Abstract
Objectives. We examined demographic and socioeconomic differences in the consumption of sugar-sweetened beverages (SSBs), its association with dental caries in children, and whether exposure to water fluoridation modifies this association.
Methods. In a cross-sectional study, we used a stratified, clustered sampling design to obtain information on 16 508 children aged 5 to 16 years enrolled in Australian school dental services in 2002 to 2005. Dental staff assessed dental caries, and parents completed a questionnaire about their child’s residential history, sources of drinking water, toothbrushing frequency, socioeconomic status (SES), and SSB consumption.
Results. Children who brushed their teeth less often and were older, male, of low SES, from rural or remote areas consumed significantly more SSBs. Caries was significantly associated with greater SSB consumption after controlling for potential confounders. Finally, greater exposure to fluoridated water significantly reduced the association between children’s SSB consumption and dental caries.
Conclusions. Consumption of SSBs should be considered a major risk factor for dental caries. However, increased exposure to fluoridated public water helped ameliorate the association between SSB consumption and dental decay. These results reconfirm the benefits of community water fluoridation for oral health.
Sugar-sweetened beverages (SSBs), including soft drinks (soda or pop), mineral waters (sweetened but noncarbonated beverages), cordials (sweet concentrates to which water is added), and sports (electrolyte) drinks, are commonly consumed in many countries, and consumption patterns have demonstrated an increase over time. In the United States, for example, soft drink consumption increased by approximately 500% between 1947 and 1999.1 Increases in consumption occurred for both children and adults. In the 25-year period between 1977 and 2002, consumption of soft drinks as a percentage of total beverage intake by children aged 6 to 11 years increased from 15% to 33%.2 In Australia, consumption of carbonated beverages increased by 240% between 1969 and 1999.3
Because of SSBs’ often high sugar content, their excessive consumption has been linked to several deleterious heath effects, most notably overweight and obesity. One US study found that for each additional serving per day of a sugar-sweetened drink, the risk for obesity increased 60% after adjustment for anthropometric, demographic, dietary, and lifestyle variables.4 In Australia, children consuming 3 or more soft drinks per day have 2.2 times the odds of being obese or overweight than children who do not consume soft drinks.5 Excessive soft drink consumption has also been linked to diabetes, metabolic dysfunction, osteoporosis, high blood pressure, and liver disease.6
In terms of oral diseases, the association between SSB consumption and both dental erosion and caries has been investigated. Consistent evidence has shown, for example, that the high acidity of many sweetened drinks, particularly soft drinks and sports drinks, can be a causal factor in dental erosion.7 However, and despite studies going as far back as the 1950s implicating the role of soft drink consumption in dental decay,8 relatively few studies have examined the cariogenicity of SSBs. One reason is that SSB consumption is often subsumed within a broader research perspective investigating the role of dietary sugars generally in the process of dental caries. Although the etiological role of sugars and other fermentable carbohydrates in caries activity has been well established,9,10 the causal role of specific foods in the overall diet can be harder to determine.11
Only a handful of studies have shown associations between SSB consumption and dental caries. For example, Ismail et al.12 found a significant positive association between the frequency of at- and between-meal consumption of soft drinks and dental caries. More recently, children aged 2 to 10 years with a predominantly high soft drink diet were found to be 1.8 times more likely to experience dental caries in the primary dentition than children with a predominantly high water consumption pattern.13 Similarly, young children with caries have significantly greater intake of soft drinks than children without caries.14 In the United Kingdom, caries prevalence in the primary dentition was found to be substantially higher among children with a higher than average frequency of SSB consumption, but little difference was found in the permanent dentition of older children.15 In Australia, higher soft drink consumption among children aged 12 years and younger was found to be positively associated with more primary tooth extractions.16 However, other studies investigating soft drink consumption and dental caries have yielded contradictory evidence.11,17 Indeed, a recent review and meta-analysis of the literature, which included only 4 studies, indicated only a small positive (r = .03) association between soft drink consumption and caries.6
One question that has received little attention is whether other risk or protective factors might modify any association between soft drink consumption and dental caries. The effect of soft drink consumption on caries might be weaker for children who come from higher income families, who brush their teeth with fluoride toothpaste more often, or who have greater exposure to fluoridated water. In particular, an overwhelming amount of research has demonstrated, and continues to demonstrate, that drinking fluoridated water offers a considerable caries preventive benefit.18–20 The US Surgeon General has declared that water fluoridation is not only efficient and cost effective but is also the single most effective means of preventing tooth decay over a person’s lifetime.21 However, surprisingly little research has been conducted into whether water fluoridation also confers benefits by reducing the impact of risk factors for dental caries. Any effect of soft drink consumption on dental caries may be mitigated to some extent when children are also receiving the benefits of consuming fluoridated water.
