Abstract
Background
To estimate the incremental cost-effectiveness of amblyopia screening at preschool and kindergarten, we compared the costs and benefits of 3 amblyopia screening scenarios to no screening and to each other: (1) acuity/stereopsis (A/S) screening at kindergarten, (2) A/S screening at preschool and kindergarten, and (3) photoscreening at preschool and A/S screening at kindergarten.
Methods
We programmed a probabilistic microsimulation model of amblyopia natural history and response to treatment with screening costs and outcomes estimated from 2 state programs. We calculated the probability that no screening and each of the 3 interventions were most cost-effective per incremental quality-adjusted life year (QALY) gained and case avoided.
Results
Assuming a minimal 0.01 utility loss from monocular vision loss, no screening was most cost-effective with a willingness to pay (WTP) of less than $16,000 per QALY gained. A/S screening at kindergarten alone was most cost-effective between a WTP of $17,000 and $21,000. A/S screening at preschool and kindergarten was most cost-effective between a WTP of $22,000 and $75,000, and photoscreening at preschool and A/S screening at kindergarten was most cost-effective at a WTP greater than $75,000. Cost-effectiveness substantially improved when assuming a greater utility loss. All scenarios were cost-effective when assuming a WTP of $10,500 per case of amblyopia cured.
Conclusions
All 3 screening interventions evaluated are likely to be considered cost-effective relative to many other potential public health programs. The choice of screening option depends on budgetary resources and the value placed on monocular vision loss prevention by funding agencies.
INTRODUCTION
Amblyopia refers to permanent visual loss caused by deficient brain learning of visual acuity associated with blocked, misaligned, or blurred images. Amblyopia develops in children as the brain adapts to visual discrepancies between the eyes by ignoring sensory input from 1 eye.1 Amblyopia is usually easily treated in young children by providing glasses and forcing the use of the amblyopic eye through patching or penalization of the better-seeing eye. However, treatment efficacy decreases as children age, and amblyopic visual loss can be irreversible if not diagnosed and treated early.1
Studies estimating the cost-effectiveness of childhood amblyopia screening have found it to be cost-effective when assuming a small utility loss associated with monocular impairment.2–12 However, no evidence currently supports the existence of such utility losses. A recent U.K. Health Technology Assessment argued that the quality-adjusted life year (QALY) losses used in these studies were derived from adult patients with progressive diseases and reflected anxiety over future bilateral blindness in addition to the utility losses (if any) from monocular impairment.2
Previous research has shown that early childhood vision screening programs can achieve high detection and referral rates at relatively low cost.13,14 However, amblyopia screening cost-effectiveness studies lack cost and referral data from real US screening implementations. At least 35 states mandate some childhood visual screening; 26 mandate screening in elementary school and 16 mandate screening in preschool. Screening technology also differs across states. Many state programs use only acuity charts, whereas 12 states mandate additional stereopsis tests, and at least 2 states use photoscreening as the primary screening test for preschoolers.15
In this paper, we model 3 amblyopia screening strategies in order to evaluate their relative cost-effectiveness when applied to a hypothetical cohort of children representative of the U.S. birth cohort. Our model uses primary programmatic cost, referral rate, and follow-up treatment data from 2 state screening programs, although we do not model the cost-effectiveness of those programs themselves.
METHODS
We developed a randomized, person-level simulation model that runs from age 3 until death or age 100. We assigned each person a race/ethnicity (non-Hispanic African American, non-Hispanic Caucasian, Hispanic), sex, and life expectancy based on the 2008 U.S. population.16 We excluded other races due to lack of data on visual outcomes. We simulated amblyopia incidence, detection with and without screening, treatment efficacy, and adult visual disease and then tracked monocular and binocular visual impairment and blindness, lifetime ophthalmologic costs, and QALYs per person. We discounted costs and QALYs to 2005 values using a 3% discount rate. We take the societal perspective except for excluding the costs of informal caregivers and lost productivity that result from adult visual impairment. These are likely to be small because of the number of years they would be discounted. We programmed the model in AnyLogic 5.4.1 (XJ Technologies Company, Ltd., St. Petersburg, Russia).
Estimation Methodology
We used probabilistic sensitivity analysis (PSA) to estimate the expected lifetime per person costs and QALYs associated with each scenario and the credible interval associated with those means. For each key model parameter, we selected a mean based on published studies and a standard error and distribution based on published simulation principles17,18 (Table 1). We selected the number of model replications (4,000 to 7,200 per scenario) and the number of patients simulated per replication (10,000) that would allow us to detect a difference of 0.0002 in lifetime QALYs between any 2 scenarios without the need to adjust standard error estimates for stochastic patient-level error.31 (Insert Table 1 here.)
Table 1.
