Abstract
Treg cells are critical homeostatic components in preventing the development of autoimmunity, and are a major focus for their therapeutic potential for autoimmune diseases. In order to enhance the efficacy of Treg cells in adoptive therapy, we developed a strategy for generating engineered Tregs that have the capacity to target autoimmune T cells in an antigen specific manner. Using a retroviral expression system encoding Foxp3 and HLA-DR1 covalently linked to the immunodominant peptide of the autoantigen type II collagen (DR1-CII), naïve T cells were engineered to become Treg cells that express DR1-CII complexes on their surface. When these cells were tested for their ability to prevent the development of collagen induced arthritis, both the engineered DR1-CII-Foxp3 and Foxp3 only Treg cells significantly reduced the severity and incidence of disease. However, the mechanism buy which these two populations of Treg cells inhibited disease differed significantly. Disease inhibition by the DR1-CII-Foxp3 Treg cells was accompanied by significantly lower numbers of autoimmune CII-specific T cells in vivo and lower levels of autoantibodies in comparison to engineered Tregs expressing Foxp3 alone. Additionally, the numbers of IFN-γ and IL-17 expressing T cells in mice treated with DR1-CII-Foxp3 Tregs were also significantly reduced in comparison to mice treated with Foxp3 engineered Treg cells or vector control cells. These data indicate that the co-expression of class II autoantigen-peptide complexes on Treg cells provides these cells with a distinct capacity to regulate autoimmune T cell responses that differs from that used by conventional Treg cells.
Introduction
Regulatory T cells (Tregs) are CD4+, CD25+, Foxp3+ cells capable of suppressing the function of T effector cells in order to enforce immunological homeostasis. The genetic absence of Tregs results in widespread disregulation of the adaptive immune response in both humans and animal models (1, 2). In humans, mutations in the Foxp3 gene, a key transcriptional regulator for the differentiation and function of Tregs (3), has been linked with IPEX syndrome (Immunodysregulation, Polyendocrinopathy, Enteropathy, X-linked) which manifests as a myriad of autoimmune disorders including diabetes, thyroiditis, and colitis (for a review see (4)). Similarly, scurfy mice, which carry an insertion mutation in the Foxp3 gene that results in a non-functional protein, also develop a variety of autoimmune disorders (3, 5). The widespread lymphoproliferation and autoimmunity that develops in the absence of Tregs has suggested that therapeutic use of Tregs might be a viable approach for the treatment of autoimmune diseases. Indeed, transfer of thymic-derived Foxp3+ cells into neonatal scurfy mice prevents development of the lymphoproliferative and autoimmune disorders that normally develop in these mice (3, 6).
Several studies have shown that adoptive transfer of Treg cells offers promise for immunotherapy of autoimmune diseases. Adoptively transferred Treg cells have been shown to alter the development of disease in several mouse models, including colitis (3, 7), experimental autoimmune encephalitis (EAE) (8–10), arthritis (11–13), diabetes (14, 15), and lupus (16). In most cases these studies have used polyclonal Tregs to inhibit the initiation of autoimmunity and most have demonstrated only a reduction in severity of disease, although some success has been achieved using Tregs to alter established disease (17). In attempts to increase the efficacy of adoptive Treg therapy in autoimmune diseases, several investigators have examined the effect of antigen specific Tregs on autoimmune responses. Several studies have demonstrated that antigen specific Tregs may be more effective than polyclonal Tregs in ameliorating or preventing autoimmunity in arthritis (18, 19), autoimmune gastritis (20), and type I diabetes models (15). Whereas polyclonal Treg cells were only minimally effective in the treatment of type I diabetes in mice, auto-antigen specific Treg cells effectively suppressed the disease (14, 15). Although the results from autoantigen specific Treg cell treatments are promising, the possibility of contamination of these cells with autoimmune T effector cells during the preparation of the therapeutic Treg cells remains a concern.
Another means by which Treg cells may target pathogenic T cells is via their expression of class II molecules. While the mouse is one of few mammalian species that do not express class II on activated T cells (21), it has been demonstrated that human Treg cells can express HLA class II, and those that do, express higher levels of Foxp3 and appear to be more efficacious in inhibiting T cell responses in vitro (22). However, it remains unclear whether or not these class II expressing Treg cells have therapeutic potential in vivo. Given the evidence for increased Treg efficacy through class II expression, and the ability to produce large numbers of Treg cells via transduction of Foxp3 (3, 15, 19, 23), we engineered targeted Treg cells by transducing naïve CD4+ T cells with a construct carrying both Foxp3 and HLA DR1 class II covalently linked to an autoantigenic peptide that drives the development of collagen induced arthritis (CIA)3. This approach provides the Treg cells with both membrane expressed HLA class II and via the autoantigenic peptide a means to specifically target the pathogenic T cells driving the autoimmune response. Our goal was to develop a system for producing large numbers of Tregs for therapeutic studies and provide a targeting mechanism that would enhance the interaction of the engineered Treg with the pathogenic T cells in a DR1-humanized autoimmune arthritis mouse model. In the following studies we demonstrate that adoptive transfer of these HLA class II targeted, engineered Tregs effectively inhibits an established autoimmune response and prevents the development of autoimmune arthritis in DR1 Tg mice. In comparison to engineered Treg cells expressing only Foxp3, the class II targeted Treg cells were more efficient and appeared to utilize a different mechanism to alter the autoimmune T cell response by both inhibiting T cell function as well as altering the functional phenotype of the autoimmune T effector cells.
Materials and Methods
Mice
The development of the B10M-DR1 (DRB1*0101) humanized mouse strain has been described previously (24). These mice express a chimeric class II molecule, with the second domains derived from I-E, enabling interaction with murine CD4 (25). The B10.M-DR1-TCR transgenic mice were obtained from Dr. Linda Myers, University of Tennessee Health Science Center. The TCR transgene was established by cloning of the Vα2Jα27 and Vβ8.1Jβ2.4 genes from a DR1-restricted, CII259-270-specific T cell hybridoma (E168) into the pVαcass and pVβcass cassette vectors (a gift from Dr. V. Kouskoff, Paterson Institute for Cancer Research, United Kingdom), respectively, and injection of the DNA into fertilized eggs of FVB/N mice. Transgenic founders were identified by PCR analysis and flow cytometry, and the transgene was backcrossed onto the B10.M-DR1 strain. These mice, named TCR-E168 DR1, express this CII-specific, DR1-restriced TCR on the majority of their CD4+ T cells, and as naïve T cells they respond to CII peptide stimulation. All mice were maintained in a pathogen-free environment in accordance with institutional guidelines.
Induction of CIA
Autoimmune arthritis was induced in 8 to 10 week old mice by immunization at the base of the tail with 100 μg of bovine CII emulsified in CFA (Difco) containing 4 mg/ml of mycobacterium (26). Severity of disease was assessed by visual scoring of each limb 3 times per week and assigning a score of 0 to 4 as described (26). For treatment with the engineered Treg cells, mice were injected with 3 x 106 GFP+ cells per each time point via the tail vein.
RNA isolation and RT-PCR
For amplification of the Foxp3 gene, CD4+ CD25+ T cells were enriched from the spleens of B10M-DR1 mice by using a CD4+CD25+ isolation kit from Miltenyi (Miltenyi Biotech). Briefly, 5x107 cells were incubated with biotinylated anti-CD8 and CD19 antibodies for 10 min at 4 °C, followed by the addition of avidin-conjugated microbeads and anti-CD25-PE. A two step procedure consisting of negative selection for CD4+ T cells followed by positive selection of the CD25+ cells using anti-PE microbeads was performed using an AutoMacs Pro (Miltenyi Biotech) according to the manufacture’s guidelines.
