Abstract
STUDY DESIGN
Case series.
BACKGROUND
It has been shown in rodent and canine models that cartilage composition is significantly altered in response to long-term unloading. To date, however, no in vivo human studies have investigated this topic. The objective of this case series was to determine the influence of unloading and reloading on T1rho and T2 relaxation times of articular cartilage in healthy young joints.
CASE DESCRIPTION
Ten patients who required 6 to 8 weeks of non–weight bearing (NWB) for injuries affecting the distal lower extremity participated in the study. Quantitative T1rho and T2 imaging of the ipsilateral knee joint was performed at 3 time points: (1) prior to surgery (baseline), (2) immediately after a period of NWB (post-NWB), and (3) after 4 weeks of full weight bearing (post-FWB). Cartilage regions of interest were segmented and overlaid on T1rho and T2 relaxation time maps for quantification. Descriptive statistics are provided for all changes.
OUTCOMES
Increases of 5% to 10% in T1rho times of all femoral and tibial compartments were noted post-NWB. All values returned to near-baseline levels post-FWB. Increases in medial tibia T2 times were noted post-NWB and remained elevated post-FWB. The load-bearing regions showed the most significant changes in response to unloading, with increases of up to 12%.
DISCUSSION
The observation of a transient shift in relaxation times confirms that cartilage composition is subject to alterations based on loading conditions. These changes appear to be mostly related to proteoglycan content and more localized to the load-bearing regions. However, following 4 weeks of full weight bearing, relaxation times of nearly all regions had returned to baseline levels, demonstrating reversibility in compositional fluctuations.
LEVEL OF EVIDENCE
Therapy, level 4.
Keywords: biomechanics, knee, medical imaging, MRI
Osteoarthritis (OA) is a degenerative disease that is characterized by cartilage thinning and biochemical compositional changes. It is estimated that 20 million individuals in the United States are living with the disease, at an annual cost of over 15 billion dollars.13,16 OA primarily affects weight-bearing joints, with the knee and hip joints being the most common sites.1
It is believed that the initiating element of cartilage degeneration is an imbalance between applied mechanical stress and the physicochemical ability of the cartilage to resist stress.1 Several studies have shown that loading of articular cartilage is necessary for stimulation of chondrocytes and normal joint function. In particular, it has been shown in cartilage explants that parameters of intermittent mechanical loading are related to proteoglycan synthesis, indicating that loading is a necessary and beneficial element for cartilage health, and that a lack of mechanical stimulation may be detrimental to cartilage health.31,32,35
Chronic unloading of cartilage has been studied using animal models. After 6 to 8 weeks of immobilization (splinting in flexion to achieve non–weight bearing [NWB]), decreases in proteoglycan content and increases in water content were observed in canines.14,33 In a review paper, Vanwanseele and colleagues37 compiled all studies on immobilization in animal models and reported a trend toward signs of early degenerative changes in articular cartilage after prolonged immobilization. Fewer studies have investigated the influence of remobilization on cartilage composition after a period of immobilization. Leroux and colleagues17 investigated the proteoglycan-water ratio in canines during an immobilization period, followed by a remobilization period. It was observed that this ratio was significantly reduced after 4 weeks of immobilization and returned to baseline levels after 3 weeks of remobilization. Jortikka and colleagues14 showed similar results in cartilage proteoglycan concentration over longer remobilization periods. This indicates that proteoglycan and water content have relatively rapid adaptation in response to the physical environment. Experimental studies to determine the role of loading on cartilage composition have traditionally been limited to in vitro and animal studies. These studies have helped to recognize many of the important attributes of the loading conditions (magnitude, frequency, duration, etc). However, only human in vivo studies can be used to definitively determine the role of loading on human cartilage composition.
Recent advancements in magnetic resonance imaging (MRI) technology have led to an improved ability to monitor early signs of OA in vivo. Of particular interest in identifying the earliest degenerative changes is the development of T1rho and T2 relaxation-time mapping.
T1rho relaxation time, or spin-lattice relaxation in the rotating frame, has been correlated with proteoglycan levels of articular cartilage. The loss in proteoglycan concentration within cartilage and the percentage increase in T1rho have been found to be correlated, with reported relationships (R2) ranging between 0.203 and 0.986.2,19 Using previously validated techniques (sodium MRI, biochemistry), several investigators have validated T1rho as an effective method to determine cartilage proteoglycan content both in vivo and ex vivo.19,38,39 It has been shown that individuals with early OA have significantly elevated T1rho relaxation times.18,30
T2 relaxation time, also known as spin-spin relaxation, has been found to distinguish between normal and OA cartilage.8 It has been established that T2 relaxation is dominated by cartilage water and collagen contents, and structural organization of the cartilage matrix.22,23,28,40,42 As these changes are known to occur early in the cartilage disease process, T2 mapping is promising in its ability to identify biochemical changes associated with early cartilage degeneration. Similar to T1rho, increased T2 relaxation times have been observed in persons with OA.9,18
These recent advancements in MRI technology allow investigation into the changes related to cartilage composition. Furthermore, these methods allow for monitoring of cartilage in vivo during periods of unloading. The purpose of this case series was to determine the influence of unloading and reloading on T1rho and T2 relaxation times of articular cartilage in healthy young adults. It is hypothesized that after 6 weeks of NWB subjects will demonstrate elevated T1rho and T2 times and that after a period of remobilization back to full weight bearing (FWB) those values will return to baseline levels.