Key Model Parameters, 2.5th and 97.5th Percentiles, and Distributions Used to Generate Simulation Results
| Parameter | Mean Value (Interval for PSA) | Source |
|---|---|---|
| Prevalence of amblyopia | 2.5* (1.5–4.0) | 19 |
| Annual QALY decrement from amblyopic monocular visual impairment | 0.010* (0.003–0.017) | Assumption |
| Annual probability a child ages 3 to 7 is examined for amblyopia without screening | 0.037† (0.035–0.040) | 20 |
| Annual probability a child ages 7 to 17 is examined for amblyopia without screening | 0.090† (0.085–0.095) | 21 |
| Sensitivity of A/S screening at age 3 | 0.72† (0.67–0.77) | 22, PBGA Data |
| Specificity of A/S screening at age 3 | 0.960 Did not vary | 22, PBGA Data |
| Sensitivity of A/S screening at age 5 | 0.75† (0.70–0.80) | 22, PBGA |
| Specificity of A/S screening at age 5 | 0.960 Did not vary | 22, PBGA |
| Sensitivity of photoscreening at ages 3 and 5 | 0.90† (0.84–0.97) | PBNC/UNC |
| Specificity of photoscreening at ages 3 and 5 | 0.950 Did not vary | PBNC/UNC |
| Rate at which positively screened patients see an eye-care professional (follow-up rate) | 0.68† (0.63–0.73) | PBNC, PBGA |
| Probability treatment is successful at age 3 years | 0.66† (0.61–0.71) | 23–26 |
| Probability treatment is successful at age 5 years (linear trend based on published data) | 0.63† (0.58–0.68) | 23–26 |
| Probability treatment is successful at age 9 years (linear trend based on published data) | 0.36† (0.33–0.39) | 27 |
| Probability treatment is successful at age 14 years (linear trend based on published data) | 0.14† (0.13–0.15) | 27 |
| Probability treatment is successful at age 18 years (linear trend based on published data) | 0 (Did not vary) | Assumption |
| Screening costs: acuity chart + stereopsis, administered in preschool | $6.72‡ ($6.18 $7.22) | PBGA |
| Screening costs: acuity chart + stereopsis, administered in kindergarten | $3.12‡ ($2.87 $3.35) | PBNC |
| Screening costs: photoscreening autorefractor administered in preschool | $17.00‡ ($15.64 $18.28) | PBNC |
| Comprehensive eye examination by an eye-care professional (current procedural terminology [CPT] code 92002) | $70.87‡ ($65.20 $76.18) | 28,29 |
| Annual cost of refractive error correction | $62.54‡ ($57.53 $67.23) | 30 |
| Amblyopia treatment costs | ||
| At age 3 years | $2,102‡ ($1,934 $2,260) | 7 |
| At age 4 years | $1,977‡ ($1,819 $2,125) | 7 |
| At age 5 years | $1,846‡ ($1,698 $1,984) | 7 |
| At age 6 years | $1,706‡ ($1,569 $1,834) | 7 |
| At age 7 years | $1,556‡ ($1,432 $1,673) | 7 |
| At age 8 years | $1,318‡ ($1,213 $1,417) | 7 |
| At age 9 years | $1,087‡ ($1,000 $1,168) | 7 |
| At ages 10 to 17 years | $775‡ ($713 $833) | 7 |
A/S = acuity/stereopsis; QALY = quality-adjusted life year; PSA = probabilistic sensitivity analysis
Gamma distribution
Logistic normal distribution
Normal distribution
Program Data
Two state-level affiliates of Prevent Blindness America (PBA)32 shared primary data on their screening costs, rates of referral and follow-up care, and epidemiological and visual functionality parameters. The first affiliate, Prevent Blindness Georgia (PBGA), conducts screenings in Georgia’s state lottery-funded preschool centers using the LEA SymbolsR Test to measure acuity and the Random Dot “E” stereopsis test to measure depth perception.33,34 PBGA screened more than 31,267 preschoolers in 2008–2009, with approximately 90% of screens conducted by paid staff and 10% by volunteers. The second affiliate, Prevent Blindness North Carolina (PBNC), conducts preschool and school-based screening for children in elementary school. In the 2007–2008 school year, PBNC screened 29,263 preschoolers and 522,437 children in elementary school. PBNC preschool screening uses paid screening staff and photorefractive camera technology,35 primarily Vision Research VisiScreen OSS-C autorefractors. They conduct elementary school screening with PBNC-certified volunteer vision screeners, school nurses, and teacher’s assistants using 10-foot acuity charts (HOTV for kindergarten and grade 1, Snellen for grades 2 and higher)36,37 and stereopsis using the Lang Stereotest II.38
Amblyopia Incidence and Progression to Visual Loss
Published amblyopia prevalence rates vary from 0.02 to 0.074 with no systematic patterns across ages.5,19,39–43 To simplify these data, we assumed a mean amblyopia prevalence of 0.025 at age 3 with no change in prevalence without treatment, equal to the prevalence measured in the only nationally representative examination survey.19 Because of its age (from 1971), this value may represent amblyopia rates that occur in the absence of systematic screening. The rate is consistent with an unpublished estimate (0.027) calculated by PBNC from primary data drawn from 600 randomly selected children evaluated by an ophthalmologist.
We set the acuity of eyes with amblyopia to 20/80, the average value in the worse-seeing eye among children with a confirmed amblyopia diagnosis identified by PBGA. Although amblyopia often presents with bilateral refractive error, our study’s focus was the prevention of permanent visual loss. We therefore assumed that amblyopia only exists in the worse-seeing eye and any visual loss in the better-seeing eye is due to refractive error and would be corrected even in the absence of screening.
Utility Losses
QALY losses have never been measured among children with amblyopic monocular visual loss.2 One study estimated a QALY decrement of 0.04 for monocular vision impairment starting in childhood, but this value was measured among parents, not those affected.44 Previous cost-effectiveness studies have used a QALY loss of 0.03 observed in adults with progressive visual disorders.45 Although a recent Health Technology Assessment argues that no evidence has documented amblyopic QALY losses,2 assuming no QALY losses is also unsupported because it implies indifference to monocular visual impairment.
