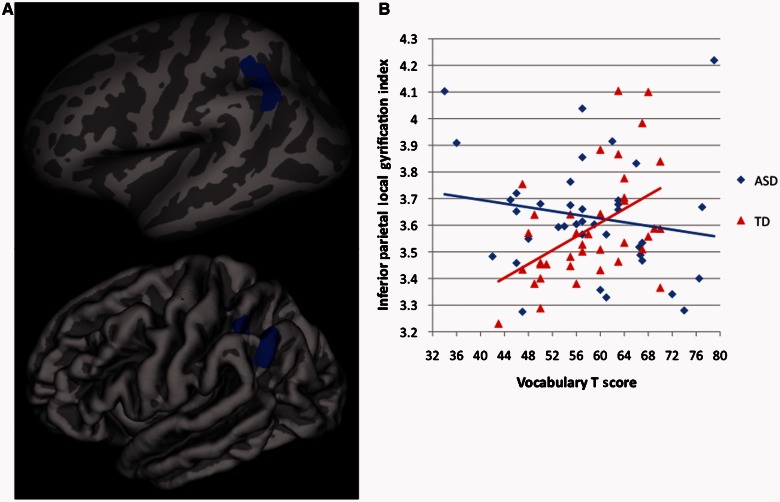
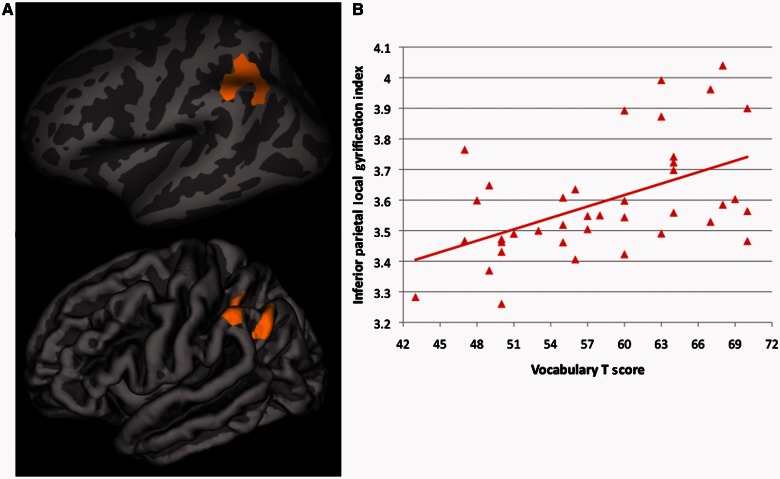
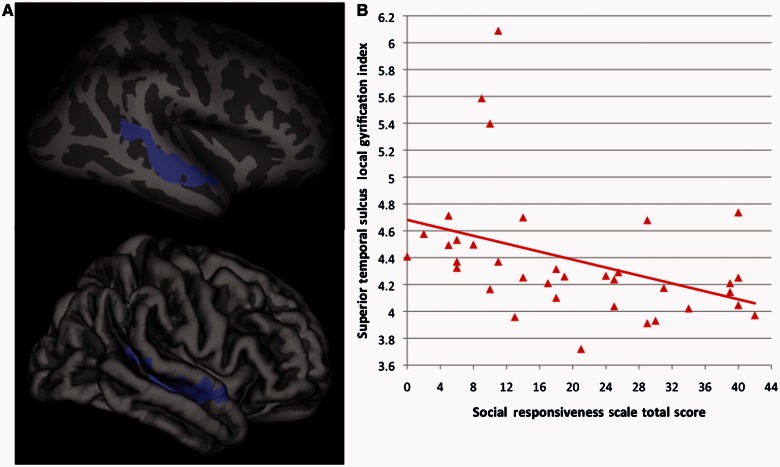
Finally, there were significant (cluster corrected P < 0.01) differences between ASD and typically developing individuals in the association between vocabulary performance and local gyrification index within left inferior parietal cortex in the vicinity of the supramarginal/angular gyri [driven by a positive association between vocabulary scores and local gyrification index in the typically developing group (r = 0.49, P = 0.001), but no significant association in the ASD group (r = −0.18, P = 0.29)] (Fig. 4). In a separate whole brain analysis within the typically developing group alone, we found that significant associations between vocabulary performance and local gyrification index (r = 0.51, P = 0.001) were isolated to left inferior parietal cortex, overlapping extensively with the area showing significant ASD-typically developing group differences in its association with vocabulary scores (Fig. 5). Further corroborating these group-specific relationships, among the three clusters where ASD-typically developing group differences in local gyrification index were observed, local gyrification index was significantly correlated with vocabulary scores in two of them, the left and right lateral occipital cortices, but only in the typically developing group (left side, typically developing: r = 0.45, P = 0.004; right side, typically developing: r = 0.43, P = 0.006). When comparing the magnitude of the correlations for the typically developing versus the ASD subjects through r to z transformations, both correlations in the typically developing group were significantly greater (left side, z difference = 2.13, P = 0.03; right side, z difference = 2.89, P = 0.004) than those found in the ASD group (left-side, ASD: r = −0.01, P = 0.95; right side, ASD: r = −0.21, P = 0.20). No group differences in the association between local gyrification index and either full-scale IQ or Matrix Reasoning performance were found. Also in contrast to vocabulary scores, we failed to find any significant (cluster corrected P < 0.01) relationship between parent ratings of primarily social-communication difficulties [assessed by the Social Responsiveness Scale total score (38)] and local gyrification index. However, after dropping the statistical threshold (to cluster-corrected P < 0.05), we did find a significant negative relationship between Social Responsiveness Scale ratings and local gyrification index (r = 0.40, P = 0.02) limited to the right superior temporal sulcus in (n = 36) typically developing subjects (Fig. 6).