CASE DESCRIPTION
Subjects
Ten subjects (4 male, 6 female; mean ± SD age, 35 ± 11 years) were recruited for the current study through referrals from the Department of Orthopaedic Surgery at the University of California, San Francisco. All subjects were prescribed 6 to 8 weeks of NWB requiring use of crutches for injuries affecting the distal lower extremity. This injury paradigm allowed for the observation of unloading effects on the ipsilateral knee joint of the injured limb. Specific diagnoses for each subject are provided in TABLE 1. Exclusion criteria included (1) any previous knee injuries or surgeries ipsilateral to the injury, (2) previous diagnosis of OA or any other disease affecting the articular cartilage of the knee, and (3) implanted biological devices that might interact with the magnetic field (eg, pacemakers, cochlear implants, or ferromagnetic cerebral aneurysm clips). All subjects were free of any knee range-of-motion restrictions and were allowed unlimited knee motion during all stages of the investigation. All procedures were approved by the Committee on Human Research at the University of California, San Francisco, and all subjects signed informed consent and Health Insurance Portability and Accountability Act (HIPAA) forms prior to data collection.
TABLE 1.
Subject Description and Timeline
| Subject | Age, y | Gender | Injury/Surgery | Time: Start NWB to Baseline MRI, d* | Time: Baseline MRI to Post-NWB MRI, d | Time: Post-NWB MRI to Post-FWB MRI, d |
|---|---|---|---|---|---|---|
| 1 | 37 | Male | Achilles rupture repair | −1 | 49 | 77 |
| 2 | 46 | Male | Peroneal tendon repair | −1 | 41 | 39 |
| 3 | 27 | Female | Tarsometatarsal arthrodesis | −2 | 42 | 36 |
| 4 | 38 | Female | Bunionectomy | −1 | 48 | 38 |
| 5 | 20 | Male | Tibiofemoral fracture | 5 | 45 | 48 |
| 6 | 43 | Male | Achilles rupture repair | −1 | 49 | 83 |
| 7 | 24 | Female | Fibula fracture | 5 | 35 | 37 |
| 8 | 50 | Female | Tarsometatarsal arthrodesis | −2 | 48 | 56 |
| 9 | 22 | Female | Lapidus procedure | −1 | 43 | 42 |
| 10 | 39 | Female | Bunionectomy | −2 | 46 | 43 |
Abbreviations: FWB, full weight bearing; MRI, magnetic resonance imaging; NWB, non–weight bearing.
Negative days indicate that baseline MRI preceded the start of NWB phase.
Magnetic Resonance Imaging
All imaging was performed at the University of California, San Francisco, Department of Radiology and Biomedical Imaging MRI facilities using a Signa HDxt 3.0T MRI scanner (GE Healthcare, Waukesha, WI) and an 8-channel phased-array knee coil (Invivo, Gainesville, FL). Sagittal high-resolution spoiled-gradient-recalled (SPGR) images (repetition time [TR], 22 milliseconds; echo time [TE], 7.0 milliseconds; flip angle, 18°; field of view, 14 cm; matrix, 512 × 512; slice thickness, 1.5 mm), sagittal T1rho maps (TR, 9.3 milliseconds; TE, 3.7 milliseconds; field of view, 14 cm; matrix, 256 × 128; slice thickness, 3 mm; time of spin lock, 0, 10, 40, and 80 milliseconds; frequency of spin lock, 500 Hz), and sagittal T2 maps (parameters were identical to T1rho parameters, with the following exceptions: TR, 11.5 milliseconds; TE, 2.8, 13.2, 23.7, and 44.5 milliseconds) were acquired using previously published protocols.34,41
Procedures
All subjects underwent imaging of the ipsilateral knee joint at 3 subject-specific time points: (1) prior to surgery (baseline), (2) immediately prior to discontinuing crutches, as determined by the referring physicians (post-NWB), and (3) after a minimum of 4 weeks of FWB, defined as elimination of all ambulatory assistive devices used at any time (post-FWB). For surgical conditions that were preplanned (peroneal tendon fixation, tarsal arthrodesis, etc), the subject’s baseline MRI was performed prior to the surgery, within 1 week of the surgery date. For subjects with traumatic injury conditions (tibial and/or fibular fractures, etc), the subject’s baseline MRI was acquired within 5 days of the injurious event, and prior to surgical intervention. The 5-day threshold was selected because it is unlikely that such a brief period would result in compositional changes within the articular cartilage but would allow subjects to arrange transportation to the MRI facilities. All subjects were contacted between 3 and 4 weeks following their baseline MRI to schedule a post-NWB MRI. By this time, most subjects had returned to the referring physician and the date of their crutch discharge could be determined. The post-NWB MRI acquisition was scheduled as close as possible to the date of crutch discharge, to allow for the maximum effects of unloading to be observed. For the post-FWB time point, subjects were contacted 2 to 3 weeks after the acquisition of the post-NWB MRI. At this time, they were interviewed to evaluate their weight-bearing status during the preceding weeks. If it was determined that the subject had been FWB since the post-NWB MRI acquisition, a final MRI scan was scheduled as close as possible to 4 weeks after crutch discharge. However, some conditions resulted in substantial partial–weight-bearing phases (eg, Achilles rupture repairs). In these cases, subjects were contacted every 1 to 2 weeks to evaluate weight-bearing status and the final MRI was scheduled as close as possible to 4 weeks following FWB. Specific time details for each subject are listed in TABLE 1.
For MRI procedures, all subjects arrived at the MRI center and were seated in a wheelchair for 30 minutes prior to data acquisition. Subjects were scanned in the supine position with the knee joint ipsilateral to the distal lower extremity injury in an MRI knee coil and positioned in 10° to 15° of flexion and neutral rotation. Following a 3-D localizer scan and a calibration scan, high-resolution SPGR images were acquired for cartilage segmentation. Next, T1rho and T2 relaxation-time mapping sequences were acquired using the identical prescription used to acquire SPGR images. Total scan time was approximately 40 minutes.