For this study we assumed an arbitrary mean QALY decrement of 0.01 per year of unresolved amblyopic monocular impairment. We calculated annual patient QALYs by multiplying 1 minus an individual’s QALY decrement times the background QALY value of all people of that age discounted and summed to the base year. This resulted in an average total lifetime loss of 0.267 discounted QALYs (0.651 undiscounted QALYs) per patient who develops monocular visual loss from amblyopia. A secondary analysis assumed 0.02 QALYs lost.
Eye Evaluation in the Absence of Screening
A report by the Vision Service Plan found that by age 12, 52% of children have been seen by an eye-care professional and a second study found that 14% of children receive a comprehensive eye exam by age 6.20,21 Assuming that no examinations occurred before age 3, we calculated an annual examination rate of 0.037 for ages 3, 4, 5 and 6 such that 14% of children receive an exam by age 6. We then calculated an annual rate of 0.093 to increase the number of children with an exam from 14% to 52% from ages 7 to 12.20,21 We applied the 0.093 examination rate to children ages 13 to 17. We assumed perfect sensitivity and specificity of evaluation for amblyopia by an eye-care professional.
Treatment and Efficacy
Children diagnosed with amblyopia by an eye-care professional received refractive error correction followed by patching/penalization.1 Based on the Pediatric Eye Disease Investigator Group studies, 66% of children treated at ages 3 to 6, 36% of children treated at ages 7 to 12, and 14% of children treated at ages 13 to 17 resolve to an acuity of 20/30 or better in the amblyopic eye.23–27,46,47 We fit a line of these success rates to the midpoints of each age interval to estimate a declining treatment efficacy with age for specific ages, resulting in a maximum efficacy of 66% at ages 3 and 4, 63% at age 5, 57% at age 6, and falling linearly thereafter to zero at age 18.
Adult Eye Diseases
To capture the impact of childhood monocular impairment on the lifetime risk of binocular impairment, we used previously published models to simulate the adult incidence and progression of age-related macular degeneration, diabetic retinopathy, and glaucoma (Rein et al., unpublished data).48,49 Because we focused our analysis on uncorrectable impairment, we excluded cataracts and uncorrected refractive error.
Treatment Costs
We used a German cost estimate of amblyopia treatment, because it allowed us to differentiate treatment costs by age, from $2,102 at age 3 to $775 at age 10 or older (converted from 2002 Euros to 2002 dollars then inflated to 2005 values using the Consumer Price Index–Medical Care Component).7,50 A U.S. study of amblyopia8 estimated nearly the same costs for the treatment of 4-year-old children ($1,922 compared to $1,977 in the German study)7 but did not differentiate costs by age.
Validation
We conducted internal validation by confirming that the model’s estimates matched the prevalence and severity of unilateral visual loss, screening, detection, and referral rates used to program it. The wide variance of amblyopia prevalence estimates (0.02 to 0.074) limited the value of performing external validity checks. Our model’s estimated amblyopia prevalence (0.025) is within the range of previous estimates.5,19,39–43
Scenarios
We compared the lifetime benefits and costs of no screening to 3 scenarios, in ascending order of costliness: (1) kindergarten acuity/stereopsis (A/S) screening, (2) preschool and kindergarten A/S screening, and (3) preschool photoscreening followed by kindergarten A/S screening. Preschool screening occured at age 3 years, and kindergarten screening occured at age 5 years. We eliminated A/S screening or photoscreening at preschool alone for simplicity because these scenarios were dominated by other screening options at any willingness-to-pay (WTP) value. We assume that each intervention is applied to all children to estimate the relative cost-effectiveness of screening the same person at different ages and intervals. To identify the best of these schedules for screening, we based our analysis on the perspective of outcomes per child screened assuming that all children would be screened at each indicated age. In reality, fewer children are likely to be screened in preschool than elementary school in statewide screening programs such as those considered here. Also, we did not consider screening subsequent to age 5 years, although PBNC does continue to support school screening in subsequent grades.
We based the sensitivity of A/S screening on rates observed in the Vision in Preschoolers (VIP) study.51 By assumption, we then reduced the sensitivity of preschool stereopsis testing to adjust for the greater difficulty of testing preschoolers than kindergartners.22 Using primary data from PBNC, we estimated the sensitivity of photoscreening in preschoolers as 0.907, similar to results for several other photorefractors evaluated by VIP.52 We calculated the specificity of A/S testing in preschool and in kindergarten and of photoscreening in preschool by assuming a true prevalence of 0.025 and the sensitivity rates described and then solving for specificity rates that would result in the observed GA and NC program referral rates.
We assume that 68% of those with a positive screening result will receive follow-up care consisting of a comprehensive eye evaluation and treatment when necessary, a rate equal to the average confirmed follow-up rates observed across the GA and NC screening interventions. Based on primary data taken from both programs, we estimated the cost of screening at $6.72 per preschool A/S screening, $17.00 per preschool photoscreening, and $3.12 per kindergarten A/S screening. Scenarios that include screening at preschool and kindergarten experience both costs.