We investigated this possible association between sweetened drink consumption and caries in both deciduous and permanent teeth in a large and representative group of Australian schoolchildren. In addition, we aimed to describe demographic and socioeconomic status (SES) differences in SSB consumption. Finally, we examined whether other protective factors for dental disease, such as higher SES, more frequent toothbrushing, and residing in an area with fluoridated water, modified any association between the consumption of sugared beverages and dental caries.
METHODS
We used cross-sectional baseline data from a longitudinal cohort study. Multisite baseline data were collected between 2002 and 2005 using a stratified random sampling of children from 4 Australian states: South Australia, Victoria, Tasmania, and Queensland. Within each state, children were stratified by metropolitan or nonmetropolitan residence and by whether they lived in a fluoridated or nonfluoridated region. Within each of the defined strata, we selected School Dental Service clinics according to probability proportional to size using the clinic’s average annual client throughput. Children were sampled when attending their routine School Dental Service examination and randomly selected using their date of birth. At the time of the study, state-based School Dental Services in Australia provided free or subsidized dental services to all children regardless of parental income or whether the children attended a public or private school.
Measures
Selected children were provided with a questionnaire that asked a parent or guardian to provide information on their child’s toothpaste use and brushing practices, exposure to other possible fluoride sources, residence history and water consumption details for each residence, food consumption in infancy, current consumption of certain foods and beverages, and household and SES characteristics.
We determined current SSB consumption with a question about the number of servings of “sweetened (non-diet) soft drinks, mineral waters, cordial, and sports drinks” the child consumed in a usual day, where a standard serving suggestion was given as 1 medium glass. SSB consumption was categorized as “0 drinks per day on average,” “1–2 drinks per day on average,” or “3 or more drinks per day on average.”
We calculated percentage of lifetime exposure to fluoridated water using a database on the fluoride level in public water for each Australian postal code and from information provided by the parents on the child’s residential history and drinking water source at each residence. We multiplied the number of years the child spent at each residence of 6 months or more by the fluoride concentration (0.0, 0.5, or 1.0 ppm) for that locality during the period of residence. Moreover, if the public water supply was not the usual source of drinking water for a child at any given residence, adjustments were made on the assumption that rainwater tanks, bores/wells, and bottled water have fluoride concentrations of less than 0.3 parts per million. We summed the product of years of residence and imputed fluoride concentration for each residence for all listed residences for each child and then divided it by the total time spent by the child at all residences. Percentage of lifetime exposure to optimally fluoridated water was categorized as either 0% to 50% of each child’s life or greater than 50% of each child’s life.
Parents were also asked how often their children currently brushed their teeth, with possible responses being “less than once a day,” “once a day,” “twice a day,” or “more than twice a day.” Average household income was requested, with possible response categories (in AU $) being “up to $20,000,” “$20,001 to $40,000,” “$40,001 to $60,000,” “$60,001 to $80,000,” “$80,001 to $100,000,” and “over $100,000.” We calculated parents’ highest educational attainment from whether any parent had attended a university. Finally, we determined residence remoteness by matching the postal code of the child’s residence to data from the Australian Bureau of Statistics, enabling any child residence to be defined as “major city,” “inner regional,” “outer regional,” “remote,” or “very remote.” For the purpose of analysis, most of these variables were dichotomized.
Oral Examinations
As part of the dental examination, data were collected on caries using visual criteria at the tooth level and included cavitated lesions, teeth that were filled because of caries, and teeth that were missing because of caries.22 Because of the large number of dentists and therapists involved in data collection around Australia, calibration of all examiners was not feasible. However, a level of standardization was attempted through the use of instruction manuals and training.
Examinations were carried out by staff of the School Dental Service, which includes dentists and dental therapists. The main outcome variables were the number of decayed, missing, and filled teeth in the primary or deciduous dentition and in the adult or permanent dentition.
Data Analysis
Because of the continued exfoliation of deciduous teeth and the eruption of permanent teeth from the age of about 6 years, we confined analyses of deciduous caries to children aged 5 to 10 years and analyses of permanent caries to children aged 11 to 16 years.
We conducted analyses between categorical variables using cross-tabulations and χ2 statistics. For analyses between categorical independent variables and continuous dependent variables, we used 1-way, 2-way, or multiple-way analysis of variance (i.e., general linear modeling). A criterion α of .05 was used to determine statistical significance.
We used univariate general linear modeling (with sweetened drink consumption used as a continuous variable) and cross-tabulations (with sweetened drink consumption categorized as 0 drinks, 1–2 drinks, and ≥ 3 drinks per day on average) to analyze differences in SSB consumption and caries by child age, gender, household income, parental educational attainment, residence remoteness, toothbrushing frequency, and adjusted percentage of lifetime exposure to fluoridated water. We computed multivariate general linear models to test whether the effect of SSB consumption on caries experience remained statistically significant after controlling for the other variables.