Total RNA was isolated from 1 x 106 CD25+ CD4+ cells using the RNeasy mini-kit (Qiagen), according to manufacturer’s instructions. cDNA was synthesized from the RNA using SuperScript II RT (Invitrogen). The full length Foxp3 cDNA was amplified using a Foxp3 forward primer 5′-CTC CCG GCA ACT TCT CCT GAC-3′ and a reverse primer 5′-TCA AGG GCA GGG ATT GGA GCA CT-3′. PCR was performed using the Phusion kit (New England BioLabs) in a 50 μl reaction with 2 μl of cDNA, 10 μl of 5x Phusion HF buffer, 1 μl of 10 mM dNTPs, 2.5 μl of 10 μM Foxp3 forward primer, 2.5 μl of 10 μM Foxp3 reverse primer, 0.5 μl of 2 U/μl Phusion DNA polymerase, and 33.5 μl of H2O. Following a 30 sec initial denaturation at 98° C, the Foxp3 cDNA was amplified using 30 PCR cycles of 10 sec at 98° C, 15 sec at 65° C, 1 min at 72° C, and a final 7 min extension at 72° C. The PCR products were purified and incubated with Taq to polyadenylate the PCR product to facilitate cloning. The final PCR products were gel purified using Bio101 (Bio101) and cloned into the pCR2.1 vector using a TA cloning kit (Invitrogen). The sequence of Foxp3 (pCR2.1-Foxp3) was verified by DNA sequencing.
Retroviral constructs
The constructs for retroviral expression of the Foxp3 and DRB1*0101 and DRA1*0101 genes were inserted into the EcoRI site of the mouse stem cell virus (MSCV) retroviral vector (27). To generate the DR1-CII retroviral construct, total RNA was isolated from the spleen of a B10.M-DR1 mouse, reverse transcribed into cDNA, and used as a template to amplify the full length DR1α chain and DR1β chain by PCR. The primers for the DR1α chain are 5′-AAA TAT GGC CAC AAT TGG AGC CCT G-3′ and 5′-TCA CAG GGC TCC TTG TCG GCG TTC-3′. The primers for the DR1β chain are 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-TCA GCT CAG GAG TCC TGT TGG CTG-3′. To construct the full length DR1β chain with the CII peptide, two rounds of PCR were performed. The DNA fragment containing the extracellular domain of DR1β with the CII peptide was generated using pRmsch-DR1-CII as template and using 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-GCA GAT GTG GAC TGT GCT TTC C-3′ as primers; the other DNA fragment containing the membrane domain and intracellular domain of DR1β was amplified using full length DR1β as the template and using 5′-CCG AAA TGG AGA CTG GAC CTT C-3′ and 5′-TCA GCT CAG GAG TCC TGT TGG CTG-3′ as primers. These two DNA fragments served as templates for the second round of PCR using 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-TCA GCT CAG GAG TCC TGT TGG CTG-3′ as primers. The PCR products were inserted into the pCR2.1 vector to generate pCR2.1-DR1β-CII and pCR2.1-DR1α. Their sequences were verified by DNA sequencing.
To enable expression of multiple genes off the same MSCV promoter, the DR1 and Foxp3 genes were concatenated using T2A sequences to separate the genes (Figure 1A) (28). This approach allows for the expression of multiple proteins from the same transcript with similar efficiencies for each gene (29). pCR2.1-DR1β-CII and pCR2.1-DR1α were used as templates to generate DR1β-CII-T2A-DR1α. The DR1β-CII-T2A fragment was amplified by using pCR2.1-DR1β-CII as the template and using 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-CTC CTC GAC GTC ACC GCA TGT TAG CAG ACT TCC TCT GCC CTC GCT CAG GAG TCC TGT TGG CTG-3′ as primers; and the T2A-DR1a fragment was amplified by using pCR2.1-DR1α as the template and using 5′-AGT CTG CTA ACA TGC GGT GAC GTC GAG GAG AAT CCT GGC CCA ATG GCC ACA ATT GGA GCC CTG-3′ and 5′-TCA CAG GGC TCC TTG TCG GCG TTC-3′ as primers. DR1β-CII-T2A-DR1α was obtained by using these two fragments as templates and using 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-TCA CAG GGC TCC TTG TCG GCG TTC-3′ as primers. The final PCR product was then cloned into pCR2.1, verified by DNA sequencing, and inserted into the EcoRI site of MSCV.
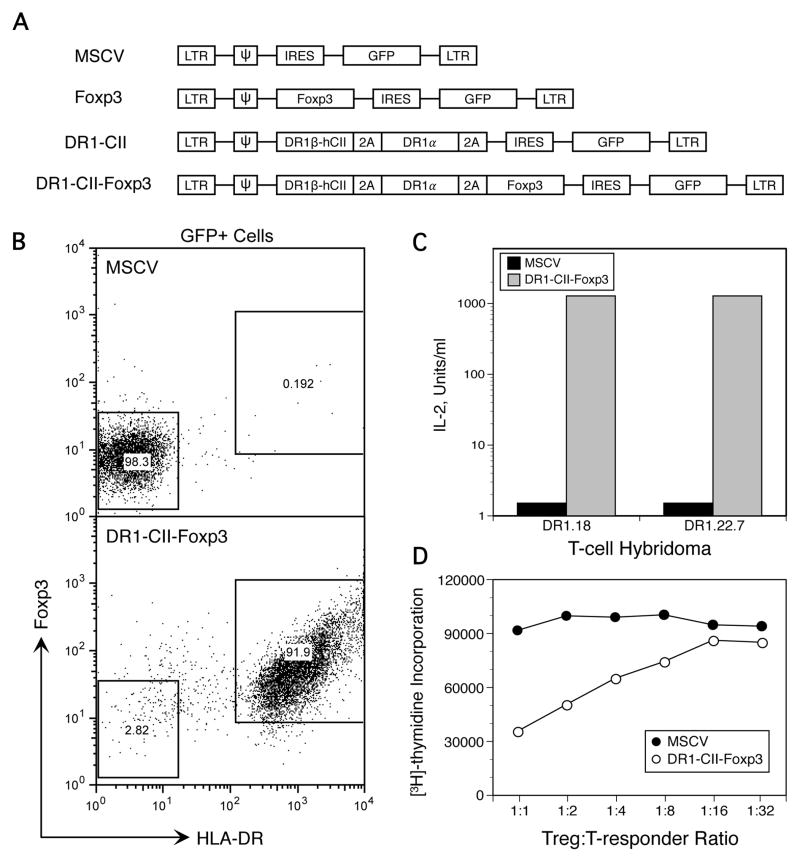
Figure 1.
Transduction of T cells with retroviral constructs encoding Foxp3 and DR1-CII. A. Constructs used to create replication-deficient retrovirus for infection of T cells. Concatenated genes (DR1a, DR1b, and Foxp3) were separated by picorna virus T2A sequences, allowing translation of all 3 genes from a single transcript driven by a single promoter. cDNA encoding the CII(257–274) peptide was integrated into the DR1b chain cDNA sequence to insure that the expressed DR1 molecules were bound to the CII peptide. B. Expression of HLA-DR1-CII and Foxp3 by GFP+ T cells infected with engineered replication-deficient retrovirus. Transduced T cells were stained with antibodies specific for HLA-DR and Foxp3. Data shown are gated on GFP+ cells. C. HLA-DR1-CII expressed by retroviral-infected T cells is functional as an antigen presentation molecule. The ability of CII-specific T cell hybridomas to be stimulated by the transfected T cells expressing DR1-CII was tested in vitro in an antigen presentation assay. Transduced T cells functioning as APC and T-hybridoma cells were incubated in 96 well plates, and after 24 hours the supernatant was collected and the quantity of IL-2 produced was measured in a bioassay as described in Materials and Methods. D. Expression of Foxp3 confers regulatory function on the transduced T cells. The ability of the engineered Treg cells transduced with Foxp3 encoded constructs to inhibit the proliferation of CII-specific T cells was tested in vitro. T cells expressing DR1-CII-Foxp3 were mixed at various ratios with CII-specific T cells from a TCR transgenic mouse in 96 well plates containing 5 x 104 responder cells, 2 x 105 irradiated antigen-presenting cells, and 1 μg/well CII peptide. Proliferation was measured using [3H]-thymidine incorporation in a 4 day assay.