Cost-effectiveness Measures and Analyses
We calculated the incremental cost-effectiveness ratio (ICER) and the incremental net benefit (INB) of each intervention as compared to no screening and as compared to the next economically efficient alternative. The INB is an alternative and potentially superior cost-effectiveness measure compared to the ICER that is easy to interpret and results in equivalent rank order of policy preferences.31,53–55 The INB is defined as
| (1) |
where λ is equal to a WTP value, ΔQ is the mean incremental difference in QALYs between 2 alternatives, and ΔC is the mean incremental difference in costs. Our baseline INB results assume a WTP value of $25,000 per incremental QALY gained and present the probability that each scenario is cost-effective compared to the next most expensive alternative given the credible estimated intervals of QALYs and costs.31 We created a second set of INB estimates in which we replaced ΔQ with a change in cases of amblyopia cured, using a range of values for the WTP per case cured. An intervention was considered more cost-effective than its alternative if its INB compared to the alternative was greater than zero.
We created a cost-effectiveness acceptability curve, which graphs the estimated probability that no screening and each given intervention was the most cost-effective alternative over a range of WTP values. Because INB measures are linear, choosing the scenario with the highest INB compared to no screening as most cost-effective gives the same results as evaluating incremental differences between scenarios. Finally, we used regression methods to determine the individual parameters most responsible for variations in costs and QALYs, and then evaluated the mean cost-effectiveness of each intervention at extreme values of those parameters using subsets of the PSA results.
RESULTS
Without screening, 1.7% of children were eventually diagnosed with and treated for amblyopia through the use of routine ophthalmological services and 0.6% of children were successfully treated. Rates of referral, diagnosis and treatment, and resolution of amblyopia were higher in each of the screening scenarios (Table 2).
Table 2.
Effectiveness and Cost Measures of Amblyopia Screening
| Scenario | No Screening | A/S at K | A/S at PS & K | Photo at PS, A/S at K |
|---|---|---|---|---|
| Amblyopia prevalence prior to screening | 0.025 | 0.025 | 0.025 | 0.025 |
| Children referred for follow-up evaluation | 0.0 | 0.056 | 0.106 | 0.119 |
| Children diagnosed with and treated for amblyopia | 0.017 | 0.021 | 0.023 | 0.024 |
| Children cured | 0.006 | 0.011 | 0.014 | 0.014 |
| Amblyopia prevalence following screening | 0.019 | 0.014 | 0.011 | 0.011 |
| Amblyopia screening and treatment costs | $16 | $36 | $55 | $69 |
| Adult visual health costs | $803 | $803 | $803 | $803 |
A/S = acuity/stereopsis; K = kindergarten; PS = preschool; Photo = photoscreening
Without screening, each child incurred an average of $16 in amblyopia-related treatment costs. Compared to no screening, costs increased by $20 for A/S screening at kindergarten, $40 for A/S screening at preschool and kindergarten, and $53 for photoscreening at preschool followed by A/S screening at kindergarten. Amblyopia screening in childhood reduced the discounted cost of adult visual disorders by less than $0.50 per child in each scenario.
Expected lifetime ophthalmological costs across the scenarios ranged from a low of $819 ($818 to $820) per child for no screening to a high of $872 ($871 to $873) per child for photoscreening at preschool and A/S screening at kindergarten (Table 3). Relative to no screening, all screening scenarios increased expected lifetime QALYs.
Table 3.
Cost-Effectiveness Results
| Costs and Effectiveness | Incremental Cost-Effectiveness Ratios | Incremental Net Benefit vs. Next Cost-Effective Scenario WTP = $25,000 | Probability of Positive Cost-Effectiveness vs. Next Cost Effective Scenario WTP = $25,000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Costs (CI) | QALYs (CI) | Cases Resolved Per 100 Screened | vs. No Screening | vs. Next Most Costly Scenario | Per QALY Gained (CI) | Per Case Resolved (CI) | Per QALY Gained | Per Case Resolved | |
| No screening | $819 ($818 $820) | 26.1261 (26.1259–26.1262) | – | – | – | – | – | – | – |
| A/S, K | $839 ($838 $840) | 26.1274 (26.1272–26.1276) | 0.4905 (0.4904–0.4906) | $15,000 | $15,000 | $2.07 ( $7.8 $12.0) | $103.0 ($101.1 $104.8) | 0.660 | 1.000 |
| A/S, PS & K | $858 ($857 $859) | 26.1283 (26.1282–26.1285) | 0.7584 (0.7583–0.7585) | $18,000 | $22,000 | $3.00 ($ 7.2 $13.2) | $55.4 ($44.8 $66.0) | 0.718 | 1.00 |
| Photo, PS + A/S at K | $872 ($871 $873) | 26.1285 (26.1283–26.1287) | 0.8247 (0.8246–0.8248) | $22,000 | $78,000 | $9.6 ( $20.2 $1.4) | $17.3 ($0.4 $34.2) | 0.041 | 0.997 |
CI = credible interval for the simulated mean, A/S= acuity/stereopsis, Photo= photoscreening, PS = preschool; K = kindergarten
We evaluated the cost-effectiveness of each scenario compared to no screening and compared to the next most costly alternative at a fixed WTP value of $25,000. At this value, all 3 screening interventions were between 90% and 99% likely to be cost-effective, with A/S screening at both preschool and kindergarten yielding the highest mean INB of $16.36 per child, and photoscreening the lowest.