We tested the potential effect modification of household income, toothbrushing frequency, and percentage of lifetime exposure to fluoridated water on the association between SSB consumption and dental caries using 3 general linear models (analyses of variance) for both deciduous dentition (children aged 5–10 years) and permanent dentition (children aged 11–16 years). For all models, for both deciduous and permanent dentition, we entered the variable of interest into an analysis of variance along with SSB consumption and an interaction term. Effect modification was assessed by the significance of the interaction term.
Data were weighted by the probability of selection of the child and the clinic. In addition, to adjust for possible bias resulting from the nonresponse rate, poststratification weighting using census-derived gold-standard estimates from the Australian Bureau of Statistics was carried out for individual-year age and gender population estimates within each of the 13 strata as defined by state, metropolitan status, and fluoridated water status. Unless stated otherwise, all results presented here use weighted data. We conducted analyses using PASW (SPSS) version 18.0 (PASW Statistics, Chicago, IL).
RESULTS
In all, 27 490 questionnaires were distributed to children with 18 541 returned, an overall response rate of 67.4%. Questionnaires for children aged 5 years or older were matched to oral examination data via unique identification numbers that were assigned to children. However, between 1.1% and 13.9% of the questionnaire data of participants in the 4 states could not be matched to an examination, so the final data set included matched questionnaire and oral health data for 16 857 children.
Table 1 shows the frequency of demographic, SES, and fluoride exposure variables among the child population. The age distribution of children shows more children aged 5 to 10 than aged 11 to 17 and is a result of differences in the ages of children seen by the School Dental Service of different states. The distribution of sweetened drink consumption demonstrated an appreciable positive skew, with 44.2% of children consuming less than 1 standard glass of SSBs per day, a similar percentage (43.2%) consuming between 1 and 2, and only a relatively small percentage of children (12.7%) consuming 3 or more. A small number of children (n = 361; 2.2%) were recorded as drinking 5 or more standard glasses of SSBs per day.
TABLE 1—
SSB Consumption and Caries Experience by Demographic, Socioeconomic Status, and Fluoride Exposure Among Children Enrolled in Australian School Dental Services: South Australia, Victoria, Tasmania, and Queensland, 2002–2005
| Daily SSB Consumption |
Caries Experience |
||||||
| Variable | No. (%) | Mean (95% CI) | 0 SSBs | 1–2 SSBs | ≥ 3 SSBs | Aged 5–10 Years, Mean dmft (95% CI) | Aged 11–16 Years, Mean DMFT (95% CI) |
| Age, y | |||||||
| 5–7 | 5152 (31.2) | 0.96*** (0.92, 0.99) | 47.9 | 40.2 | 11.9 | 1.73 (1.66, 1.81) | … |
| 8–10 | 5217 (31.6) | 1.01 (0.97, 1.04) | 44.7 | 44.0 | 11.3 | 1.82 (1.75, 1.88) | … |
| 11–13 | 4407 (26.7) | 1.15 (1.11, 1.19) | 40.8 | 45.3 | 13.9 | … | 0.91*** (0.86, 0.95) |
| 14–16 | 1732 (10.5) | 1.25 (1.18, 1.32) | 40.2 | 43.8 | 16.1 | … | 1.61 (1.50, 1.72) |
| Gender | |||||||
| Male | 8471 (51.3) | 1.14*** (1.11, 1.17) | 42.6 | 42.5 | 14.9 | 1.91*** (1.84, 1.99) | 1.08 (1.02, 1.14) |