Data Analysis
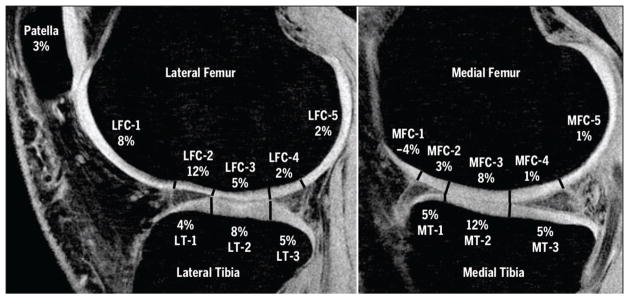
SPGR images were rigidly registered to the T1rho relaxation-time map using the Visualization Toolkit registration software (Kitware, Inc, Clifton Park, NY).7 A blinded investigator (T.B.), using a semi-automatic process (automatic edge detection and manual correction) custom-developed in MATLAB software (The MathWorks, Inc, Natick, MA), segmented 5 compartments: medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibia (MT), lateral tibia (LT), and patella (PAT). Next, the femoral condyles and tibiae were further divided into regions, based on load-bearing relationships (subcompartmental analysis), and into 2 equal layers (laminar analysis) for regional analysis. Subcompartments were defined with regard to meniscus position, as reported in the literature and detailed in FIGURE 1.5 Laminar analysis consisted of dividing each cartilage plate into 2 equally spaced layers (superficial and deep), as has been previously described.12
FIGURE 1.
Subcompartment analysis of T1rho change scores between baseline and post-NWB time point. Values indicate mean change in T1rho value as a percentage of baseline relaxation time. Abbreviations: LFC, lateral femoral condyle; LT, lateral tibia; MFC, medial femoral condyle; MT, medial tibia; NWB, non–weight bearing.
The T1rho and T2 maps were reconstructed voxel by voxel, by fitting T1rho- and T2-weighted images to the following standardized equation: , where S is the image signal at a given time point (TSL for T1rho maps or TE for T2 maps).
Segmented cartilage contours were overlaid on T1rho and T2 maps for relaxation-time quantification. Careful correction of segmented regions of interest was performed on the relaxation-time map to eliminate any abnormally high relaxation values (defined as T1rho values greater than 150 milliseconds and T2 values greater than 120 milliseconds), which might indicate inclusion of synovial fluid. The average T1rho and T2 values in each cartilage plate were recorded as a primary variable of interest. Secondary variables of interest included T1rho and T2 relaxation-time values for the subcompartmental and laminar analyses.
Reproducibility
Previous studies have examined the reproducibility of these quantitative MRI measures of cartilage composition. Specifically, Li and colleagues20 reported an overall T1rho coefficient of variation value of 1.6%, with compartmental and sub-compartmental variation ranging from 3.0% to 6.4% in regions similar to those analyzed in the current study. In a similar study, Pai and colleagues29 reported T2 reproducibility across compartments to be approximately 4.2% using a T2-mapping pulse sequence similar to that used in the current study.
Descriptive Analysis
Differences in primary variables of interest across time points were explored using descriptive statistics. All differences are expressed in average or percentage change in dependent variables. For percentage change scores, these were calculated as the average of each individual pairwise percentage change. Note that this calculation results in a different value from that determined by the percentage change from the group average values.
OUTCOMES
Compartmental Analysis
Baseline Versus Post-NWB
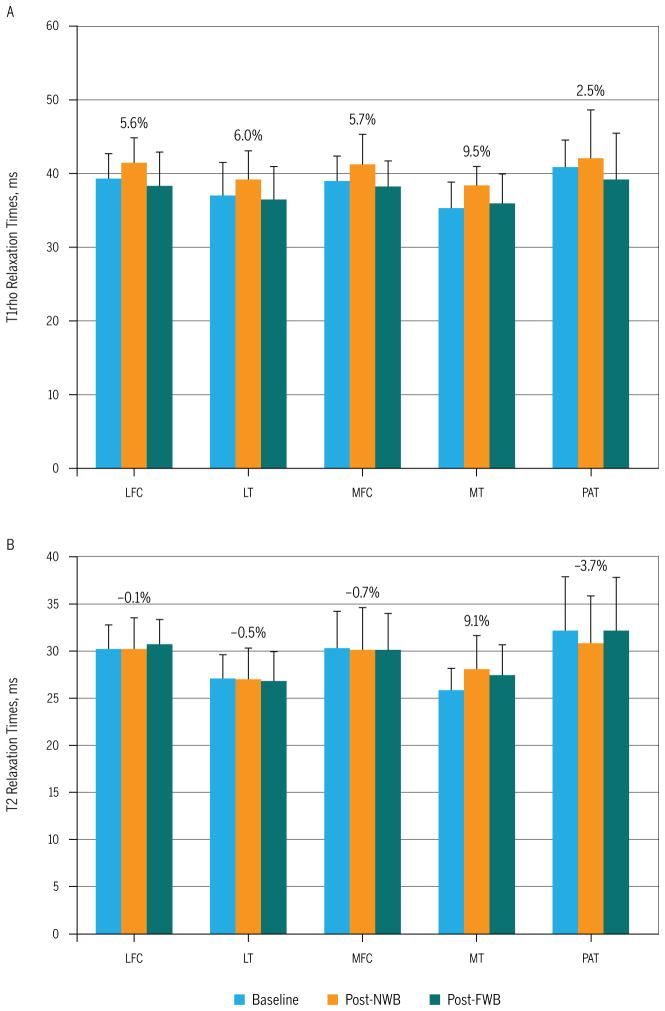
When compared to baseline values, average T1rho relaxation times following a period of NWB were notably higher in the LFC (mean percentage change ± SD, 5.6% ± 8.4%; range, −1.6% to 25.0%), LT (6.0% ± 7.0%; range, −3.0% to 17.4%), MFC (5.7% ± 5.7%; range, −2.0% to 18.0%), and MT (9.5% ± 7.7%; range, −3.1% to 25.2%). Changes were less remarkable in the PAT and showed substantial variability (2.5% ± 9.0%; range, −11.2% to 14.2%) (FIGURE 2A). There were minimal differences in average T2 relaxation times between baseline and post-NWB measures in the LFC (−0.1% ± 4.1%; range, −8.4% to 6.4%), LT (−0.5% ± 5.3%; range, −8.7% to 8.3%), and MFC (−0.7% ± 3.2%; range, −5.1% to 4.0%). However, we observed higher T2 values in the MT (9.1% ± 13.0%; range, −15.4% to 30.4%), slightly lower values in the PAT (−3.7% ± 5.2%; range, −12.5% to 1.7%), and substantial variability in changes among subjects was noted in all compartments (FIGURE 2B).