Given a fixed WTP of $25,000 per QALY gained, the expected INB of A/S screening at kindergarten compared to no screening was $13.4 per child screened, and the probability that the scenario was cost-effective was 0.996. The INB of A/S screening at preschool and kindergarten compared to screening in kindergarten alone was $3.0, and the probability it was cost-effective was 0.718. Photoscreening at preschool and A/S screening at kindergarten compared to A/S screening at both preschool and kindergarten resulted in an expected INB of $9.6 with a probability of being cost-effective of 0.041. The likelihood that A/S was cost-effective compared to no screening exceeded 0.50 at a WTP of $15,000. The likelihood that A/S screening at preschool and kindergarten was cost-effective compared to A/S screening in kindergarten alone exceeded 50% at a WTP of $22,000. The likelihood that photoscreening at preschool and A/S screening at kindergarten was cost-effective compared to A/S screening at both preschool and kindergarten exceed 50% at a WTP of $75,000.
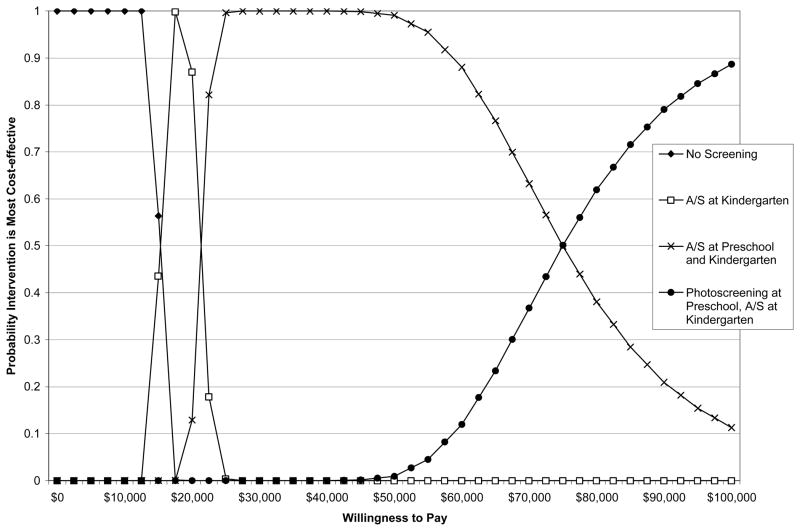
When looking across a range of possible WTP values, no screening was the most likely of the scenarios to be cost-effective between a WTP of $0 and < $16,000 per QALY gained; A/S screening at kindergarten alone was the scenario most likely to be cost-effective between a WTP of $16,000 and <$21,000; A/S screening in both preschool and kindergarten was the scenario most likely to be cost-effective between a WTP of $22,000 and <$75,000; and photoscreening in preschool followed by A/S screening at kindergarten was most likely to be cost-effective at a WTP of ≥$75,000 (Figure 1).
Figure 1.
Probability That No Screening or Each of Three Screening Alternatives is the Most Cost-Effective Given Different Willingness-to-Pay Values Per Incremental QALY Gained
When we evaluated the WTP per case of amblyopic visual impairment cured as opposed to the WTP per QALY gained, we found that compared to no screening, A/S screening at kindergarten, A/S screening at preschool and kindergarten, and photoscreening at preschool and A/S screening at kindergarten all had a greater than 0.99 probability of being cost-effective at a WTP of $25,000 per case cured. The probability that photoscreening at preschool and A/S screening at kindergarten was cost-effective compared to A/S screening in both settings exceeded 0.50 at a WTP of $10,500 per case cured.
Compared to no screening, increasing the mean annual QALY loss resulting from amblyopic monocular vision loss to 0.02 substantially increased the cost-effectiveness of all scenarios. The use of the higher QALY decrement had the most impact when comparing photoscreening at preschool followed by A/S screening at kindergarten to A/S screening at both settings. In that scenario, increasing the mean QALY losses to 0.02 resulted in a 0.50 probability that photoscreening at preschool followed by A/S screening at kindergarten was cost-effective as compared to A/S screening at both settings at a WTP of $36,000 per QALY gained. The probability exceeded 0.99 at a WTP of $40,000 per QALY gained.
The ICERs generated by our model were similar to those estimated by earlier studies: $15,000 to $22,000 per QALY compared to no screening and $15,000 to $78,000 per QALY when scenarios were directly compared.2–11 Variations in total costs were driven primarily by variations in screening and treatment costs. No other variables had a substantial impact on costs. Variations in QALYs were driven by differences in the QALY weight and by differences in treatment efficacy.
DISCUSSION
Our study suggests that when conservatively assuming a minimal amblyopia utility loss of 0.01, A/S screening for amblyopia in kindergarten is likely to be cost-effective compared to no screening at a WTP of $15,000 per QALY gained and that A/S screening at preschool and kindergarten is likely to be cost-effective when compared to kindergarten screening alone at a WTP of $22,000 per QALY gained.
In comparison, the choice to replace A/S screening in preschool with photoscreening (both scenarios retain A/S screening at kindergarten) appears costly when using our baseline assumption of 0.01 QALYs lost per year of monocular impairment. This conclusion is sensitive to our assumption of QALY losses and our estimated program costs for photoscreening. Photoscreening appeared more favorable in scenarios in which we assumed a QALY loss of 0.02 per year of monocular impairment and when we evaluated interventions based on their INB per case resolved as opposed to per QALY gained. When using an assumption of 0.02 QALYs lost per year of monocular impairment, we found that replacing A/S screening in preschool with photoscreening was more than 50% likely be cost-effective at a WTP of >$36,000 per QALY gained. It may also be possible to implement photoscreening at lower cost than that used in our model using different screening technology.