| Female | 8037 (48.7) | 0.96 (0.94, 0.99) | 45.9 | 43.8 | 10.3 | 1.63 (1.56, 1.70) | 1.13 (1.07, 1.20) |
| Household income, AU $ | |||||||
| ≤ 40 000 | 7078 (46.4) | 1.20*** (1.17, 1.24) | 40.0 | 44.5 | 15.5 | 2.15*** (2.06, 2.23) | 1.21*** (1.14, 1.28) |
| 40 001–80 000 | 5976 (39.2) | 0.99 (0.96, 1.03) | 46.0 | 42.7 | 11.3 | 1.55 (1.47, 1.62) | 1.03 (0.96, 1.11) |
| > 80 000 | 2186 (14.3) | 0.76 (0.72, 0.81) | 52.9 | 39.3 | 7.8 | 1.21 (1.11, 1.32) | 0.95 (0.84, 1.05) |
| Parental education | |||||||
| High school | 9506 (58.9) | 1.20*** (1.17, 1.23) | 39.3 | 45.3 | 15.4 | 1.98*** (1.92, 2.05) | 1.22*** (1.16, 1.28) |
| Some university | 6635 (41.1) | 0.84 (0.81, 0.87) | 51.3 | 39.9 | 8.8 | 1.44 (1.37, 1.51) | 0.91 (0.84, 0.97) |
| Remoteness | |||||||
| Major city | 10 429 (64.0) | 1.00*** (0.98, 1.02) | 44.9 | 43.6 | 11.5 | 1.55*** (1.49, 1.61) | 0.95*** (0.89, 1.00) |
| Inner regional | 3467 (21.3) | 1.16 (1.11, 1.21) | 42.4 | 42.5 | 15.1 | 2.23 (2.11, 2.34) | 1.39 (1.29, 1.50) |
| Outer regional to very remote | 2402 (14.7) | 1.11 (1.05, 1.17) | 44.1 | 42.2 | 13.7 | 2.04 (1.90, 2.19) | 1.33 (1.21, 1.44) |
| Tooth brushing/d | |||||||
| ≤ once | 4660 (29.7) | 1.25*** (1.20, 1.29) | 39.5 | 43.9 | 16.6 | 1.85 (1.76, 1.95) | 1.29*** (1.20, 1.38) |
| ≥ twice | 11 010 (70.3) | 0.97 (0.95, 0.99) | 46.0 | 42.9 | 11.1 | 1.75 (1.69, 1.81) | 0.99 (0.94, 1.04) |
| Fluoridated water exposure, % | |||||||
| 0–50 | 7285 (45.0) | 1.04 (1.01, 1.07) | 45.9 | 41.9 | 12.2 | 2.18*** (2.10, 2.27) | 1.26*** (1.19, 1.33) |
| > 50 | 8916 (55.0) | 1.07 (1.04, 1.09) | 42.7 | 44.2 | 13.1 | 1.44 (1.38, 1.49) | 0.96 (0.90, 1.06) |
Note. CI = confidence interval; dmft = decayed, missing, and filled deciduous teeth; DMFT = decayed, missing, and filled permanent teeth; SSB = sugar-sweetened beverage. The sample size was n = 16 508.
***P < .001.
Several demographic, socioeconomic, and behavioral variables were associated with sweetened drink consumption (Table 1). Children drinking more SSBs tended to be older, male, be from lower income households, have parents with lower educational attainment, be from regional and remote residences, and to brush their teeth less often. We found no significant association between fluoridated water exposure and SSB consumption.
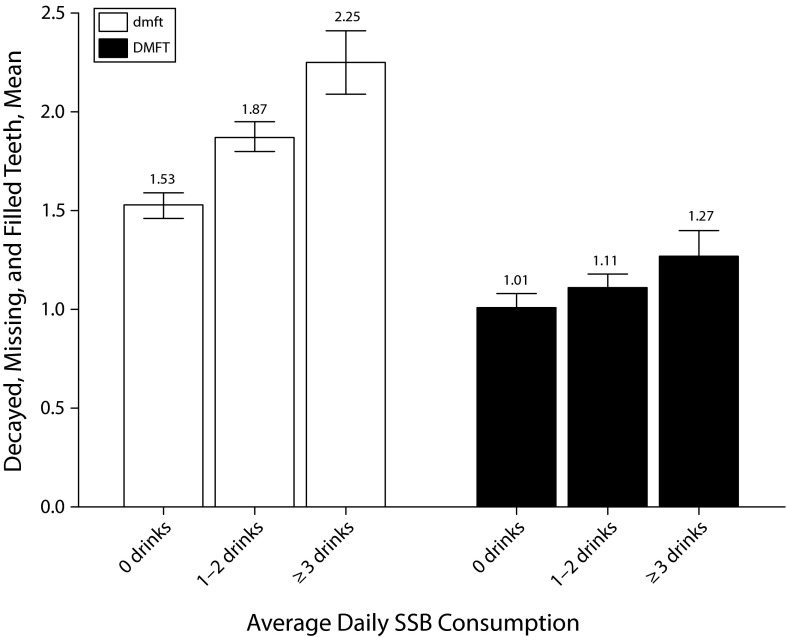
As shown in Figure 1, greater SSB consumption was significantly associated with greater deciduous caries (F[2,10236] = 47.16; P < .001). Young children consuming 3 or more sweetened drinks per day had on average 47.1% more decayed, missing, and filled deciduous teeth than children who did not consume sweetened drinks (Tukey honestly significant difference post hoc comparison, P < .001). The association was not as strong in children aged 11 to 16 years with permanent dentition, although it was still statistically significant (F[2,6081] = 7.60; P = .001). Older children drinking 3 or more sweetened drinks a day had a decayed, missing, and filled permanent teeth score 25.7% higher than that of children who did not consume sweetened drinks (Tukey honestly significant difference post hoc comparison, P < .001).
FIGURE 1—
Caries experience by sugar-sweetened beverage consumption among children enrolled in Australian School Dental Services: South Australia, Victoria, Tasmania, and Queensland, 2002–2005.