FIGURE 2.
(A) T1rho and (B) T2 relaxation times of cartilage at baseline, following approximately 6 weeks of non–weight bearing (post-NWB), and after 4 weeks of returning to full weight bearing (post-FWB). The values above each set of bars indicate the percentage change score between baseline and post-NWB. Abbreviations: FWB, full weight bearing; LFC, lateral femoral condyle; LT, lateral tibia; MFC, medial femoral condyle; MT, medial tibia; NWB, non–weight bearing; PAT, patella.
Baseline Versus Post-FWB
When compared to baseline values, average T1rho relaxation times following 4 weeks of FWB were similar for all cartilage plates except PAT (LFC mean ± SD percentage change [range], −2.2% ± 11.8% [−23.7% to 20.0%]; LT, −1.3% ± 7.6% [−18.8% to 8.5%]; MFC, −1.9% ± 4.1% [−6.7% to 6.8%]; MT, 2.3% ± 12.4% [−22.8% to 22.7%]; and PAT, −4.5% ± 8.9% [−18.6% to 7.1%]). Additionally, there were minimal differences in T2 relaxation times when comparing baseline values to post-FWB values in the LFC (1.8% ± 4.1% [−6.8% to 7.4%]), LT (−0.8% ± 6.9% [−12.1% to 9.2%]), MFC (−0.4% ± 5.1% [−5.6% to 8.6%]), and PAT (−0.3% ± 8.7% [−11.8% to 22.3%]). However, T2 values in the MT, although less than those following the NWB period, remained above baseline values (percentage change, 6.3% ± 7.9% [−7.2% to 19.6%]).
Laminar Analysis
When evaluating T1rho relaxation-time change scores from baseline to post-NWB, the deep layer showed, on average, larger percentage changes than those of the superficial layer (7.4% versus 4.6%) (TABLE 2). Greater changes in the deep layer compared to the superficial layer were measured in all compartments, except the MT, which demonstrated large changes in both the deep (9.6%) and superficial (10.0%) layers.
TABLE 2.
Relaxation-Time Change Scores From Laminar Analysis
| T1rho Relaxation Times
|
T2 Relaxation Times
|
|||
|---|---|---|---|---|
| Superficial Layer | Deep Layer | Superficial Layer | Deep Layer | |
| LFC | ||||
| Baseline, ms | 44.9 | 33.6 | 32.7 | 27.7 |
| Post-NWB, ms | 46.3 | 36.5 | 32.6 | 27.8 |
| Change, %* | 3.3 | 8.9 | −0.2 | 0.4 |
| LT | ||||
| Baseline, ms | 42.6 | 31.6 | 30.3 | 23.9 |
| Post-NWB, ms | 44.4 | 34.2 | 29.1 | 25.0 |
| Change, %* | 4.2 | 9.1 | −4.1 | 4.8 |
| MFC | ||||
| Baseline, ms | 44.8 | 33.0 | 32.0 | 28.5 |
| Post-NWB, ms | 46.6 | 35.8 | 31.7 | 28.5 |
| Change, %* | 3.7 | 8.9 | −1.1 | −0.1 |
| MT | ||||
| Baseline, ms | 41.2 | 30.0 | 29.1 | 22.9 |
| Post-NWB, ms | 45.2 | 32.4 | 31.5 | 25.0 |
| Change, %* | 10.0 | 9.6 | 8.8 | 9.5 |
| PAT | ||||
| Baseline, ms | 47.0 | 34.6 | 36.2 | 28.1 |
| Post-NWB, ms | 48.1 | 35.7 | 34.7 | 27.0 |
| Change, %* | 1.9 | 3.5 | −3.7 | −3.2 |
Abbreviations: LFC, lateral femoral condyle; LT, lateral tibia; MFC, medial femoral condyle; MT, medial tibia; NWB, non–weight bearing; PAT, patella.
Percentages listed are calculated as the mean of the individual percentage change scores on a pairwise basis.
Laminar analysis of T2 relaxation times revealed slightly higher change scores between baseline and post-NWB values in the deep layer compared to the superficial layer, but the laminar changes in individual compartments varied greatly (TABLE 2). The MT was the only compartment that showed change scores greater than 5% and had similar values in the deep and superficial layers (9.5% and 8.8%, respectively).
Subcompartmental Analysis
The highest T1rho change scores between baseline and post-NWB were observed in the LFC-2, MT-2, MFC-3, and LT-2 (FIGURE 1, TABLES 3 and 4). Similarly, the largest change scores for T2 relaxation times were observed in the LFC-2, LT-2, and MT-2. All other subcompartments had T2 changes less than 5% (TABLES 3 and 4).
TABLE 3.