We also estimated the cost-effectiveness of each intervention in terms of the WTP for each case of monocular impairment from amblyopia averted. We found that the probability that A/S screening was cost-effective compared to no screening exceeded 0.50 at a WTP value of $4,010 per case averted and that adding A/S screening at preschool compared to screening at kindergarten alone had a greater than 0.50 probability of being cost-effective at a WTP value of $4,400 per case averted. The probability that photoscreening in preschool and A/S screening in kindergarten was cost-effective compared to A/S screening in both settings exceeded 0.50 at a WTP of $10,500 per case averted. These cost-effectiveness estimates are in line with what individual patients would pay out of pocket to avert similar but less serious conditions.56,57
Limitations
Our study is limited by at least the following factors. First, like earlier studies of amblyopia screening (which used QALY decrements ranging from 0.00 to 0.03), our analysis suffers from a lack of scientific evidence regarding the utility impacts of amblyopic monocular visual loss. This limits our ability to definitively state that amblyopia screening results in positive benefits. However, our results show that some forms of screening were cost-effective even when our simulations assumed only a small mean QALY decrement of 0.01, a value roughly one-third that used in most previous studies. To offset this limitation, we present the probability that screening is cost-effective given a range of WTP values to prevent a case of amblyopic impairment. These estimates require no assumption that QALY losses result from amblyopia, only that there is a monetary value at which some payer (be it insurance or a parent) would pay to avert a case of amblyopia.
Second, our analysis uses data collected from only 2 states when at least 35 states conduct some form of childhood screening. Furthermore, our model represents an idealized screening situation in which all children are reached at preschool, kindergarten, or at both ages. While we feel our results are informative to policy making, they cannot be directly inferred to any individual program, as each real instance of screening will experience its own costs and effectiveness.
Third, by assumption, our analysis excludes the benefits of detecting uncorrected refractive error because the benefits of early detection of refractive error are unclear. Because the costs of treating refractive error are included in the normal treatment costs of amblyopia, including the benefits of detection would lead to better cost-effectiveness results for all scenarios.
Fourth, while our analysis includes lower rates of amblyopia treatment success, we do not differentiate the expected level of acuity gain by age of treatment initiation. Long-term follow-up of an amblyopia treatment cohort showed significantly higher level of resolved acuity (approximately 20/25) in children treated at age 3 than in children treated at age 5 or older (approximately 20/30).58
Finally, we made a number of simplifying assumptions to describe the natural history, detection, and treatment of amblyopia. We assumed that amblyopia has a constant prevalence of 0.025 at age 3, that no additional incidence occurs, and that those cases will not resolve without treatment. We chose a constant prevalence rate and to ignore incidence because of the ambiguity of the data regarding each of these parameters. Our assumed value falls toward the lower end of published prevalence values (0.02 to 0.074).5,19,39–43 Lower overall prevalence values would reduce the cost-effectiveness of all screening, while increasing incidence with age may lead to a conclusion that screening in kindergarten is preferable to screening in preschool and could potentially undermine the rationale for screening in preschool. In addition, if a proportion of cases resolve without treatment, then screening could lead to overtreatment, which would also lead to a less favorable cost-effectiveness of screening. We assume that all children have the same background rate of ophthalmologic examinations, although it is possible that children with amblyopia would be more likely to undergo an examination and be diagnosed in the absence of screening, a finding that would lead to a less favorable cost-effectiveness ratio.
Implications
Our findings suggest that amblyopia screening is likely to be cost-effective to a policy maker willing to pay $15,000 per QALY averted (assuming a QALY loss from untreated amblyopia of 0.01 per year) or $4,005 per case of amblyopic visual loss averted. Areas that have established kindergarten screening programs in place may wish to consider adding combined acuity and stereopsis screening for preschoolers. Photoscreening in preschool is more costly but likely results in greater benefits than A/S screening.
Acknowledgments
We wish to thank Mary Bregantini of Prevent Blindness America, Jennifer Talbot of Prevent Blindness North Carolina, and Jennifer Pomeroy of Prevent Blindness Georgia for their generosity in sharing programmatic data with us for the purposes of this study.
Financial Support: Funding to support research was provided by the Centers for Disease Control and Prevention, Atlanta, GA (contract no. 200-2008-F-26421). Funding to support the development of probabilistic sensitivity analysis was provided by the National Eye Institute, Bethesda, MD (contract no. REY019173A).
Footnotes
Conflict of Interest: No authors have any financial/conflicting interests to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
David B. Rein, RTI International.
John S. Wittenborn, RTI International.
Xinzhi Zhang, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion.
Michael Song, RTI International.
Jinan B. Saaddine, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion.