Figure 4.
(A) Inflated and pial surface maps (dark grey = sulci; light grey = gyri) of the cluster-corrected (P < 0.01) group difference in associations between gyrification and Vocabulary score (peak: x = −44.8, y = −55.7, z = 40.7) and (B) scatterplot of these correlations for each group (autism spectrum disorders group r2 = 0.03; typically developing group r2 = 0.24). TD = typically developing.
Figure 5.
Within the typically developing males only, (A) inflated and pial surface maps (dark grey = sulci; light grey = gyri) of the cluster-corrected (P < 0.01) association between gyrification and Vocabulary score (peak: x = −46.6, y = −40.1, z = 40.6) and (B) scatterplot of this correlation (R2 = 0.26).
Figure 6.
Within the typically developing males only, (A) inflated and pial surface maps (dark grey = sulci; light grey = gyri) of the cluster-corrected (P < 0.05) association between gyrification and total score from the Social Responsiveness Scale (peak: x = 45.8, y = −39.0, z = 12.4) and (B) scatterplot of this correlation (R2 = 0.16), which does not change after removing the three highest gyrification values.
Discussion
In the first study to assess surface area and gyrification at high-resolution in ASD, we found greater gyrification in left precuneus and bilateral posterior temporal/lateral occipital regions of adolescent and young adult males with ASD as compared to age and IQ matched typically developing males, while surface area did not differ between groups. Moreover, whereas a robust positive correlation between vocabulary knowledge and gyrification was found in left inferior parietal cortex in the typically developing group, no such relationship was found in the ASD group. Finally, we found no group by age interactions for surface area and gyrification and noted gyrification decreases with age across large portions of bilateral frontal, posterior temporal and parietal cortices in the combined sample of ASD and typically developing individuals.
The current findings corroborate some earlier studies by showing increased gyrification in ASD (Hardan et al., 2004), particularly in posterior parietal cortices (Kates et al., 2009), and no differences in surface area (Raznahan et al., 2009, 2010; Mak-Fan et al., 2012). However, previous studies were limited in their resolution (e.g. lobar-based surface area and gyrification values), methods (e.g. for gyrification: not whole-brain; only selected coronal slices utilized), and/or sample size. Although not assessing gyrification, Nordahl et al. (2007) found deeper bilateral intraparietal sulci in a group of children and adolescents with Asperger’s syndrome compared to typically developing controls, which is consistent with increased gyrification in ASD, as documented here. Furthermore, unlike cortical thickness (Wallace et al., 2010), there was no ASD-typically developing group difference in the relationship between age and both surface area and gyrification during adolescence and young adulthood. For surface area, this is similar to at least one previous study (Mak-Fan et al., 2012). Both groups demonstrated decreasing gyrification with increasing age, consistent with prior studies demonstrating decreasing whole-brain (White et al., 2010; Raznahan et al., 2011) and lateralized lobar-level (Su et al., 2013) gyrification during this age range in typically developing children and adolescents. This suggests that the greater gyrification among individuals with ASD is maintained during adolescence and young adulthood. Whether ASD and typically developing groups show comparable age-local gyrification index relationships at younger ages remains an open question for future research to explore.