Relaxation-Time Change Scores From Femoral Subcompartments
| T1rho Relaxation Times | T2 Relaxation Times | |
|---|---|---|
| LFC-1 | ||
| Baseline, ms | 42.5 | 33.3 |
| Post-NWB, ms | 45.2 | 33.7 |
| Change, %* | 7.6 | 1.5 |
| LFC-2 | ||
| Baseline, ms | 36.5 | 25.1 |
| Post-NWB, ms | 40.7 | 26.4 |
| Change, %* | 12.2 | 6.7 |
| LFC-3 | ||
| Baseline, ms | 40.8 | 29.2 |
| Post-NWB, ms | 42.9 | 28.5 |
| Change, %* | 5.5 | −2.6 |
| LFC-4 | ||
| Baseline, ms | 40.7 | 31.3 |
| Post-NWB, ms | 41.3 | 30.0 |
| Change, %* | 1.8 | −3.4 |
| LFC-5 | ||
| Baseline, ms | 38.5 | 30.4 |
| Post-NWB, ms | 39.3 | 28.7 |
| Change, %* | 2.3 | −4.9 |
| MFC-1 | ||
| Baseline, ms | 41.4 | 32.0 |
| Post-NWB, ms | 39.4 | 30.4 |
| Change, %* | −4.1 | −4.6 |
| MFC-2 | ||
| Baseline, ms | 37.2 | 27.3 |
| Post-NWB, ms | 38.2 | 27.0 |
| Change, %* | 3.1 | −0.6 |
| MFC-3 | ||
| Baseline, ms | 39.6 | 30.3 |
| Post-NWB, ms | 42.7 | 29.9 |
| Change, %* | 8.2 | −1.2 |
| MFC-4 | ||
| Baseline, ms | 39.5 | 29.7 |
| Post-NWB, ms | 39.8 | 29.6 |
| Change, %* | 0.6 | −0.4 |
| MFC-5 | ||
| Baseline, ms | 40.4 | 31.6 |
| Post-NWB, ms | 40.9 | 31.4 |
| Change, %* | 1.3 | −0.9 |
Abbreviations: LFC, lateral femoral condyle; MFC, medial femoral condyle; NWB, non–weight bearing.
Percentages listed are calculated as the mean of the individual percentage change scores on a pairwise basis.
TABLE 4.
Relaxation-Time Change Scores From Tibial Subcompartments
| T1rho Relaxation Times | T2 Relaxation Times | |
|---|---|---|
| LT-1 | ||
| Baseline, ms | 38.0 | 26.1 |
| Post-NWB, ms | 39.2 | 25.1 |
| Change, %* | 3.8 | −4.1 |
| LT-2 | ||
| Baseline, ms | 34.9 | 25.6 |
| Post-NWB, ms | 37.3 | 27.1 |
| Change, %* | 8.0 | 6.3 |
| LT-3 | ||
| Baseline, ms | 39.9 | 28.8 |
| Post-NWB, ms | 41.7 | 30.0 |
| Change, %* | 4.8 | 4.0 |
| MT-1 | ||
| Baseline, ms | 34.8 | 24.2 |
| Post-NWB, ms | 36.6 | 25.3 |
| Change, %* | 5.2 | 4.5 |
| MT-2 | ||
| Baseline, ms | 35.7 | 27.5 |
| Post-NWB, ms | 39.7 | 33.2 |
| Change, %* | 11.9 | 20.9 |
| MT-3 | ||
| Baseline, ms | 36.7 | 25.9 |
| Post-NWB, ms | 38.3 | 26.6 |
| Change, %* | 4.6 | 2.9 |
Abbreviations: LT, lateral tibia; MT, medial tibia; NWB, non–weight bearing.
Percentages listed are calculated as the mean of the individual percentage change scores on a pairwise basis.
DISCUSSION
This study evaluated cartilage relaxation-time parameters before and after a period of NWB, and again after a period of FWB. The results show that T1rho relaxation times of all load-bearing cartilage plates were greater following NWB, suggesting a decrease in proteoglycan content. With the exception of the MT, which showed increased T2 values following NWB (suggesting disorganization of the collagen network), all other cartilage plates had fairly unchanged T2 relaxation times. Following the NWB period, nearly all T1rho and T2 times had returned to values similar to those at baseline levels within 4 weeks of FWB, with the most notable exception being T2 of the MT (FIGURES 2 and 3). These findings suggest that there may be a window of time in which articular cartilage of a lower extremity undergoing weight-bearing restrictions experiences compositional changes that subsequently place it at risk for injury.
FIGURE 3.
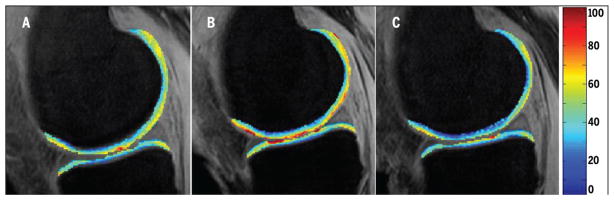
Representative T1rho color map of the medial femoral condyle and medial tibia (A) before non–weight bearing, (B) after non–weight bearing, and (C) after returning to full weight bearing. The unit for the color scale is milliseconds.
As hypothesized, we observed increases in T1rho relaxation times in articular cartilage following a 6-week period of NWB. Interestingly, these findings were most notable in the weight-bearing cartilage surfaces. The patella, which is relatively unloaded during quiet standing, showed the smallest differences in T1rho relaxation times following NWB. This suggests that cartilage that habitually experiences loading during weight-bearing activities may respond differently to episodes of unloading, when compared to cartilage that is not a primary load-bearing surface. Furthermore, the findings from our subcompartmental analysis support this premise, as the areas of primary load bearing (LFC-2 to LFC-4, MFC-2 to MFC-4, LT-2, and MT-2) showed the greatest average change scores after the period of NWB. These findings are consistent with a framework of OA development after injury to anterior cruciate ligament, as proposed by Andriacchi and colleagues,3 in which the initiating element of cartilage degeneration is a shift of weight-bearing loads on the articular surface of the cartilage plate from one region to another. Our findings support this framework, in that primary load-bearing regions that go unloaded appear to experience compositional changes that are similar to quantitative MRI findings associated with early OA.