Note. dmft = decayed, missing, and filled deciduous teeth; DMFT = decayed, missing, and filled permanent teeth; SSB = sugar-sweetened beverage. Whiskers indicate 95% confidence intervals.
The association between deciduous and permanent caries and the demographic, socioeconomic, and fluoride exposure variables is shown in Table 1. In the deciduous dentition of younger children, all the variables showed statistically significant associations with caries with the exception of toothbrushing frequency. Caries was higher in children who were male, were from lower income families, had parents without a university education, were from regional or remote residences, and had lower fluoridated water exposure. The pattern of associations was similar in children aged 11 to 16 years with permanent dentition. However, among the older children, we found no association between gender and caries, but toothbrushing was significantly associated with caries, being higher for children who brushed their teeth less often.
After controlling for the effect of the other variables, children who consumed 1 or 2 sweetened drinks per day had 0.34 more decayed, missing, and filled deciduous teeth, and children who consumed 3 or more sweetened drinks per day had 0.46 more decayed, missing, or filled deciduous teeth than did children who consumed zero sweetened drinks per day (Table 2). The association was also statistically significant among children with permanent dentition, although it was not as strong.
TABLE 2—
General Linear Modeling of Association of Deciduous and Permanent Caries With Demographic, Socioeconomic Status, Fluoride Exposure, and Soft Drink Consumption Variables Among Children Enrolled in Australian School Dental Services: South Australia, Victoria, Tasmania, and Queensland, 2002–2005
| Variable | Aged 5–10 Years dmft, B (95% CI) | Aged 11–16 Years DMFT, B (95% CI) |
| Age | 0.01 (−0.03, 0.03) | 0.17*** (0.14, 0.20) |
| Gender | ||
| Male (Ref) | 1.00 | 1.00 |
| Female | −0.27*** (−0.37, −0.16) | 0.09 (−0.00, 0.18) |
| Household income, AU $ | ||
| ≤ 40 000 (Ref) | 1.00 | 1.00 |
| 40 001–80 000 | −0.51*** (−0.63, −0.39) | −0.13* (−0.23, −0.03) |
| > 80 000 | −0.64*** (−0.80, −0.48) | 0.03 (−0.12, 0.18) |
| Parental education | ||
| High school (Ref) | 1.00 | 1.00 |
| Some university | −0.29*** (−0.41, −0.18) | −0.16** (−0.26, −0.06) |
| Remoteness | ||
| Major city (Ref) | 1.00 | 1.00 |
| Inner regional | 0.20** (0.06, 0.34) | 0.43*** (0.31, 0.55) |
| Outer regional to Very remote | 0.20* (0.01, 0.38) | 0.18* (0.04, 0.31) |
| Toothbrushing/d | ||
| ≤ once (Ref) | 1.00 | 1.00 |
| ≥ twice | 0.04 (−0.07, 0.16) | −0.27*** (−0.37, −0.17) |
| Fluoridated water exposure, % | ||
| 0–50 (Ref) | 1.00 | 1.00 |
| > 50 | −0.66*** (−0.77, −0.54) | −0.10* (−0.20, 0.00) |
| SSB consumption, no. drinks | ||
| 0 | 1.00 | 1.00 |
| 1–2 | 0.34*** (0.23, 0.45) | 0.16** (0.06, 0.26) |
| ≥ 3 | 0.46*** (0.29, 0.64) | 0.27*** (0.13, 0.41) |
Note. CI = confidence interval; dmft = decayed, missing, and filled deciduous teeth; DMFT = decayed, missing, and filled permanent teeth; SSB = sugar-sweetened beverage.
*P < .05; **P < .01; ***P < .001.
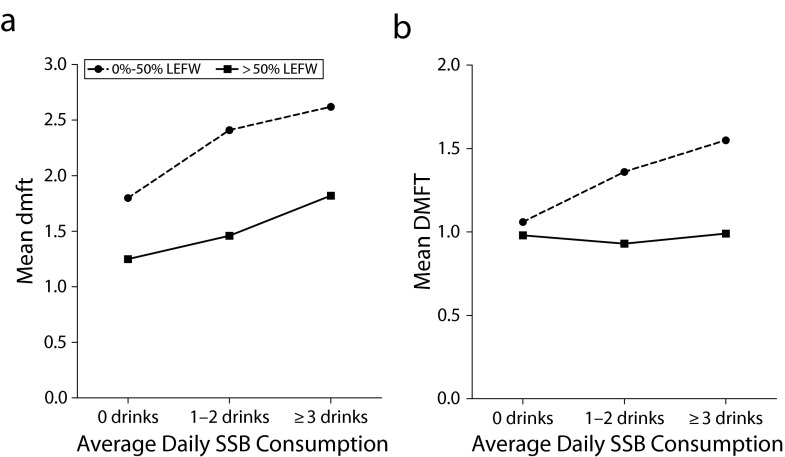
Analyses examining possible effect modification demonstrated no statistically significant effect modification for either household income or toothbrushing behavior (both P > .05). However, the interaction term between lifetime exposure to fluoridated water and sweetened drink consumption was statistically significant for children with both deciduous dentition (F[2,9800] = 7.20; P = .001) and permanent dentition (F[2,5762] = 8.63; P < .001). Figure 2 shows the interaction effect to be much more apparent in the permanent dentition of children aged 11 to 16 years than in the deciduous dentition of those aged 5 to 10 years. We found almost no association between sweetened drink consumption and decayed, missing, and filled permanent teeth for older children with more than a 50% lifetime exposure to fluoridated water. By contrast, for children with lower fluoridated water exposure, the number of decayed, missing, and filled permanent teeth was 46.3% higher among children consuming 3 or more sweetened drinks a day than for children who were not consuming sweetened drinks.