The DR1-CII-Foxp3 construct was produced by combining the DR1β-CII-T2A-DR1α construct and the Foxp3 cDNA by another T2A sequence. The DR1β-CII-T2A-DR1αT2A fragment was produced using MSCV-DR1-CII as the template and 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-CTC CTC GAC GTC ACC GCA TGT TAG CAG ACT TCC TCT GCC CTC CAG GGC TCC TTG TCG GCG TTC-3′ as primers, and the T2A-Foxp3 fragment was generated using MSCV-Foxp3 as the template and 5′-AGT CTG CTA ACA TGC GGT GAC GTC GAG GAG AAT CCT GGC CCA ATG CCC AAC CCT AGG CCA GCC-3′ and 5′-TCA AGG GCA GGG ATT GGA GCA CT-3′ as primers. These two fragments were then used as templates to produce the DR1-CII-Foxp3 construct using 5′-AAA ATG GTG TGG CTC CCC AG-3′ and 5′-TCA AGG GCA GGG ATT GGA GCA CT-3′ as primers. The final PCR product was cloned into the pCR2.1 vector, verified by DNA sequencing, and cloned into the EcoRI site of MSCV to generate MSCV-DR1-CII-Foxp3.
Production of retrovirus and infection of T cells
The retroviral constructs were co-transfected into 293T cells with two helper DNAs, pcDNA-gag-pol and pcDNA-Env (gifts of Dr. Lorraine Albritton, University of Tennessee Health Science Center) at a molar ratio of 1:1:1 by using Fugene HD according to manufacturer’s instructions (Roche). Briefly, 10 μg of DNA were diluted into 500 μl of Opti-Medium and then 40 μl of Fugene HD was added to the DNA solution. After a 15 min incubation, the DNA-Fugene mixture was added to the 293T cells drop by drop. Sixteen hours later, the DNA-Fugene containing medium was replaced with fresh EHAA medium with 10% FBS and penicillin/streptomycin. Viral supernatant was harvested 24 hours and 48 hours later.
Prior to infection, T cells from the spleens of DR1 mice were activated by plate-bound anti-CD3 (clone 145-2C11, 5 μg/ml) and anti-CD28 (clone PV1, 5 μg/ml) and cultured in EHAA medium with 10% FBS, penicillin/streptomycin, 5 x 10−5 M 2-ME, and 15 U/ml of IL-2 for two days. Cells were washed twice with EHAA medium without 2-ME and resuspended into freshly harvested viral supernatant with 10 μg/ml of polybrene (Sigma) and 15 U/ml of IL-2 at a density of 1x106 cells per 1 ml of viral supernatant. Infection was aided by spin-inoculation at 700 x g for 90 min at RT followed by overnight incubation at 37° C. The infection procedure was repeated 24 hr later with fresh viral supernatant. After a second 24 hr incubation period, cells were collected, their efficiency of transduction evaluated by analyzing GFP expression using flow cytometry (FacsCalibur or LSRII, BD Biosciences), and used in experiments. Transduction efficiency averaged 60% for cells used in experiments.
Immunofluorescence and Flow Cytometry
Nuclear staining of Foxp3 was performed using a mouse Foxp3 staining kit (eBioscience) according to manufacture’s instructions. Prior to nuclear staining, cells were stained with the anti-DR antibody L243. Cells were analyzed by flow cytometry with a minimum of 10,000 events collected.
For IL-17 and IFN-γ staining, cells from draining lymph nodes of CII-immunized mice were seeded into 48-well plates at a density of 2x106 cells per well and incubated with 400 μg/well of CII peptide 257–274 overnight. The next day, PMA, ionomycin, and monensin were added at concentrations of 5 ng/ml, 500 ng/ml, and 0.66 μg/ml, respectively. Cells were incubated for 4 hr at 37° C. Cells were then washed and stained with anti-CD4 FITC, anti-CD8-PerCP, and CD19-PerCP-conjugated antibodies for 30 min at 4° C. After this incubation, cells were washed twice with PBS with 2% FBS, resuspended into 500 μl of Cytofix/Cytoperm (BD Biosciences), incubated for 20 min at 4° C, and washed twice with 1x Perm/Wash (BD Biosciences). Cells were then incubated with anti-IFN-γ-APC and anti-IL17A-PE (BD Biosciences) antibodies for 30 min at 4° C and analyzed by flow cytometry.
For staining with the DR1-CII tetramer, 1x106 cells from draining lymph nodes were incubated with 1 μg of DR1 tetramer in 50 μl of complete medium with 5 mM NaN3 as previously described (30). After 2.5 hr incubation at 37° C, anti-CD4-APC, anti-CD8-PerCP-Cy5.5, anti-CD19-PerCP-Cy5.5, anti-Vβ8-FITC, and anti-Vβ14-FITC antibodies were added, cells were incubated for 30 min at 4° C, and analyzed by flow cytometry. For tetramer studies a minimum of 100,000 cells were analyzed from each sample.
Proliferation assay
Ten days after immunization, cells were harvested from draining LN of CII-immunized mice and seeded in 96-well plates at a density of 4.5 x 105 cells per well. Cells were incubated for 4 days with 100 μg of CII peptide 257–274 in HL-1 medium (BioWhittaker) supplemented with 50 μM of 2-ME and 0.1% BSA. During the last 16 hr, 1 μCi of 3H-thymidine (New England Nuclear, Boston, MA) was added to each well. Cells were harvested and incorporation of 3H was measured by a Matrix 96 direct ionization beta counter (Packard Instrument, Meriden CT).
Antigen presentation assay
Antigen presentation experiments were performed in 96-well microtiter plates in a total volume of 0.3 ml containing 105 antigen presenting cells, 105 T-hybridoma cells, and 100 μl of the CII(257-274) peptide at various concentrations in complete DMEM (DMEM supplemented with 10% FBS, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 0.05 mM β-mercaptoethanol, and 2 mM L-glutamine). Cell cultures were maintained at 37° C in 10% humidified CO2 for 24 hours, after which 80 μl supernatant was removed from each well and two-fold serial dilutions were made through each row of the plate. IL-2-dependent HT-2 cells (5 x 103) were then added to each well of the 96 well plate and following an 18 hour incubation, HT-2 cell viability was assessed by cleavage of MTT and quantitation at 690 nm with background absorption at 560 nm subtracted (31, 32). IL-2 titers were quantified by the reciprocal of the highest two-fold serial dilution maintaining HT-2 cell viability greater than two fold over control cultures. Results are presented as units of IL-2 per ml of undiluted supernatant as described by Kappler et al. (33).
Suppression assay
Effector CD4+ T cells were enriched from the spleens of TCR-E168 DR1 transgenic mice using a CD4+ T cell isolation kit (Miltenyi) and were seeded in 96-well plates at a density of 5x104 cells per well. These cells were mixed with 5x104 irradiated APC and various numbers of engineered Treg cells, and incubated for 4 days in the presence of 1 μg/well of the CII(257-274) peptide. One μCi of 3H-thymidine was added for the last 16 hr of incubation. Cells were then harvested onto filters and the incorporation of 3H-thymidine was measured by a Matrix 96 direct ionization beta counter (Packard Instrument).
CII antibody measurements
Sera from mice were collected at days 28 and 42 post-immunization and the quantity of anti-CII IgG antibodies was determined using a solid phase ELISA as previously described (24). Briefly, microtiter plates were coated with 500 ng of bCII at 4° C overnight. After extensive washing with 0.5 M saline/0.1% Tween 20, wells were blocked by the addition of 2% BSA for 30 min at 4° C and washed. A 1:2000 dilution of the mouse sera in 2% goat serum was then added to each well and the assay was incubated overnight at 4° C. After washing with saline and Tween 20, an HRP-labeled goat anti-mouse IgG (1:10,000 dilution, Southern Biotech) was added. After 2 hours the plates were washed and developed by the addition of o-phenyldiamine (Sigma). After stopping the reaction with 2.5 N H2SO4, the degree of color development was measured using a spectrophotometric plated reader (Spectra Max, Molecular Devices). Data are expressed as optical density (OD) at 490 nm with background absorbance of 650 nm subtracted.