The increase in average T1rho relaxation times following NWB may reflect a decrease in proteoglycan content, as previous studies have shown a very close relationship between these 2 variables (R2 = 0.986).23 These findings are consistent with the literature that has reported 30% and 50% reductions in proteoglycan content and synthesis following periods of experimental NWB in small animals.4,11 Furthermore, these works reveal evidence of reduced biomechanical properties of articular cartilage, whereby reductions of 17% to 75% in elastic and equilibrium shear modulus have been reported following similar periods of NWB.10,15,17 This is consistent with the data published by Tang and colleagues,36 who found a significant relationship (R2 = 0.908) between cartilage T1rho relaxation times and the viscoelastic properties of articular cartilage measured ex vivo. However, it should be noted that it is unknown how a 5% to 10% increase in T1rho relaxation times may affect cartilage biomechanics. Clearly, further work is needed to elucidate the clinical implication of these findings.
When comparing our relaxation-time data to those reported in the literature on persons with OA, it is important to compare data acquired with similar pulse sequences and scanners. A previous data set on persons with OA acquired by Bolbos and colleagues,6 using a nearly identical pulse sequence and the same MRI scanner as that used in the current investigation, found that average T1rho relaxation times of persons with OA were approximately 43 milliseconds for LFC, 38 milliseconds for LT, 45 milliseconds for MFC, and 36 milliseconds for MT (outcomes for PAT were not reported). Interestingly, for some compartments (LT and MT), these values are very similar to those observed in the current investigation after a period of NWB (LFC, 41.4 milliseconds; LT, 39.1 milliseconds; MFC, 41.2 milliseconds; and MT, 38.4 milliseconds). However, it is important to note that substantial variability was observed in both cohorts, and no threshold of T1rho relaxation times has been identified as being a criterion for diagnostic purposes.
In contrast to our T1rho results, we observed minimal differences in T2 relaxation times following NWB in most cartilage plates. However, the MT, the cartilage plate that showed the largest T1rho relaxation change score, showed increased values compared to baseline values, and these values remained elevated after the period of FWB. These findings, along with the T1rho findings, suggest that collagen composition is less likely to be influenced by periods of unloading than proteoglycan content is, but that, when changes do occur, they are more permanent. It is difficult to compare our results to those reported in the literature, as there is a paucity of data regarding the influence of NWB on relaxation times of cartilage. Several investigators have shown that increased loading results in changes in T2 times. For example, it has been reported that static loading of cartilage results in immediate decreases in T2 relaxation times.26,27,34 Furthermore, it has been shown that increased dynamic loads, such as performing bouts of running, result in longer-term increases in cartilage T2 times.21,24 Finally, our data are consistent with an unloading study in canines by Muller and colleagues,25 which reported that after 4 weeks of NWB there was a decrease in proteoglycan content but no change in collagen content. However, no human studies on the response of cartilage T2 relaxation during long-term unloading have been performed.
Despite remarkable changes in T1rho relaxation times following NWB, all compartments had values that returned to baseline levels following 4 weeks of FWB. This has important clinical implications because it implies that periods of NWB may result in altered cartilage composition of the limb being unloaded, but that normal loading rapidly reverses these alterations. Nonetheless, there appears to be a period immediately following NWB in which the cartilage may be at risk for injury, given its decreased proteoglycan content and potentially reduced ability to resist loads. Further work is needed to evaluate this very sensitive period to determine the time frame of altered cartilage composition. Interestingly, the only region that was observed to have higher T2 values following NWB remained elevated after a period of FWB. This suggests that collagen disorganization occurring from NWB may be a slower process or, potentially, a permanent change. Longer follow-up is needed to address this uncertainty. Conversely, the PAT showed slightly lower values following NWB, which may reflect an increase in collagen organization or a decrease in tissue hydration, and returned to baseline levels following FWB. The PAT is the only cartilage plate evaluated that is not loaded in quiet standing, and the differing response of this plate to unloading underscores the biochemical dependence of cartilage on mechanical loading.
This study evaluated the regional dependence on changes in cartilage relaxation times with unloading. The deep layer appears to be more sensitive to changes associated with unloading, particularly with respect to T1rho relaxation times. With the exception of the MT, which showed substantial changes in both layers, all other cartilage surfaces had deep-layer change values of approximately twice those of their superficial layer. The laminar analysis of the T2 data is more variable and the findings are less clear. In the MT, the changes in T2 between the deep and superficial layers were large and consistent. However, layers within other cartilage plates showed differing patterns of T2 changes, with magnitudes in the reproducibility error range.
With regard to subcompartments, the current data reveal the largest changes following NWB within the weight-bearing regions. For both T1rho and T2 changes, the largest percentages were noted in the cartilage-on-cartilage surfaces (MT-2, LT-2, and MFC-3) (FIGURE 1) and the meniscal horn–covered, load-bearing surfaces (LFC-2), indicating that the habitual loading associated with these areas may play a role in the compositional changes observed with unloading. One surprising result observed was that MFC-1 showed an opposite trend when compared to the rest of the MFC subcompartments. However, it must be noted that due to the anatomy of the anterior MFC, MFC-1 is a very small subcompartment that at times may consist of only a few pixels. Therefore, these results might have been influenced by undersampling in this region and should be interpreted in light of this limitation.
There are several important clinical implications of this research. First, these findings should inform the clinician of the potential for injury following periods of NWB. In as little as 6 weeks, we observed deficits in proteoglycan content in young asymptomatic joints. This needs to be considered when implementing rehabilitation strategies following prolonged NWB, with particular attention not only to the joint involved, but also to the other load-bearing joints that experienced unloading. Although the 2 subjects with Achilles rupture repairs underwent prolonged partial–weight-bearing phases, leading to a much longer time between post-NWB and post-FWB MRIs (TABLE 1), many other subjects went from NWB to FWB immediately on surgeon approval. These data suggest that a transition period may be warranted in these individuals.