FIGURE 2—
Interactions between SSB consumption and lifetime exposure to fluoridated water on children enrolled in Australian School Dental Services who were (a) aged 5–10 years with dmft and (b) aged 11–16 years with DMFT: South Australia, Victoria, Tasmania, and Queensland, 2002–2005.
Note. dmft = decayed, missing, and filled deciduous teeth; DMFT = decayed, missing, and filled permanent teeth; LEFW = lifetime exposure to fluoridated water; SSB = sugar-sweetened beverage.
DISCUSSION
Although the findings reported in the literature to date have been conflicting, we found consistent dose–response associations between the consumption of SSBs and dental caries. However, the consumption of fluoridated water modified this association, and we found no association between SSB consumption and dental decay in the permanent dentition of older children with the greatest fluoridated water exposure.
The association between SSBs and dental caries was stronger for decay in the deciduous teeth of younger children than in the permanent dentition of older children. Nonetheless, the effect was statistically significant for both younger and older children after controlling for child age, gender, SES, residential location, toothbrushing frequency, and fluoridated water exposure. These findings add to the extensive body of research indicating that SSBs are associated with a range of deleterious lifestyle diseases and conditions. Given that dental decay is one of the most widely experienced diseases in the community, with considerable associated personal and social cost, the role of SSBs as a caries risk indicator needs to be addressed. In particular, and on the basis of a common risk factor approach to health promotion, the risk of dental caries should be incorporated into messages concerning the importance of a healthy diet.
Perhaps the most important finding from this study was that greater exposure to water fluoridation significantly reduced the association between sweetened drink consumption and both caries in both deciduous and permanent teeth. In particular, we found no association between drink consumption and caries in permanent teeth for children aged 11 to 16 years who had resided for more than half their life in optimally fluoridated areas. Although water fluoridation has long been shown to be effective at reducing caries in its own right, much less attention has been paid to how it might either enhance the effectiveness of other preventive measures or mitigate the detrimental effects of some risk factors. However, evidence has shown that water fluoridation can reduce the socioeconomic inequalities in dental caries23 and that it can improve the effectiveness of fissure sealants in preventing decay in children.24 The finding here that greater exposure to fluoridated water might help compensate for a cariogenic diet provides still further support for the continued benefit of water fluoridation as an effective population preventive approach to reducing dental disease.
We found a reasonably high consumption of sweetened (nondiet) drinks among Australian children. Approximately 56% of children consumed at least 1 sugared drink per day, and 12.6% consumed 3 or more on average per day. These results are consistent with those of other Australian studies.25,26 Our study found that boys consumed more sweetened drinks than girls, which has also been reported previously in the literature25 and may be related to differences in the beliefs and attitudes of boys and girls.27
We found considerable differences in sweetened drink consumption across all socioeconomic groupings. Children from the lowest income families consumed almost 60% more servings of sugared drinks than did children from families with a household income of more than $80 000 per year. In addition, children with parents who had attended a university consumed 43% fewer servings of SSBs per day. This finding is consistent with those of studies in several other countries that have demonstrated socioeconomic gradients in soft drink consumption.28–30
We also found that children with higher SSB consumption brushed their teeth less frequently, which is an example of how risk behaviors tend to cluster together in many at-risk individuals. Although we found toothbrushing frequency to only significantly predict dental caries in the permanent dentition of older children, the evidence that effective toothbrushing with fluoride toothpaste is beneficial in reducing caries experience is considerable. Indeed, toothbrushing with fluoridated toothpaste is the single most widely practiced oral health behavior among Australian children.31
Strengths and Limitations
One of the strengths of this study is that rather than restricting our investigation solely to the effects of soft drinks, which is a common approach in much of the literature, we looked at the effects of a broader range of sweetened beverages. Certainly, in Australia, children commonly consume both cordials and sports drinks. Although soft drinks are usually singled out for attention because they are well-identified products, readily available, and marketed aggressively to teenagers, other SSBs have generally been regarded as having a similar impact on energy and nutrient intake.32 Another strength of this study is the large sample size and the representativeness of the children who participated. Because the School Dental Service in Australia sees children in government-funded public schools, religious schools, and private schools, the study results are likely to be generalizable across the Australian child population.