Results
Generation of retrogenic Treg cells expressing MHC class II
Targeted Treg cells where engineered using a replication deficient retrovirus encoding Foxp3 and an HLA-DR1 peptide-ligand complex (constructs shown in Figure 1A). This DR1-CII peptide complex in tetrameric form has been previously shown to bind selectively to the TCR of CII-specific, CD4+ pathogenic T cells (30, 34). cDNA encoding the immunodominant CII peptide and a linker sequence were inserted near the amino terminus of the DRB1 chain in order to covalently link the CII peptide to the DR1 molecule (30, 35). Empty vector (MSCV) and vector encoding only the DR1-CII construct were used as controls (Figure 1A), and all of the vectors expressed GFP in a second cistron using an IRES promoter.
Flow cytometric analysis of the transduced T cells indicated that nearly all GFP-positive cells expressed the retrovirally encoded genes (Figure 1B). To determine if the Foxp3 and DR1-CII proteins expressed by the retrovirus were functional, antigen presentation experiments and Treg suppression assays were performed (Figure 1C and 1D). As shown in Figure 1C, T cells transduced with a construct containing DR1-CII-Foxp3 are highly efficient in their ability to stimulate DR1-restricted, CII-specific T cell hybridomas to produce IL-2. The inclusion of the Foxp3 construct had no effect on the T-hybridomas as these cells are not susceptible to Treg function (data not shown). In comparison, T cells transduced with the empty MSCV control vector (Figure 1C) or Foxp3 alone vector (data not shown) were incapable of stimulating the T-hybridoma cells. These data indicate that the DR1 molecule is expressed as a functional molecule and that the covalently linked CII peptide is properly folded into the DR1 binding pocket. To determine if the Foxp3 gene is functional and converts naïve CD4+ T cells to Treg cells, T cells transduced with either the control MSCV vector or the vector containing the DR1-CII-Foxp3 construct were tested for their ability to suppress the in vitro proliferative response of DR1-restricted, CII-specific T cells recovered from TCR-E168 DR1 mice. As shown in Figure 1D, T cells transduced by the control MSCV vector did not inhibit the proliferation of CII-specific T cells at any ratio of engineered Treg to T cell tested, nor did cells transduced with DR1-CII alone (data not shown). In contrast, cells transduced with the DR1-CII-Foxp3 vector significantly suppressed the proliferation of the CII-specific T cells and suppression was still evident at a ratio of 1:8, Treg:T-responder cell, indicating that the Foxp3 expressed by the construct is functional and transduction of naïve CD4+ T cells with this construct produces cells with Treg function.
Retrogenic Tregs expressing HLA-DR1-CII suppress autoimmune arthritis in DR1 Tg mice
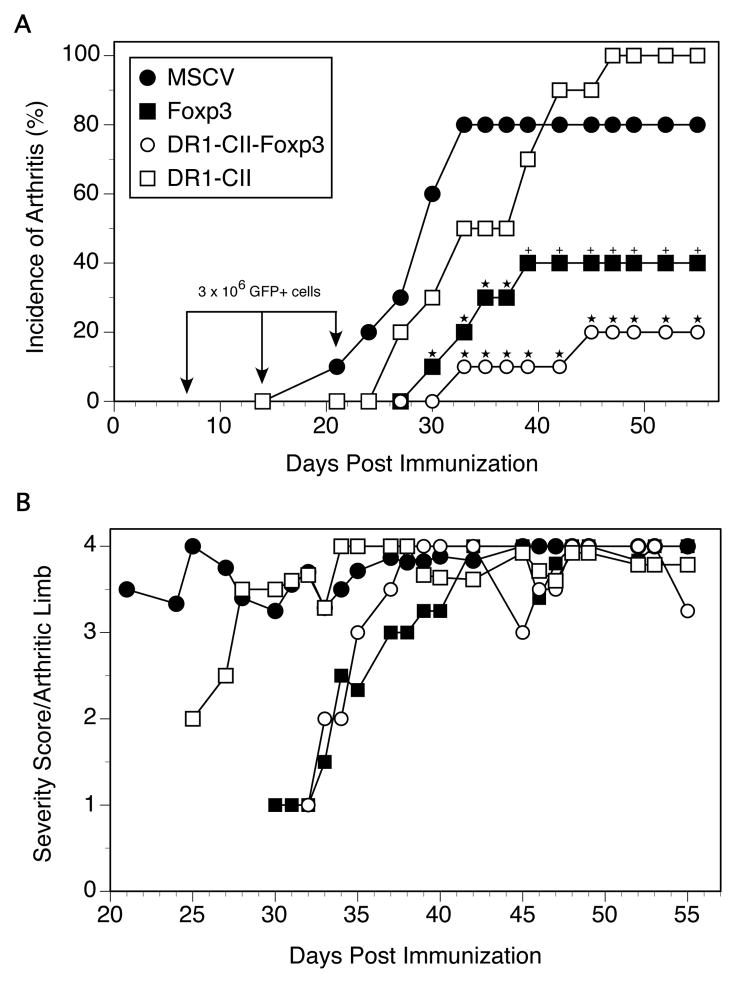
To determine if the engineered Treg cells could alter the development of autoimmune arthritis, humanized DR1 Tg mice were immunized with bCII and treated with 3 x 106 GFP+ transduced T cells i.v. on days 7, 14, and 21 post-immunization. As shown in Figure 2, engineered Tregs expressing the DR1-CII-Foxp3 construct were highly efficient in inhibiting the development of autoimmune arthritis (Figure 2A). High incidences of disease were observed in both groups of control animals that received either T cells transduced with the MSCV vector (80% incidence) or T cells transduced with the vector containing only the DR1-CII construct (100% incidence). In contrast, mice receiving T cells transduced with either Foxp3 alone or DR1-CII-Foxp3 had significantly reduced incidences of disease (p ≤ 0.03, Figure 2A). While the arthritis incidence was reduced to 40% for mice receiving the Foxp3-transduced T cells, when DR1-CII and Foxp3 were co-expressed by the engineered Treg cells, only 20% of the mice developed arthritis. While this difference did not reach statistical significance within a single experiment, this trend was observed in multiple experiments. Of the few mice that did develop arthritis in the Foxp3 and DR1-CII-Foxp3 groups, the onset of disease was delayed (mean of 33.75 and 38.5 days, respectively, p < 0.02) in comparison to the control group (mean of 28.1, MSCV group), and the severity of disease was reduced at the early stages of disease (Figure 2B). However, as disease progressed in these mice, no differences in severity were observed in comparison to the control groups. Thus these data indicate that co-expression of Foxp3 and DR1-CII by the Treg cells enhances their efficacy in inhibiting the development of arthritis in this model.
Figure 2.
Engineered Treg cells expressing DR1-CII are efficient inhibitors of collagen induced arthritis. A. DR1 Tg mice were immunized with CII/CFA, and on days 7, 14 and 21 after immunization mice were treated with T cells infected with replication deficient virus encoding either MSCV alone, Foxp3 alone, DR1-CII alone, or DR1-CII- Foxp3. 3 x 106 GFP+ cells were injected at each time point. Mice were evaluated 3 times per week for the presence and severity of arthritis. N=10 for all groups in this experiment and each experimental group was repeated 2 to 3 times. ★ indicates p ≤ 0.03 compared with MSCV control; + indicates p = 0.08 compared with MSCV control (both by Fisher’s Exact test). B. Arthritis severity score per arthritic limb is reduced at early time points after treatment with engineered Tregs. Arthritis severity was determined by adding the severity scores of all arthritic limbs for a given time point and dividing by the number of arthritic limbs. Limbs were scored for severity on a scale of 0 to 4 as described in Materials and Methods.