The increased gyrification in ASD may represent one downstream consequence of an aberrant developmental trajectory of brain growth in these disorders. More specifically, recent work shows increased numbers of cortical neurons in ASD (Courchesne et al., 2011), confirming some earlier post-mortem studies (for review, see Palmen et al., 2004), though aberrant dendritic arborization and/or synaptic pruning may be the more likely cellular mechanisms driving atypical brain growth trajectories in ASD (Schumann et al., 2011). There is also increasingly strong evidence of structural connectivity abnormalities in ASD at various developmental windows based on diffusion imaging studies (Booth et al., 2011; Vissers et al., 2012). Given theories of gyrification resulting from cortical fibre tension (Van Essen, 1997), it may be that aberrant cortical folding is one result of these micro- and macro-level structural brain differences in ASD. These gyrification differences may, in turn, correlate with functional connectivity, which many studies indicate is aberrant in ASD (including many of the subjects included in this report) (Gotts et al., 2012; for review, see Vissers et al., 2012). Speculatively, it may be that the types of disruptions in regional cortical folding patterns found here result in less homogeneous endogenous fluctuations. In order to assess these possible associations, future studies could link the types of high-resolution gyrification metrics used here with both structural (e.g. using diffusion tensor imaging or diffusion spectrum imaging) and functional (e.g. using functional MRI or magnetoencephalography) connectivity.
Utilizing other structural metrics, we found that increased local gyrification index in ASD is directly associated with differences in both pial surface area and by sulcal/gyral morphometry. Within one of the three regions of interest (precuneus) where groups differed, the ASD group had increased pial surface area compared with typically developing control subjects. Furthermore, all three regions of interest demonstrated significant associations between local gyrification index and pial surface area across the groups. A different region of interest (left lateral occipital cortex) demonstrated not only increased sulcal depth/gyral height in the ASD group relative to controls, but also a significant association between local gyrification index and sulcal depth/gyral height across the groups. Taken together, these findings suggest that gyrification increases in ASD may be the result of the aetiological mechanisms, such as the number of radial units along the ventricular zone, that drive regionally specific surface areal expansion (Rakic, 1988).
Additionally, we found a discrepancy between groups in the vocabulary–local gyrification index correlation. Within left inferior parietal cortex, in the vicinity of the supramarginal/angular gyri we found that vocabulary knowledge was robustly correlated with local gyrification index in the typically developing group, though not in the ASD group. Similarly, within two of the three clusters (i.e. left and right lateral occipital cortices) where increased local gyrification index was found in the ASD group, there were also discrepant vocabulary–local gyrification index correlations (significant positive associations in the typically developing group, not in the ASD group). These significantly positive correlations in the typically developing group fit with previous postulations about the relationship between gyrification levels and cognitive abilities (White et al., 2010). Moreover, the location (inferior parietal cortex) of the independent analyses revealing not only a group discrepancy in vocabulary-local gyrification index associations but also a strong correlation between vocabulary knowledge and gyrification in the typically developing group alone was highly concordant. Other measures of cortical structure (e.g. grey matter density) in this same region have been linked with individual differences in vocabulary knowledge (Mechelli et al., 2004; Lee et al., 2007). However, this is the first study, to our knowledge, to establish this relationship with gyrification and to show its dissociation in ASD. Placed in the context of the broader literature, our findings extend one prior study that found ASD-typically developing group discrepancies in IQ-lobar-level local gyrification index correlations in the temporal, parietal, and occipital lobes (Kates et al., 2009). However, whereas their study included ASD and control participants with IQs that differed by >50 points, on average, the current study matched participants on IQ (with average range or higher scores). Taken together, these studies suggest that aberrant gyrification in ASD may disrupt the typically significant positive association between IQ-related measures (vocabulary knowledge in the current study) and cortical folding as observed in the typically developing controls here, in earlier studies of typically developing individuals (Luders et al., 2008; Kates et al., 2009), and in other clinical groups (e.g. bipolar disorder; McIntosh et al., 2009).
Finally, we also found an indication that parent ratings of primarily social-communication difficulties were negatively correlated with local gyrification index in the right superior temporal sulcus of typically developing subjects. This preliminary finding was particularly noteworthy because it replicated—in a smaller group of subjects—our recent report of a strong relationship between Social Responsiveness Scale ratings and cortical structure (cortical thickness in that case) in right superior temporal sulcus in a much larger cohort of typically developing subjects (Wallace et al., 2012).