One limitation that should be considered when interpreting these findings is that measurement error may have occurred as a result of a discrepancy between the parent’s report of the child’s beverage consumption and the child’s actual consumption. Even when parents may have asked the child, self-report may have biased the findings because adolescents in particular have been found to overreport dietary intake.33 In addition, we did not take into account a child’s source of drinking water at school, which may have led to an underestimate of fluoridated water consumption for those children drinking little public water at home but more considerable quantities of fluoridated tap water at school.
A further possible limitation of the study is the use of the tooth-level decayed, missing, and filled teeth index as a measure of dental caries experience. Using the number of decayed, missing, and filled surfaces, in comparison, provides greater sensitivity and can produce stronger associations. However, the decayed, missing, and filled teeth index is generally considered to be more robust than the decayed, missing, and filled surfaces index, and we considered this important because of the large number of examiners involved in this study. A more robust index is believed to provide higher interexaminer reproducibility, which can be crucial to the quality of data collected.34
Conclusions
We found that SSB consumption was highest for children from lower SES backgrounds and those who brushed their teeth less often. Greater SSB consumption was associated with children having more dental disease in both the deciduous and the permanent dentition. However, increased exposure to fluoridated water helped ameliorate the apparent deleterious effect of SSB consumption on child dental caries. These results underscore the importance of considering SSB consumption as a major risk indicator for dental caries. The results also reconfirm the continued benefits of community water fluoridation in preventing caries and support the idea that exposure to fluoridated water confers additional benefits in helping to reduce the impact of dental disease.
Acknowledgments
This research was supported by the National Health and Medical Research Council (project grant no. 207806).
We thank the management and staff of the School Dental Service in each of the 4 Australian states involved in this research for their contribution.
Human Participant Protection
Ethics approval for the study was obtained from the University of Adelaide Human Research Ethics Committee.
References
- 1.Putnam JJ, Allshouse JE. Food Consumption, Prices, and Expenditures, 1970–97. Washington, DC: US Department of Agriculture, Economic Research Service, Food and Consumers Economics Division; 1999 [Google Scholar]
- 2.Sebastian RS, Cleveland LE, Goldman JD, Moshfegh AJ. Trends in the food intakes of children 1977-2002. Consumer Interests Annual. 2006;52:433–434 [Google Scholar]
- 3.Hector D, Rangan A, Louie J, Flood V, Gill T. Soft Drinks, Weight Status and Health: A Review. Sydney, New South Wales, Australia: NSW Centre for Public Health Nutrition; 2009 [Google Scholar]
- 4.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357(9255):505–508 [DOI] [PubMed] [Google Scholar]
- 5.Sanigorski AM, Bell AC, Swinburn BA. Association of key foods and beverages with obesity in Australian schoolchildren. Public Health Nutr. 2007;10(2):152–157 [DOI] [PubMed] [Google Scholar]
- 6.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon MEJ. Soft drinks and dental health: a review of the current literature. J Dent. 2006;34(1):2–11 [DOI] [PubMed] [Google Scholar]
- 8.Bibby BG, Goldberg HJV, Chen E. Evaluation of caries-producing potentialities of various foodstuffs. J Am Dent Assoc. 1951;42(5):491–509 [DOI] [PubMed] [Google Scholar]
- 9.Rugg-Gunn AJ. Diet and dental caries. In: Murray JJ, ed. Prevention of Oral Disease. Oxford, England: Oxford University Press; 1996:3–31 [Google Scholar]
- 10.Sheiham A. Dietary effects on dental diseases. Public Health Nutr. 2001;4(2B):569–591 [DOI] [PubMed] [Google Scholar]
- 11.Heller KE, Burt BA, Eklund SA. Sugared soda consumption and dental caries in the United States. J Dent Res. 2001;80(10):1949–1953 [DOI] [PubMed] [Google Scholar]
- 12.Ismail AI, Burt BA, Eklund SA. Cariogenicity of soft drinks in the United States. J Am Dent Assoc. 1984;109(2):241–245 [DOI] [PubMed] [Google Scholar]
- 13.Sohn W, Burt BA, Sowers MR. Carbonated soft drinks and dental caries in the primary dentition. J Dent Res. 2006;85(3):262–266 [DOI] [PubMed] [Google Scholar]