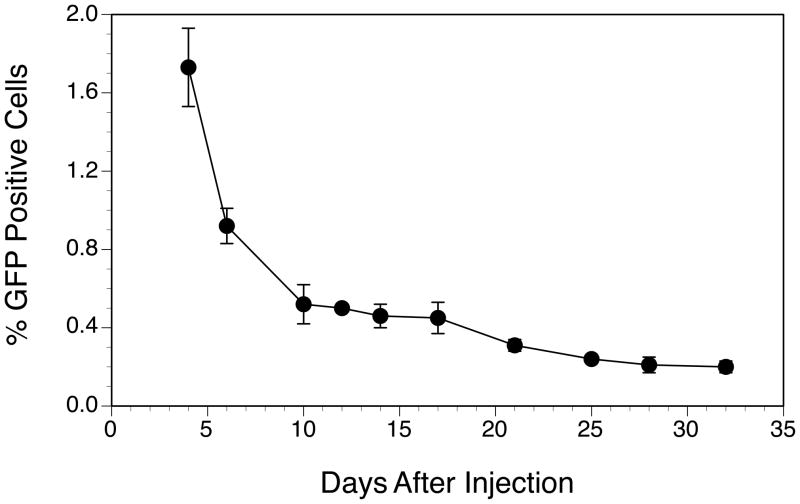
Since a delayed onset and a prolonged inhibition of arthritis in animals treated with the T cells transduced with the Foxp3 or DR1-CII-Foxp3 construct was observed, we sought to determine how long these engineered Tregs persisted in the mouse after transfer. Mice were injected with a single dose of 3 x 106 engineered Treg cells expressing DR1-CII-Foxp3 and peripheral blood was collected at several time points after, and the percentage of GFP+ cells present was assessed by flow cytometry. As shown in Figure 3, the engineered Treg cells survived well beyond the time frame during which arthritis developed in all four groups (Figure 2A). Five days after injection, GFP+ (transduced) cells comprised approximately 1.8% of peripheral blood lymphocytes and persisted at low levels at least through day 32 (0.3%). While it is not clear if the regulatory function of these cells is maintained over this time, these data are consistent with the sustained inhibition of arthritis observed in Figure 2A.
Figure 3.
Engineered Tregs survive for long periods in recipient mice. DR1 Tg mice were injected with 3 x 106 engineered GFP+ Treg cells expressing DR1-CII and Fox P3, and the number of GFP+ cells in the peripheral blood was measured at several time points. Data are based on total numbers of lymphocytes in the peripheral blood. Error bars indicate standard deviations of a minimum of 3 measurements at each time point.
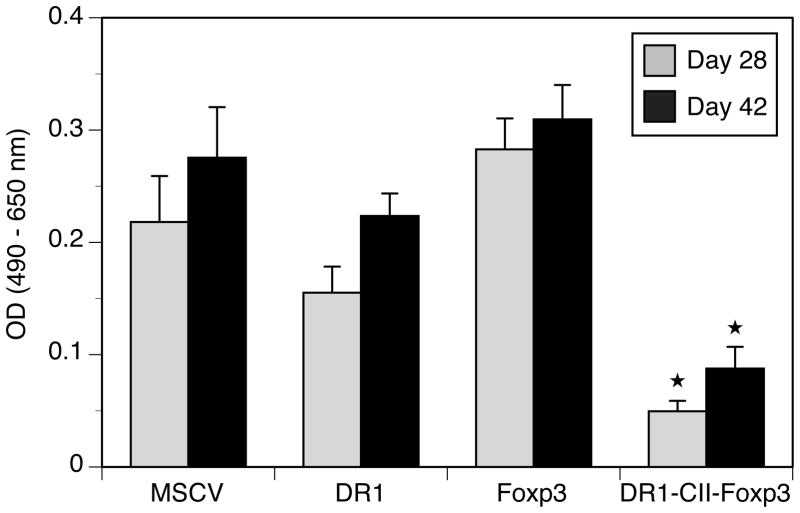
Since autoantibodies to CII are a major component of the arthritis pathogenesis in this model, we sought to determine if inhibition of disease by the engineered Treg cells was accompanied by changes in the anti-CII IgG response in these mice. Sera from mice treated with the engineered Tregs, DR1-CII alone, and MSCV control cells were collected at days 28 and 42 post-immunization and the quantity of CII specific antibody was measured using a solid phase ELISA. As is shown in Figure 4, mice injected with DR1-CII-Foxp3 transduced T cells produced significantly less CII-specific IgG on days 28 and 42 post-immunization compared to all other groups. The levels of anti-CII antibody production in the MSCV and DR1-CII control groups correlated with the high level of disease observed in these mice (Fig. 2A). Interestingly, although mice injected with the Foxp3-transduced T cells had significantly lower arthritis incidence (40%) than mice treated with the MSCV of DR1-CII control T cells (80% and 100%, respectively), anti-CII antibody levels were similar between these three groups. These data suggests that different mechanisms are utilized between the Foxp3 and DR1-CII-Foxp3 engineered Treg cells, and imply a role for pathogenic T cells in addition to autoantibody in this model.
Figure 4.
Decrease in CII-specific autoantibody in mice treated with engineered Treg cells. Sera were obtained from the mice on days 28 and 42 after immunization, and the quantity of CII-specific antibody measured using a CII specific ELISA. Data are expressed as optical density (OD) at 490 nm absorbance minus absorbance at 650 nm. ★ indicates p < 0.05 between DR1-Foxp3 and MSCV groups at day 28, and p < 0.00005 at day 42.
DR1-CII-Foxp3 Treg cells reduce the number and function of CII-specific autoimmune T cells in vivo
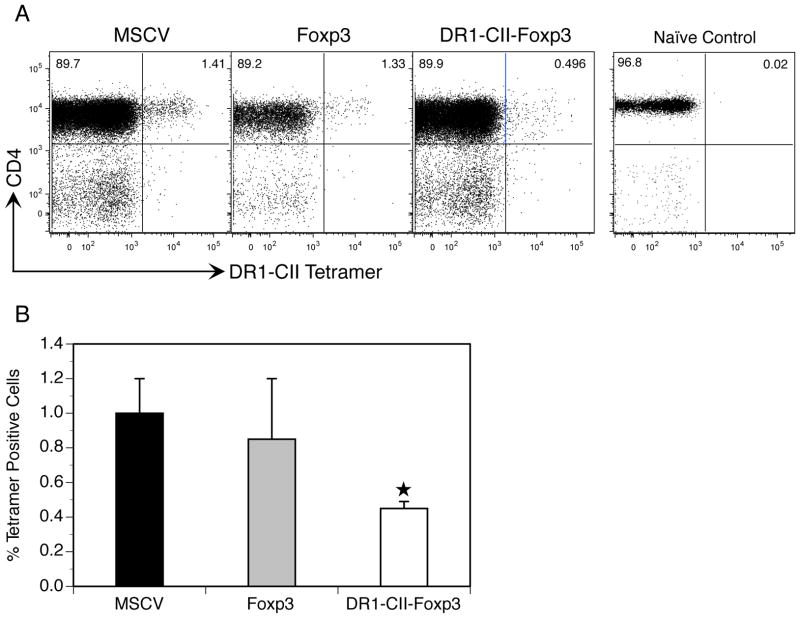
Since CII-specific antibody concentrations in the sera were significantly lower in the DR1-CII-Foxp3 group, we examined the magnitude and functional state of the CD4+ CII-specific T cell response in mice treated with the engineered Treg cells. To determine the magnitude of the T cell response in vivo, mice were immunized with CII and treated on day 1 and day 7 after immunization with T cells transduced with either MSCV, Foxp3, or DR1-CII-Foxp3. On day 10, the peak of the CII-specific T cell response in vivo (30), cells from the draining lymph nodes were recovered and labeled with a PE labeled HLA-DR1-CII tetramer and fluorochrome labeled antibodies specific for CD4, CD8, and CD19. Cells were then analyzed by flow cytometry and the data were analyzed by negative gating on CD8 and CD19 expression. As shown in Figures 5A and 5B, CII-specific autoimmune T cells comprised 1% of the CD4+ T cells from the draining lymph nodes of immunized mice treated with MSCV transduced T cells, and averaged 0.8% from mice treated with Foxp3 engineered Treg cells. In contrast, the mice treated with the DR1-CII-Foxp3 engineered Treg cells had 50% fewer CII-specific T cells, comprising less than 0.5% of the CD4+ population. These data indicate that one mechanism governing the effectiveness of these engineered Treg cells appears to be inhibition of the expansion of the autoimmune CII-specific T cells in vivo.