There are several limitations to consider when evaluating this research. The ASD sample of this study was limited to males who had average to above average IQ; therefore, these findings may not apply to lower functioning individuals and/or females on the autism spectrum. We chose to limit our sample to males because of earlier work demonstrating sex differences in surface area and gyrification (Raznahan et al., 2011) during typical development. This study focused on ASD individuals with average range or higher IQ in order to isolate ASD-specific effects on surface area and local gyrification index and because of previous demonstrations of both surface area (Fahim et al., 2012; Meda et al., 2012) and gyrification (Bonnici et al., 2007; Zhang et al., 2010) reductions among individuals with syndromic and idiopathic intellectual impairments. Finally, the present cross-sectional study assessed age-related changes in surface area and gyrification among ASD and typically developing individuals, but the most stringent test of developmental effects requires longitudinal designs that could be utilized in future investigations.
Nevertheless, the present study makes a novel contribution to the existing literature by demonstrating increased gyrification but intact surface area at high-resolutions in adolescents and young adults with ASD and by showing a dissociation in the link between vocabulary knowledge and local gyrification index for those with ASD (no association) versus typically developing controls (a positive correlation). Furthermore, the negative correlation between age and gyrification is comparable for ASD and typically developing groups during adolescence/young adulthood, which stands in stark contrast to our previous findings of age-related cortical thinning in this same ASD sample (Wallace et al., 2010) and suggests that there are developmentally dissociable cortical atypicalities in ASD. Future studies should link these types of gyrification measures with metrics of structural and functional connectivity and examine gyrification in younger ASD samples to further explore the age-dependence of this atypical increase in cortical folding. Finally, given the extensive smoothing field inherent to the local gyrification index quantification used here, future research could use complementary high-resolution metrics, such as intrinsic curvature (Ronan et al., 2011), to further characterize ASD-specific atypicalities in cortical surface morphometry.
Acknowledgements
We would like to thank Marie Schaer and Douglas Greve for their many helpful insights on processing and analysis of local gyrification and surface area, respectively. We would also like to thank the children/adults and their families who so kindly gave their time and energy to assist in completing this research.
Glossary
Abbreviation
- ASD
autism spectrum disorders
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
References
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Alberts BM, Andreasen NC, Bargmann C, Benes F, Goldman-Rakic P, et al. Workshop on schizophrenia. Proc Natl Acad Sci USA. 1997;94:1612–4. doi: 10.1073/pnas.94.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, William T, Moorhead J, Stanfield AC, Harris JM, Owens DG, et al. Pre-frontal lobe gyrification index in schizophrenia, mental retardation and comorbid groups: an automated study. Neuroimage. 2007;35:648–54. doi: 10.1016/j.neuroimage.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Booth R, Wallace GL, Happé F. Connectivity and the corpus callosum in autism spectrum conditions: insights from comparison of autism and callosal agenesis. Prog Brain Res. 2011;189:303–17. doi: 10.1016/B978-0-444-53884-0.00031-2. [DOI] [PubMed] [Google Scholar]
- Casanova MF, El-Baz A, Mott M, Mannheim G, Hassan H, Fahmi R, et al. Reduced gyral window and corpus callosum size in autism: possible macroscopic correlates of a minicolumnopathy. J Autism Dev Disord. 2009;39:751–64. doi: 10.1007/s10803-008-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouchoux C, Kudelski D, Gholipour A, Warfield SK, Viseur S, Bouyssi-Kobar M, et al. Quantitative in vivo MRI measurement of cortical development in the fetus. Brain Struct Funct. 2012;217:127–39. doi: 10.1007/s00429-011-0325-x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004;45:719–26. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011;306:2001–10. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis-I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Ecker C, Marquand A, Mourão–Miranda J, Johnston P, Daly EM, Brammer MJ, et al. Describing the brain in autism in five dimensions—magnetic resonance imaging-assisted diagnosis of autism spectrum disorder using a multiparameter classification approach. J Neurosci. 2010;30:10612–23. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, Yoon U, Nashaat NH, Khalil AK, El-Belbesy M, Mancini-Marie A, et al. Williams syndrome: a relationship between genetics, brain morphology and behaviour. J Intellect Disabil Res. 2012;78:74–84. doi: 10.1111/j.1365-2788.2011.01490.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis-II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–25. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Jou RJ, Keshavan MS, Varma R, Minshew NJ. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 2004;131:263–8. doi: 10.1016/j.pscychresns.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biol Psychiatry. 2012;72:207–14. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Ikuta I, Burnette CP. Gyrification patterns in monozygotic twin pairs varying in discordance for autism. Autism Res. 2009;2:267–78. doi: 10.1002/aur.98. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: a study by the collaborative program of excellence in autism. Am J Med Genet A. 2006;140A:2257–74. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism Diagnostic Interview—a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–87. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27:1184–9. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, et al. Cortical sulcal maps in autism. Cereb Cortex. 2003;13:728–35. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised–a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Szeszko PR, Gurbani MN, Hamilton L, et al. Mapping the relationship between cortical convolution and intelligence: effects of gender. Cereb Cortex. 2008;18:2019–26. doi: 10.1093/cercor/bhm227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak-Fan KM, Taylor MJ, Roberts W, Lerch JP. Measures of cortical grey matter structure and development in children with autism spectrum disorder. J Autism Dev Disord. 2012;42:419–27. doi: 10.1007/s10803-011-1261-6. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, McKirdy J, Hall J, Sussmann JE, Stanfield AC, et al. Prefrontal gyral folding and its cognitive correlates in bipolar disorder and schizophrenia. Acta Psychiatr Scand. 2009;119:192–8. doi: 10.1111/j.1600-0447.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, et al. Neurolinguistics: structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Meda SA, Pryweller JR, Thornton-Wells TA. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with Williams syndrome. PLoS One. 2012;7:e31913. doi: 10.1371/journal.pone.0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguid N, Fahim C, Yoon U, Nashaat NH, Ibrahim AS, Mancini-Marie A, et al. Brain morphology in autism and fragile X syndrome correlates with social IQ: first report from the Canadian-Swiss-Egyptian Neurodevelopmental Study. J Child Neurol. 2010;25:599–608. doi: 10.1177/0883073809341670. [DOI] [PubMed] [Google Scholar]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27:11725–35. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 2011;69:974–79. doi: 10.1016/j.biopsych.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–83. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Piven J, Berthier ML, Starkstein SE, Nehme E, Pearlson G, Folstein S. Magnetic resonance imaging evidence for a defect of cerebral cortical development in autism. Am J Psychiatry. 1990;147:734–49. doi: 10.1176/ajp.147.6.734. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–40. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Proitsi P, Powell J, Paus T, Bolton P, et al. A functional polymorphism of the brain derived neurotrophic factor gene and cortical anatomy in autism spectrum disorder. J Neurodev Disord. 2009;1:215–23. doi: 10.1007/s11689-009-9012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, et al. Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry. 1986;143:862–6. doi: 10.1176/ajp.143.7.862. [DOI] [PubMed] [Google Scholar]
- Ronan L, Pienaar P, Williams G, Bullmore E, Crow TJ, Roberts N, et al. Intrinsic curvature: a marker of millimeter-scale tangential cortico-cortical connectivity? Int J Neural Syst. 2011;21:351–66. doi: 10.1142/S0129065711002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27:161–70. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schaer M, Glaser B, Cuadra MB, Debbane M, Thiran JP, Eliez S. Congenital heart disease affects local gyrification in 22q11.2 deletion syndrome. Dev Med Child Neurol. 2009;51:746–53. doi: 10.1111/j.1469-8749.2009.03281.x. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Nordahl CW. Bridging the gap between MRI and postmortem research in autism. Brain Res. 2011;1380:175–86. doi: 10.1016/j.brainres.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, White T, Schmidt M, Kao CY, Sapiro G. Geometric computation of human gyrification indexes from magnetic resonance images. Hum Brain Mapp. 2013;34:1230–44. doi: 10.1002/hbm.21510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36:604–25. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–54. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Shaw P, Lee NR, Clasen LS, Raznahan A, Lenroot RK, et al. Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. J Neurosci. 2012;32:4856–60. doi: 10.1523/JNEUROSCI.6214-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23:339–52. doi: 10.1017/S0954579410000842. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain Cogn. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EL, El-Baz A, Nitzken M, Switala AE, Casanova MF. Spherical harmonic analysis of cortical complexity in autism and dyslexia. Transl Neurosci. 2012;3:36–40. doi: 10.2478/s13380-012-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BT, Fischl B, Greve DN, Kochunov P, et al. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage. 2012;61:1428–43. doi: 10.1016/j.neuroimage.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou Y, Yu C, Lin L, Li C, Jiang T. Reduced cortical folding in mental retardation. Am J Neuroradiol. 2010;31:1063–7. doi: 10.3174/ajnr.A1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Armstrong E, Schleicher A, Kretschmann HJ. The human pattern of gyrification in the cerebral cortex. Anat Embryol. 1988;179:173–9. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]