- 14.Marshall TA, Levy SM, Broffitt Bet al. Dental caries and beverage consumption in young children. Pediatrics. 2003;112(3 pt 1):e184–e191 [DOI] [PubMed] [Google Scholar]
- 15.Walker A, Gregory J, Bradnock G, Nunn J, White D. National Diet and Nutrition Survey: Young People Aged 4–18 Years. Vol. 2: Report of the Oral Health Survey. London, England: The Stationery Office, 2000 [Google Scholar]
- 16.Slater PJ, Gkolia PP, Johnson HL, Thomas AR. Patterns of soft drink consumption and primary tooth extractions in Queensland children. Aust Dent J. 2010;55(4):430–435 [DOI] [PubMed] [Google Scholar]
- 17.Forshee RA, Storey ML. Evaluation of the association of demographics and beverage consumption with dental caries. Food Chem Toxicol. 2004;42(11):1805–1816 [DOI] [PubMed] [Google Scholar]
- 18.Locker D. Benefits and risks of water fluoridation: an update of the 1996 Federal-Provincial Sub-committee Report. Toronto, ON, Canada: Community Dental Health Services Research Unit; 1999 [Google Scholar]
- 19.McDonagh MS, Whiting PF, Wilson PMet al. Systematic review of water fluoridation. BMJ. 2000;321(7265):855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health and Medical Research Council A Systematic Review of the Efficacy and Safety of Fluoridation. Canberra, Australian Capital Territory, Australia: Australian Government; 2007 [Google Scholar]
- 21.Carmona R. Surgeon General Statement on Community Water Fluoridation. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2004 [Google Scholar]
- 22.World Health Organization Oral Health Surveys—Basic Methods. 4th ed. Geneva, Switzerland: World Health Organization; 1997 [Google Scholar]
- 23.Slade GD, Spencer AJ, Davies MJ, Stewart JF. Influence of exposure to fluoridated water on socioeconomic inequalities in children’s caries experience. Community Dent Oral Epidemiol. 1996;24(2):89–100 [DOI] [PubMed] [Google Scholar]
- 24.Armfield JM, Spencer AJ. Community effectiveness of fissure sealants and the effect of fluoridated water consumption. Community Dent Health. 2007;24(1):4–11 [PubMed] [Google Scholar]
- 25.Australian Bureau of Statistics National Nutrition Survey: Foods Eaten, Australia, 1995. Canberra, Australian Capital Territory, Australia: Australian Bureau of Statistics, 1999. Catalogue No. 4804.0. Available at: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/4804.01995?OpenDocument. Accessed October 18, 2012 [Google Scholar]
- 26.Food Standards Australia New Zealand Consumption of Intense Sweeteners in Australia and New Zealand: Benchmark Survey 2003. Evaluation Report Series no. 8. Canberra, Australian Capital Territory, Australia: Food Standards Australia New Zealand; 2004. Available at: http://www.foodstandards.gov.au/_srcfiles/Intense_sweetener_Report_feb04.pdf. Accessed October 18, 2012 [Google Scholar]
- 27.Booth M, Okely AD, Denney-Wilson E, Hardy L, Yang B, Dobbins T. NSW Schools Physical Activity and Nutrition Survey (SPANS) 2004: Full Report. Sydney, New South Wales, Australia: NSW Department of Health; 2006. Available at: http://www.health.nsw.gov.au/pubs/2006/pdf/spans_report.pdf. Accessed October 18, 2012 [Google Scholar]
- 28.Ortiz-Hernández L, Gómez-Tello BL. Food consumption in Mexican adolescents. Rev Panam Salud Publica. 2008;24(2):127–135 [DOI] [PubMed] [Google Scholar]
- 29.Rehm CD, Matte TD, Van Wye G, Young C, Frieden TR. Demographic and behavioral factors associated with daily sugar-sweetened soda consumption in New York City adults. J Urban Health. 2008;85(3):375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vereecken CA, Inchley J, Subramanian SV, Hublet A, Maes L. The relative influence of individual and contextual socio-economic status on consumption of fruit and soft drinks among adolescents in Europe. Eur J Public Health. 2005;15(3):224–232 [DOI] [PubMed] [Google Scholar]
- 31.Armfield JM, Spencer AJ. Dental Health Behaviours Among Children. Dental statistics and research series no. 56. Canberra, Australian Capital Territory, Australia: Australian Institute of Health and Welfare; 2012 [Google Scholar]
- 32.Gill TP, Rangan AM, Webb KL. The weight of evidence suggests that soft drinks are a major issue in childhood and adolescent obesity. Med J Aust. 2006;184(6):263–264 [DOI] [PubMed] [Google Scholar]
- 33.Livingstone MB, Robson PJ. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59(2):279–293 [DOI] [PubMed] [Google Scholar]
- 34.Bian JY, Wang WH, Wang WJ, Rong WS, Lo EC. Effect of fluoridated milk on caries in primary teeth: 21-month results. Community Dent Oral Epidemiol. 2003;31(4):241–245 [DOI] [PubMed] [Google Scholar]