References
- 1.American Academy of Ophthalmology (AAO) Preferred practice pattern guidelines: Amblyopia. San Francisco, CA: American Academy of Ophthalmology; 2007. [Google Scholar]
- 2.Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J. The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: A systematic review and economic evaluation. Health Technol Assess. 2008;12(25):1–214. doi: 10.3310/hta12250. [DOI] [PubMed] [Google Scholar]
- 3.White A. Eye exams for children: Their impact and cost effectiveness. Cambridge, MA: Abt Associates; 2004. Prepared for the Vision Council of America. [Google Scholar]
- 4.König HH, Barry JC. Cost-utility analysis of orthoptic screening in kindergarten: A Markov model based on data from Germany. Pediatrics. 2004;113(2):e95–108. doi: 10.1542/peds.113.2.e95. [DOI] [PubMed] [Google Scholar]
- 5.König HH, Barry JC, Leidl R, Zrenner E. Economic evaluation of orthoptic screening: Results of a field study in 121 German kindergartens. Invest Ophthalmol Vis Sci. 2002;43(10):3209–3215. [PubMed] [Google Scholar]
- 6.König HH, Barry JC. Economic evaluation of different methods of screening for amblyopia in kindergarten. Pediatrics. 2002;109(4) doi: 10.1542/peds.109.4.e59. [DOI] [PubMed] [Google Scholar]
- 7.König HH, Barry JC. Cost effectiveness of treatment for amblyopia: An analysis based on a probabilistic Markov model. Br J Ophthalmol. 2004;88(5):606–612. doi: 10.1136/bjo.2003.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Membreno JH, Brown MM, Brown GC. A cost-utility analysis of therapy for Amblyopia. Ophthalmology. 2002;109:2265–2271. doi: 10.1016/s0161-6420(02)01286-1. [DOI] [PubMed] [Google Scholar]
- 9.Joish VN. Reply to comment. J AAPOS. 2004;8:74–75. [Google Scholar]
- 10.Joish VN, Malone DC, Miller JM. A cost-benefit analysis of vision screening methods for preschoolers and school-age children. J AAPOS. 2003;7:283–290. doi: 10.1016/s1091-8531(03)00116-2. [DOI] [PubMed] [Google Scholar]
- 11.Gandjour A, Schlichtherle S, Neugebauer A, Russmann W, Lauterbach KW. A cost-effectiveness model of screening strategies for amblyopia and risk factors and its application in a German setting. Optom Vis Sci. 2003;80(3):259–269. doi: 10.1097/00006324-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Baltussen R, Naus J. Cost-effectiveness of screening and correcting refractive errors in school children in Africa, Asia, America and Europe. Health Policy. 2009;89:201–215. doi: 10.1016/j.healthpol.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Arnold R, Armitage M, Gionet E, Balinger A. The Cost and Yield of Photoscreening: Impact of Photoscreening on Overall Pediatric Ophthalmic Costs. Pediatric Ophthalmology & Strabismus. 2005;42(2):103–111. doi: 10.3928/01913913-20050301-05. [DOI] [PubMed] [Google Scholar]
- 14.Longmuir SQ, Pfeifer W, Leon A, Olson RJ. Network photoscreening program of 147809 children using a photoscreener in Iowa. Ophthalmology. 2010;117:1869–1875. doi: 10.1016/j.ophtha.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Naser N, Hartmann EE. Comparison of state guidelines and policies for vision screening and eye exams: Preschool through early childhood. Paper presented at Association for Research in Vision and Ophthalmology annual meeting; 2008. [Google Scholar]
- 16.U.S. Census Bureau Population Division. Annual estimates of the population by sex and age for the United States: April 1, 2000 to July 1, 2007. Washington, DC: U.S. Census Bureau; Released: May 1, 2008. [Google Scholar]
- 17.Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5(2):157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 18.Briggs A, Claxton K, Schulpher M. Decision modeling for health economic evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- 19.NCHS. Eye conditions and related need for medical care. Vital Health Stat. 1983;228:1–69. [PubMed] [Google Scholar]
- 20.Ciner EB, Dobson V. A survey of vision screening policy of preschool children in the United States. Surv Ophthalmol. 1999;43(5):445–457. doi: 10.1016/s0039-6257(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 21.Martinez V. Children's Vision Awareness Study: Quantitative Research Results. Sacramento, CA: Vision Service Plan; 2002. [Google Scholar]
- 22.Vision in Preschoolers (VIP) Study Group. Testability of preschoolers on stereotests used to screen vision disorders. Optometry and Vision Sciences. 2003;80:753–757. doi: 10.1097/00006324-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Pediatric Eye Disease Investigator Group (PEDIG_ATS1a) A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children. Arch Ophthalmology. 2002;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 24.Pediatric Eye Disease Investigator Group (PEDIG_ATS2B) A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmology. 2003;121:603–611. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 25.Pediatric Eye Disease Investigator Group (PEDIG_ATS4) A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–2085. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Pediatric Eye Disease Investigator Group (PEDIG_ATS5) Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pediatric Eye Disease Investigator Group (PEDIG_ATS3) Randomized trial aged 7 to 17 years. Arch Ophthalmology. 2005;123:437–447. [Google Scholar]
- 28.American Medical Association. CPT 2006: Current procedural terminology (standard edition) Vol. 17. Florence, KY: Thomson Delmar Learning; 2007. [Google Scholar]
- 29.Gray L, Parkinson J. The essential RBRVS. Salt Lake City, UT: St. Anthoney’s Publishing; 2003. [Google Scholar]
- 30.Rein DB, Zhang P, Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124(12):1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 31.O'Hagan A, Stevenson M, Madan J. Monte Carlo probabilistic sensitivity analysis for patient level simulation models: Efficient estimation of mean and variance using ANOVA. Health Econ. 2007;16(10):1009–1023. doi: 10.1002/hec.1199. [DOI] [PubMed] [Google Scholar]