A second, albeit very small, population for which these findings have significant implications is those who perform space travel. Clearly, these individuals are at risk for cartilage compositional changes if countermeasures are not implemented. Currently, focused-task missions range from 1 to 2 weeks, and longer missions to the International Space Station may last 6 months or more. These cartilage compositional changes need to be considered when evaluating the health of these individuals. Additionally, with travel to Mars becoming a real goal of NASA, travel times would take approximately 6 months, with total missions lasting 18 months to 3 years. Cartilage-specific countermeasures may be necessary to prevent catastrophic compositional changes.
The data from this study must be viewed in the light of their respective reproducibility and inherent errors. As noted previously, the coefficient of variation for T1rho relaxation times has been reported to be between 1.6% and 6.4%.20 The reproducibility for T2 relaxation times has been reported to be approximately 4.2% across all compartments.29 In the current study, many of the T2 change scores and several of the superficial T1rho change scores are within this error range and should be evaluated carefully, keeping in mind these limitations. However, we observed several compartments and subcompartments with change scores of 8% to 12%, particularly in the MT, deep laminar layer, and load-bearing subcompartments of the cartilage. As such, these changes are probably not the result of reproducibility error and likely reflect true compositional changes occurring in response to unloading.
Several potential limitations need to be noted for the current study. First, the influence of lower-leg/ankle injury in the current model of unloading has not been thoroughly evaluated. The T1rho and T2 relaxation times in participants who might have been taking pain medications immediately following their injury might have been influenced. However, given the previous literature on the determinants of T1rho and T2 imaging, this would not be expected to influence the findings. Second, 2 of the subjects in the current study had been NWB for several days prior to the baseline scan, due to an unexpected traumatic event (TABLE 1). It is unclear if changes in composition from unloading could have manifested in this short a time, which might have resulted in larger differences between baseline and post-NWB follow-up. Finally, it is unclear how a 12% increase in relaxation times might have affected cartilage biomechanical properties and influenced injury risk potential. Further studies are needed during this post-NWB period to evaluate the potential for secondary injury.
CONCLUSION
We observed substantial fluctuations in cartilage composition following a period of NWB. These changes appear to have been mostly related to proteoglycan content and more localized to the load-bearing surfaces and deep layer of the cartilage. However, following 4 weeks of FWB, relaxation times of nearly all cartilage plates had returned to baseline levels, suggesting an important reversibility in compositional fluctuations. These findings suggest that there may be a window of time in which articular cartilage of a lower extremity that has undergone weight-bearing restrictions experiences compositional changes that place it at risk for injury upon return to FWB status.
Acknowledgments
This work was supported by the National Space Biomedical Research Institute through NASA NCC 9-58 and the National Institutes of Health through NIH AG17762. This study was approved by the Committee on Human Research of the University of California, San Francisco.
References
- 1.Aigner T, Sachse A, Gebhard PM, Roach HI. Osteoarthritis: pathobiology–targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58:128–149. doi: 10.1016/j.addr.2006.01.020. http://dx.doi.org/10.1016/j.addr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46:419–423. doi: 10.1002/mrm.1208. http://dx.doi.org/10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 4.Behrens F, Kraft EL, Oegema TR., Jr Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7:335–343. doi: 10.1002/jor.1100070305. http://dx.doi.org/10.1002/jor.1100070305. [DOI] [PubMed] [Google Scholar]
- 5.Bolbos RI, Link TM, Ma CB, Majumdar S, Li X. T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage. 2009;17:12–18. doi: 10.1016/j.joca.2008.05.016. http://dx.doi.org/10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolbos RI, Zuo J, Banerjee S, et al. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 2008;16:1150–1159. doi: 10.1016/j.joca.2008.02.018. http://dx.doi.org/10.1016/j.joca.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carballido-Gamio J, Bauer JS, Stahl R, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. http://dx.doi.org/10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22:673–682. doi: 10.1016/j.mri.2004.01.071. http://dx.doi.org/10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 9.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. http://dx.doi.org/10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haapala J, Arokoski J, Pirttimaki J, et al. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int J Sports Med. 2000;21:76–81. doi: 10.1055/s-2000-8860. http://dx.doi.org/10.1055/s-2000-8860. [DOI] [PubMed] [Google Scholar]
- 11.Haapala J, Arokoski JP, Hyttinen MM, et al. Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999:218–229. [PubMed] [Google Scholar]
- 12.Holtzman DJ, Theologis AA, Carballido-Gamio J, Majumdar S, Li X, Benjamin C. T(1rho) and T(2) quantitative magnetic resonance imaging analysis of cartilage regeneration following microfracture and mosaicplasty cartilage resurfacing procedures. J Magn Reson Imaging. 2010;32:914–923. doi: 10.1002/jmri.22300. http://dx.doi.org/10.1002/jmri.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001:S14–S25. [PubMed] [Google Scholar]
- 14.Jortikka MO, Inkinen RI, Tammi MI, et al. Immobilisation causes longlasting matrix changes both in the immobilised and contralateral joint cartilage. Ann Rheum Dis. 1997;56:255–261. doi: 10.1136/ard.56.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurvelin J, Kiviranta I, Tammi M, Helminen JH. Softening of canine articular cartilage after immobilization of the knee joint. Clin Orthop Relat Res. 1986:246–252. [PubMed] [Google Scholar]