Figure 5.
Treatment of mice with engineered Tregs reduces the number of CII-specific T cells in vivo. Mice were immunized with CII/CFA, treated with the engineered Treg cells on days 1 and 7 post-immunization, and on day 10 draining lymph node cells were recovered and stained with antibodies specific for CD4, CD8, and CD19 and with an HLA-DR1-CII tetramer. Naïve T cells from an unimmunized DR1 Tg mouse was used as a control for tetramer staining. Data shown are based on negative gating of CD8 and CD19 expressing cells. A. Representative experiment of tetramer staining by CD4+ T cells. B. Cumulative data represented by average percent of CD4+, DR1-CII tetramer+ T cells, compiled from 3 or more experiments. ★ indicates p < 0.002 relative to MSCV control.
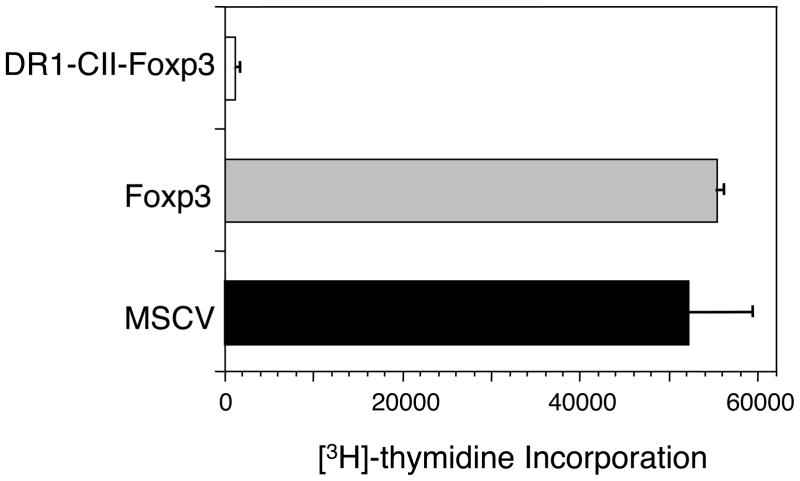
While the CII-T cell response was reduced in the mice treated with the DR1-CII-Foxp3 Tregs, a 0.5% population still represents the presence of a significantly expanded population of T cells driven by antigen stimulation in vivo. This raised the questions as to whether or not these CII-T cells were not only reduced in number but also rendered functionally inactive by the engineered Tregs. To assess their functional state, cells were recovered from the draining lymph nodes of CII-immunized mice that had been treated with engineered T cells, and the CII-specific T cells present were tested for their ability to proliferate in response to stimulation by the immunodominant CII peptide in vitro. When T cells from mice treated with MSCV or Foxp3 transduced T cells were stimulated with the CII peptide, a strong proliferative response was observed in each case (Figure 6). However, T cells from mice treated with DR1-CII-Foxp3 Treg cells were highly refractory to CII peptide stimulation, responding minimally above the background level of unstimulated cells (Figure 6). The lack of a response by the T cells from these mice is not a simple result of the reduced numbers of CII-specific T cells, as we have found that as few as 0.1% tetramer positive cells will still generate an appreciable antigen specific T cell proliferative response in vitro (E.R., unpublished observation). Thus these data indicate that treatment of mice with the DR1-CII-Foxp3 Tregs renders the autoimmune T cells unresponsive to antigen stimulation and that this unresponsive state is likely playing a role in the suppression of the autoimmune arthritis.
Figure 6.
Proliferative responses of CII-specific T-cells recovered from the draining lymph nodes of immunized mice treated with engineered Treg cells. Mice were immunized with CII/CFA, treated twice (days 1 and 7) with the engineered Treg cells, and lymph node cells were recovered 10 days post-immunization and tested for their ability to proliferate in vitro when stimulated with the CII peptide. Proliferation was measured by [3H]-thymidine incorporation after 4 days of culture.
Retrogenic Treg cells alter the cytokine phenotype of autoimmune T cells
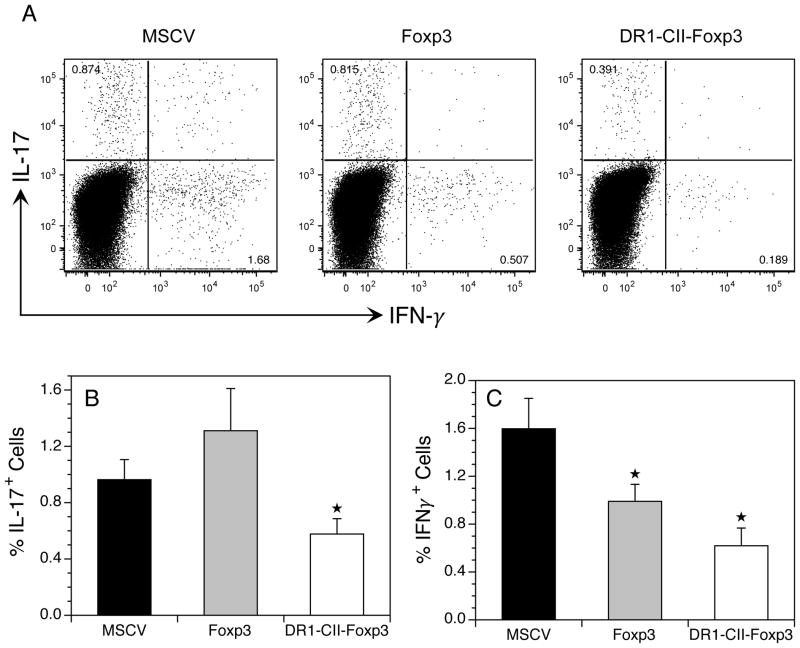
To determine if treatment of the mice with the engineered Treg cells altered the Th1 and Th17 T cell response in these mice, we measured the number of CD4+ T cells producing IFN-γ and Th17 in mice treated with the engineered T cells. Based on our observation that the engineered Treg cells expressing only Foxp3 inhibited arthritis development without changes in antibody production or T cell responses, we hypothesized that reductions in proinflammatory Th1 and Th17 T cells may be associated with this inhibition of arthritis. To test this concept, mice were immunized with CII and treated with engineered Tregs, and 10 days later lymph node cells were recovered, restimulated with CII peptide in vitro, and analyzed for intracellular expression of IL-17 and IFN-γ by flow cytometry. Day 10 was chosen for analysis because it is the peak of the CD4+ T cell response in this model (30). As shown in Figure 7A and 7B, Th17 cells from immunized mice receiving MSCV control T cells comprised 1% of the CD4+ T cells (Figure 7B) and IFN-γ+ Th1 cells were 1.6% (Figure 7C). In contrast, mice receiving the Foxp3 or DR1-CII-Foxp3 engineered Treg cells had significant decreases in IFN-γ+ Th1 cells, or Th1 and Th17 T cells, respectively. While the Foxp3 transduced Treg cells had similar numbers of Th17 cells (1.2%) compared to the controls (Figure 7B), the IFN-γ+ Th1 cells comprised only 0.8% of the CD4+ T cells, a significant decreased of 50% in comparison to the control mice (Figure 7C). Interestingly, although fewer mice in both the Foxp3 group and the DR1-CII-Foxp3 group developed arthritis relative to the vector control group, only the DR1-CII-Foxp3 treated group had reduced percentages of Th17 and Th1 cells. The DR1-CII-Foxp3 group had the lowest percentage of IFN-γ+ Th1 cells (0.6%) and a 50% decrease in Th17 cells (0.5%) compared to the control group. Unlike the relationship among these three groups observed with the Th17 data, the percentages of IFN-γ+ Th1 cells (Figure 7C) were directly proportional to the arthritis incidence in Figure 2. The significant decrease in both IFN-γ+ cells and IL-17+ cells in the DR1-CII-Foxp3 group suggests that part of the mechanism mediating the enhanced inhibition of arthritis by these cells may involve interference or redirection of the generation of both Th1 and Th17 cells.