- 32.Prevent Blindness America. The economic impact of vision problems: The toll of major eye disorders, visual impairment, and blindness on the US economy. Chicago: Prevent Blindness America; 2007. [Google Scholar]
- 33.Bertuzzi F, Orsoni JG, Porta MR, Paliaga GP, Miglior S. Sensitivity and specificity of a visual acuity screening protocol performed with the Lea Symbols 15-line folding distance chart in preschool children. Acta Ophthalmol Scand. 2006;84(6):807–811. doi: 10.1111/j.1600-0420.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt P, Maguire M, Kulp MT, Dobson V, Quinn G. Random Dot E stereotest: Testability and reliability in 3- to 5-year-old children. J AAPOS. 2006;10(6):507–514. doi: 10.1016/j.jaapos.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim AH, Chen J, Ottar-Pfeifer W, et al. Screening for amblyopia in preverbal children with photoscreening photographs: IV. Interobserver variability in photograph grading: Origin and method of reduction. Binocul Vis Strabismus Q. 2005;20(2):71–80. [PubMed] [Google Scholar]
- 36.Cyert L, Dobson V, Kulp MT, et al. Preschool visual acuity screening with HOTV and lea symbols: Testability and between-test agreement. Optom Vis Sci. 2004;81(9):678–683. doi: 10.1097/01.opx.0000144746.80718.67. [DOI] [PubMed] [Google Scholar]
- 37.McGraw P, Winn B, Whitaker D. Reliability of the Snellen chart. BMJ. 1995;310(6993):1481–1482. doi: 10.1136/bmj.310.6993.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huynh SC, Ojaimi E, Robaei D, Rose K, Mitchell P. Accuracy of the Lang II stereotest in screening for binocular disorders in 6-year-old children. Am J Ophthalmol. 2005;140(6):1130–1132. doi: 10.1016/j.ajo.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 39.Abrahamsson M, Fabian G. A longitudinal study of a population based sample of astigmatic children. II. The changeability of anisometropia. Acta Ophthalmol (Copenh) 1990;68(4) doi: 10.1111/j.1755-3768.1990.tb01672.x. [DOI] [PubMed] [Google Scholar]
- 40.Vision in Preschoolers (VIP) Study Group. Preschool vision screening tests administered by nurse screeners compared with lay screeners in the vision in preschoolers study. Invest Ophthalmol Vis Sci. 2005;46:2639–2648. doi: 10.1167/iovs.05-0141. [DOI] [PubMed] [Google Scholar]
- 41.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia: The Blue Mountains Eye Study. Ophthalmology. 1996;103:357–364. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 42.Kvarnstrom G, Jakobsson P. Visual screening of Swedish children: An ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79:240–244. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- 43.Stewart-Brown S, Butler N. Visual acuity in a national sample of 10 year old children. J Epidemiol Community Health. 1985;39:107–112. doi: 10.1136/jech.39.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll AE, Downs SM. Improving Decision Analyses: Parent Preferences (Utility Values) for Pediatric Health Outcomes. J Pediatr. 2009;155(1):21–25. doi: 10.1016/j.jpeds.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48(2):204–223. doi: 10.1016/s0039-6257(02)00457-5. [DOI] [PubMed] [Google Scholar]
- 46.Pediatric Eye Disease Investigator Group (PEDIG_ATS1b) Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmology. 2005;123:149–157. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- 47.Pediatric Eye Disease Investigator Group (PEDIG_ATS2A) A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Rein DB, Saaddine JB, Wittenborn JS, et al. Cost-effectiveness of vitamin therapy for age-related macular degeneration. Ophthalmology. 2007;114(7):1319–1326. doi: 10.1016/j.ophtha.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 49.Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116(5):823–832. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Bureau of Labor Statistics. [Accessed October 6, 2009.];Consumer price index—all urban consumers, US medical care, 1982–84=100-CUUR0000SAM. http://146.142.4.24/cgi-bin/surveymost?cu.
- 51.Vision in Preschoolers (VIP) Study Group. Sensitivity of screening tests for detecting vision in preschoolers-targeted vision disorders when specificity is 94% Optom Vis Sci. 2005;85:432–438. doi: 10.1097/01.OPX.0000162660.14378.30. [DOI] [PubMed] [Google Scholar]
- 52.Vision in Preschoolers (VIP) Study Group. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the vision in preschoolers study. Ophthalmology. 2004;111:637–650. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 53.O'Hagan A, Stevens JW, Montmartin J. Inference for the cost-effectiveness acceptability curve and cost-effectiveness ratio. Pharmacoeconomics. 2000;17(4):339–349. doi: 10.2165/00019053-200017040-00004. [DOI] [PubMed] [Google Scholar]
- 54.Claxton K. Exploring uncertainty in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):781–798. doi: 10.2165/00019053-200826090-00008. [DOI] [PubMed] [Google Scholar]
- 55.Willan AR, Lin DY. Incremental net benefit in randomized clinical trials. Stat Med. 2001;20(11):1563–1574. doi: 10.1002/sim.789. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell WA, Waycaster CR, D'Souza AO, Meissner BL, Hileman K. A United States cost-benefit comparison of an apodized, diffractive, presbyopia-correcting, multifocal intraocular lens and a conventional monofocal lens. J Cataract Refract Surg. 2008 Nov;34(11):1855–1861. doi: 10.1016/j.jcrs.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Segre L, Haddrill M, Thompson V. [Accessed November 18, 2009.];Cost of LASIK and other corrective eye surgery. 2009 http://www.allaboutvision.com/visionsurgery/cost.htm.
- 58.Pediatric Eye Disease Investigator Group (PEDIG) A randomized trial of atropine versus patching for treatment of moderate amblyopia: Follow-up at 10 years of age. Arch Ophthalmol. 2008;126(8):1039–1044. doi: 10.1001/archopht.126.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]