- 16.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. http://dx.doi.org/10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Leroux MA, Cheung HS, Bau JL, Wang JY, Howell DS, Setton LA. Altered mechanics and histomorphometry of canine tibial cartilage following joint immobilization. Osteoarthritis Cartilage. 2001;9:633–640. doi: 10.1053/joca.2001.0432. http://dx.doi.org/10.1053/joca.2001.0432. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. http://dx.doi.org/10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Cheng J, Lin K, et al. Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging. 2011;29:324–334. doi: 10.1016/j.mri.2010.09.004. http://dx.doi.org/10.1016/j.mri.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008;59:298–307. doi: 10.1002/mrm.21414. http://dx.doi.org/10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke AC, Stehling C, Stahl R, et al. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38:2273–2280. doi: 10.1177/0363546510372799. http://dx.doi.org/10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 22.Mlynarik V, Szomolanyi P, Toffanin R, Vittur F, Trattnig S. Transverse relaxation mechanisms in articular cartilage. J Magn Reson. 2004;169:300–307. doi: 10.1016/j.jmr.2004.05.003. http://dx.doi.org/10.1016/j.jmr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. http://dx.doi.org/10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 24.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. http://dx.doi.org/10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller FJ, Setton LA, Manicourt DH, Mow VC, Howell DS, Pita JC. Centrifugal and biochemical comparison of proteoglycan aggregates from articular cartilage in experimental joint disuse and joint instability. J Orthop Res. 1994;12:498–508. doi: 10.1002/jor.1100120406. http://dx.doi.org/10.1002/jor.1100120406. [DOI] [PubMed] [Google Scholar]
- 26.Nag D, Liney GP, Gillespie P, Sherman KP. Quantification of T(2) relaxation changes in articular cartilage with in situ mechanical loading of the knee. J Magn Reson Imaging. 2004;19:317–322. doi: 10.1002/jmri.20000. http://dx.doi.org/10.1002/jmri.20000. [DOI] [PubMed] [Google Scholar]
- 27.Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in knee cartilage T2 in response to mechanical loading. J Magn Reson Imaging. 2008;28:175–180. doi: 10.1002/jmri.21418. http://dx.doi.org/10.1002/jmri.21418. [DOI] [PubMed] [Google Scholar]
- 28.Nissi MJ, Toyras J, Laasanen MS, et al. Proteoglycan and collagen sensitive MRI evaluation of normal and degenerated articular cartilage. J Orthop Res. 2004;22:557–564. doi: 10.1016/j.orthres.2003.09.008. http://dx.doi.org/10.1016/j.orthres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Pai A, Li X, Majumdar S. A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging. 2008;26:1215–1220. doi: 10.1016/j.mri.2008.02.017. http://dx.doi.org/10.1016/j.mri.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. http://dx.doi.org/10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 31.Sauerland K, Raiss RX, Steinmeyer J. Proteoglycan metabolism and viability of articular cartilage explants as modulated by the frequency of intermittent loading. Osteoarthritis Cartilage. 2003;11:343–350. doi: 10.1016/s1063-4584(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 32.Sauerland K, Steinmeyer J. Intermittent mechanical loading of articular cartilage explants modulates chondroitin sulfate fine structure. Osteoarthritis Cartilage. 2007;15:1403–1409. doi: 10.1016/j.joca.2007.05.004. http://dx.doi.org/10.1016/j.joca.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage. 1997;5:1–16. doi: 10.1016/s1063-4584(97)80027-1. [DOI] [PubMed] [Google Scholar]
- 34.Souza RB, Stehling C, Wyman BT, et al. The effects of acute loading on T1rho and T2 relaxation times of tibiofemoral articular cartilage. Osteoarthritis Cartilage. 2010;18:1557–1563. doi: 10.1016/j.joca.2010.10.001. http://dx.doi.org/10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Steinmeyer J, Knue S, Raiss RX, Pelzer I. Effects of intermittently applied cyclic loading on proteoglycan metabolism and swelling behaviour of articular cartilage explants. Osteoarthritis Cartilage. 1999;7:155–164. doi: 10.1053/joca.1998.0204. http://dx.doi.org/10.1053/joca.1998.0204. [DOI] [PubMed] [Google Scholar]
- 36.Tang SY, Souza RB, Ries M, Hansma PK, Alliston T, Li X. Local tissue properties of human osteoarthritic cartilage correlate with magnetic resonance T(1) rho relaxation times. J Orthop Res. 2011;29:1312–1319. doi: 10.1002/jor.21381. http://dx.doi.org/10.1002/jor.21381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanwanseele B, Lucchinetti E, Stussi E. The effects of immobilization on the characteristics of articular cartilage: current concepts and future directions. Osteoarthritis Cartilage. 2002;10:408–419. doi: 10.1053/joca.2002.0529. http://dx.doi.org/10.1053/joca.2002.0529. [DOI] [PubMed] [Google Scholar]
- 38.Wheaton AJ, Casey FL, Gougoutas AJ, et al. Correlation of T1rho with fixed charge density in cartilage. J Magn Reson Imaging. 2004;20:519–525. doi: 10.1002/jmri.20148. http://dx.doi.org/10.1002/jmri.20148. [DOI] [PubMed] [Google Scholar]
- 39.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54:1087–1093. doi: 10.1002/mrm.20678. http://dx.doi.org/10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 40.Xia Y, Moody JB, Alhadlaq H. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med. 2002;48:460–469. doi: 10.1002/mrm.10216. http://dx.doi.org/10.1002/mrm.10216. [DOI] [PubMed] [Google Scholar]
- 41.Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–1416. doi: 10.1016/j.joca.2010.07.012. http://dx.doi.org/10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng S, Xia Y, Badar F. Further studies on the anisotropic distribution of collagen in articular cartilage by muMRI. Magn Reson Med. 2011;65:656–663. doi: 10.1002/mrm.22648. http://dx.doi.org/10.1002/mrm.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]