Figure 7.
Treatment of mice with engineered Tregs expressing DR1-CII and Foxp3 inhibits the development of IL-17 and IFN-γ expressing CD4+ T cells. Mice were immunized with CII/CFA and treated with engineered Treg cells on days 1 and 7 post-immunization. On day 10 post-immunization, cells from draining lymph nodes were recovered, stimulated overnight with CII peptide, and stained with antibodies specific for CD4, CD8, CD19, IL-17 and IFN-γ. Data are based on negative gates for CD8 and CD19 expressing cells, and CD4+ cells. GFP+ engineered Treg cells were removed from the data analysis by negative gating. A. Representative experiment measuring Th17 and IFN-γ expression by CD4+ T cells. B. Cumulative data from 3 or more experiments represented by average percent of CD4+ T cells expressing IL-17 or IFN-γ. ★ indicates p < 0.05 relative to MSCV control.
Discussion
While the potential of adoptively transferred Treg cells to be utilized therapeutically for autoimmunity and transplantation is promising, there are a number of obstacles to overcome, including generation of sufficient numbers of autologous Treg cells and improved efficacy in ameliorating the disease. Given that the total absence of Treg cells results in profound autoimmunity in both man and in mouse models (1, 2), it is clear that Treg cells play a significant role in homeostasis of the immune system and prevent the onset of multi-organ autoimmunity. While most adoptive transfer studies of Tregs in animal models have focused on the use of polyclonal Tregs, there is some evidence that monoclonal Tregs expressing TCR specific for the autoantigen are more efficacious in inhibiting autoimmune responses via the “targeting” capabilities of these cells (15, 18–20). In the study described here, we engineered targeted Treg cells using a complex of the MHC ligand and peptide antigen that drives the pathogenic T cell response in an autoimmune arthritis model. These targeted engineered Treg cells were produced by transduction of naïve CD4+ T cells with Foxp3 and HLA-DR covalently linked to an immunodominant peptide derived from CII that drives the pathogenic T cell response in an autoimmune arthritis model. This approach generated engineered T cells with both the functional properties of a Treg cell as well as a targeting mechanism (DR1-CII) to promote their interaction with the CII-specific, pathogenic T cells. While it is generally accepted that functional maturation of Treg cells requires stimulation via the T cell receptor, it appears that anti-CD3/CD28 activation of the naïve T cells just prior to transduction with Foxp3 suffices to create fully functional regulatory T cells (3, 36). When these engineered Treg cells were used as an adoptive therapy after the initiation of an autoimmune response in the arthritis model, they significantly reduced the incidence of disease and were consistently more effective than engineered Treg cells expressing Foxp3 alone. Not only were the class II expressing Tregs effective in preventing the autoimmune response from becoming overt disease, but the mechanism by which they inhibited the autoimmune response appears to be very different than that of the classical Tregs expressing Foxp3 only. Unlike the Foxp3 only Tregs, the DR1-CII-Foxp3 Treg cells inhibited the expansion of the T effector response to the autoantigen in vivo, significantly reduced the autoantibody response, and prevent the development of both Th1 and Th17 cells. None of these mechanisms were evident in the mice treated with the Foxp3 only engineered Tregs, despite the fact that they were efficient inhibitors of autoimmune arthritis. Thus the expression of the HLA-DR:peptide ligand by the engineered Treg cells generated a highly effective regulatory cell with additional mechanistic capabilities of altering an autoimmune response.
While mouse T cells do not normally express MHC class II molecules, the expression of class II molecules by activated T cells is normal in most all other species (21, 37). It has been recognized for many years that activated human T cells express class II, and mostly recently it was been demonstrated that this extends to at least a subpopulation of human Treg cells (22). These DR+ Treg cells express high levels of Foxp3 and appear to be a functionally distinct lineage of CD4+CD25+ T cells based on their capacity to inhibit T cell proliferative responses in vitro. Whether or not the DR expression indicates that these Tregs are antigen experienced iTregs is not clear, but like our engineered DR-CII-Foxp3 Tregs, the DR+ human Tregs have functional mechanisms that differ from DR-negative Treg cells. Functionally, there are a number of similarities between the human DR+ Tregs and our engineered murine DR1+ Tregs. In our studies, autoantigen specific T cells from mice treated with the DR1-CII-Foxp3 engineered Treg cells were completely unresponsive to stimulation with antigen in vitro, while the autoantigen specific T cells from mice treated with “conventional” Foxp3 only Tregs responded vigorously. This is similar to the studies of human Treg cells where DR1+ Tregs strongly inhibited the anti-CD3/CD2 response of naïve T cells while DR1-negative Tregs did not (22). Additionally, treatment with the DR1+ engineered Tregs caused a decrease in the number of Th1 cells producing IFN-γ as did human DR+ Tregs, but not the DR-negative Tregs in both cases. Thus it appears that Treg cells that express class II molecules represent a functionally distinct subset of Tregs responsible for immune homeostasis in man.
The mechanisms by which our Foxp3 only and DR1-CII-Foxp3 engineered Tregs function appear to be significantly different. While the Foxp3 only Tregs significantly reduced the incidence of arthritis, the changes in the autoimmune response brought about by these cells were difficult to identify. No significant changes in autoantibody levels, numbers of activated CII-specific T cells, nor the ability of these T cells to be stimulated by antigen in vitro were observed. Numbers of Th17 T-cells produced in mice treated with Foxp3 engineered Tregs were also unchanged, although there was a significant decrease in Th1 IFN-γ producing cells. Surprisingly, this is not an uncommon observation in studies where adoptive transfer of syngeneic Treg cells where used to successfully inhibited the development of an autoimmune disease. It has been suggested that the mechanism of inhibition for Treg cells occurs at the site of inflammation (38), and this may explain the apparent lack of effect in peripheral lymphoid organs. In contrast to the Foxp3 engineered Tregs, the DR1-CII-Foxp3 engineered Treg cells drastically altered the autoimmune response in the periphery. Significant decreases in autoantibody levels were observed, the development of the autoimmune T cells response in draining lymph nodes was reduced, and the CII-specific T cells that were present were totally refractory to stimulation by autoantigen in vitro. In addition, adoptive transfer of the DR1-CII-Foxp3 Treg cells reduced the numbers of both Th17 and Th1 T cells in the recipient mice to nearly background levels. How the expression of the DR1-CII ligand on the Treg cells confers this regulatory mechanism is not clear. While the expression of class II in the absence of costimulatory molecules would be expected to induce a negative signal in naïve T cells, the engineered Treg cells were adoptively transferred into the DR1 Tg mice after the initiation of the autoimmune response, a time when the autoimmune T cells should no longer be dependent on co-stimulation. However, the function of class II expression by T cells is still unknown, and it remains possible that an interaction between a T effector cells and class II+ T cell results in a negative signal to the T effector cell regardless of its activation state (39), perhaps through an undefined co-stimulatory/regulatory receptor ligand interaction. In our system, this undefined receptor/ligand would have to involve Treg cells since cells transduced with DR1-CII alone did not alter the develop of autoimmune arthritis. Arthritis inhibition required co-expression of DR1-CII with Foxp3 resulting in Treg cells that efficiently shut down the autoimmune T cell response over a long period.
The majority of studies aimed at targeting or enhancing the efficacy of adoptive Treg therapy in autoimmunity have focused on the use of antigen specific Tregs. In the MBP or MOG based EAE models, Tregs expressing TCR specific for either antigen have been shown to be very effective in inhibiting primary disease as well treating relapsing disease (36, 40). Similar results have been observed in autoimmune diabetes (14) and antigen specific arthritis (19). The proposed mechanism by which antigen specific Tregs may be more efficient in down regulating an autoimmune response is their ability to migrate to the sites of inflammation because of their antigen specificity, and inhibit the pathogenic T effector cell function in situ. Indeed, it has been suggested that Treg inhibition of T effector cell function is more pronounced at the site of inflammation than in the draining lymph nodes (41). Whether or not our engineered Tregs are more effective at the site of arthritis development or the draining lymph nodes is unknown at this point, however we have previously demonstrated that there is a highly selective clonal population of CII specific T cells infiltrating the joints at the onset of disease (34) and these cells would represent a prime target for the DR1-CII-Foxp3 Treg cells to down regulate the development of autoimmune arthritis in this model.
Similar to our approach to targeting T effector cells with class II expressing Tregs, LaGuern et al. achieved enhanced Treg function in allogeneic transplantation studies using Treg cells transfected with murine class II (42). However, the class II expression in their system was restricted to the intracellular space; it was not expressed on the surface of the Treg cells. How the mechanism of this enhanced Treg function in this study relates to ours described here is unclear. Since the class II is not expressed on the cell surface of the Treg cells in this transplantation model, it is unlikely to be serving as a targeting mechanism for the Tregs to selectively interact with the T effector cells. The authors propose that the intracellular class II is being degraded into peptides that are accessible to the class I biosynthesis pathway, and that class I presentation of class II-derived peptides is the mechanism that mediates the enhanced Treg activity. Although our transduced DR1 molecules would also be accessible to the class I antigen presentation pathway, this mechanism is unlikely to be functioning in our system as adoptive transfer of Treg cells transfected with only DR1-CII did not inhibit the development of arthritis.
While the studies described here rely on a defined autoantigen for generating the therapeutic DR1-CII-Foxp3 Treg cells, it is not clear yet if the antigen is required or if class II expression alone on Treg cells will enhance their function. Clearly in the absence of Foxp3, T cells expressing the DR1-peptide ligand did not inhibit the development of arthritis, and may have promoted the autoimmune response. Additionally, the mechanism by which the class II expressing Treg cells inhibited the autoimmune response appears to be different or at least supplementary to mechanisms used by normal Treg cells, similar to the observations made in the study of human DR-expressing Treg cells (22). It is conceivable that the class II expressed by the human Treg cells is capable of capturing autoantigenic peptides and expressing sufficient numbers of these class II-autoantigen peptide complexes on their cell surface to serve as a bait and trap for autoimmune pathogenic T cells. Whether this is a natural occurring homeostatic function of the immune system or simply represents a therapeutic exploit remains to be determined. Regardless, these data imply that treatment of autoimmune diseases by expansion of endogenous class II+ Tregs may be an effective therapeutic approach. Development of a means of stimulating their expansion in vivo, or expansion in vitro followed by peptide loading and transfer back to the patient are attractive approaches for novel therapy in autoimmunity.
Abbreviations
- CIA
collagen induced arthritis
- bCII
bovine CII
- CII
type II collagen
- DR1-CII
HLA-DR1 covalently linked to CII(257–273) peptide
References
- 1.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 2.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: a model of immune dysregulation. Current opinion in allergy and clinical immunology. 2002;2:481–487. doi: 10.1097/00130832-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc Natl Acad Sci U S A. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 9.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 11.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 12.Frey O, Petrow PK, Gajda M, Siegmund K, Huehn J, Scheffold A, Hamann A, Radbruch A, Brauer R. The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res Ther. 2005;7:R291–301. doi: 10.1186/ar1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, Song J. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 16.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 17.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Herwijnen MJ, Wieten L, van der Zee R, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, den Braber I, Anderton SM, Singh M, Meiring HD, van Els CA, van Eden W, Broere F. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012;109:14134–14139. doi: 10.1073/pnas.1206803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, Ehrenstein MR, Stauss HJ. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 21.Benoist C, Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- 22.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 24.Rosloniec EF, Brand DD, Myers LK, Whittington KB, Gumanovskaya M, Zaller DM, Woods A, Altmann DM, Stuart JM, Kang AH. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med. 1997;185:1113–1122. doi: 10.1084/jem.185.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods A, Chen HY, Trumbauer ME, Sirotina A, Cummings R, Zaller DM. Human major histocompatibility complex class II-restricted T cell responses in transgenic mice. J Exp Med. 1994;180:173–181. doi: 10.1084/jem.180.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosloniec EF, Cremer M, Kang A, Myers LK. Collagen-induced arthritis. Curr Protoc Immunol. 2001;Chapter 15(Unit 15):15. doi: 10.1002/0471142735.im1505s20. [DOI] [PubMed] [Google Scholar]
- 27.Persons DA, Allay JA, Allay ER, Smeyne RJ, Ashmun RA, Sorrentino BP, Nienhuis AW. Retroviral-mediated transfer of the green fluorescent protein gene into murine hematopoietic cells facilitates scoring and selection of transduced progenitors in vitro and identification of genetically modified cells in vivo. Blood. 1997;90:1777–1786. [PubMed] [Google Scholar]
- 28.Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D, Ryan MD. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly ML, Luke G, Mehrotra A, Li X, Hughes LE, Gani D, Ryan MD. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol. 2001;82:1013–1025. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 30.Latham KA, Whittington KB, Zhou R, Qian Z, Rosloniec EF. Ex Vivo Characterization of the Autoimmune T Cell Response in the HLA-DR1 Mouse Model of Collagen-Induced Arthritis Reveals Long-Term Activation of Type II Collagen-Specific Cells and Their Presence in Arthritic Joints. J Immunol. 2005;174:3978–3985. doi: 10.4049/jimmunol.174.7.3978. [DOI] [PubMed] [Google Scholar]
- 31.Denizot F, Rita L. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Z, Latham KA, Whittington KB, Miller DC, Brand DD, Rosloniec EF. An autoantigen-specific, highly restricted T cell repertoire infiltrates the arthritic joints of mice in an HLA-DR1 humanized mouse model of autoimmune arthritis. J Immunol. 2010;185:110–118. doi: 10.4049/jimmunol.1000416. [DOI] [PubMed] [Google Scholar]
- 35.Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- 36.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, Harris RA, Magnusson PU, Brittebo E, Loskog AS. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. Journal of neuroinflammation. 2012;9:112. doi: 10.1186/1742-2094-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holling TM, Schooten E, van Den Elsen PJ. Function and regulation of MHC class II molecules in T-lymphocytes: of mice and men. Hum Immunol. 2004;65:282–290. doi: 10.1016/j.humimm.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–2710. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 40.Stephens LA, Malpass KH, Anderton SM. Curing CNS autoimmune disease with myelin-reactive Foxp3+ Treg. Eur J Immunol. 2009;39:1108–1117. doi: 10.1002/eji.200839073. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeGuern C, Akiyama Y, Germana S, Tanaka K, Fernandez L, Iwamoto Y, Houser S, Benichou G. Intracellular MHC class II controls regulatory tolerance to allogeneic transplants. J Immunol. 2010;184:2394–2400. doi: 10.4049/jimmunol.0803664. [DOI] [PMC free article] [PubMed] [Google Scholar]