Abstract
Purpose
Virtual reality (VR) during chemotherapy has resulted in an elapsed time compression effect, validating the attention diversion capabilities of VR. Using the framework of the pacemaker–accumulator cognitive model of time perception, this study explored the influence of age, gender, state anxiety, fatigue, and cancer diagnosis in predicting the difference between actual time elapsed during receipt of intravenous chemotherapy while immersed in a VR environment versus patient’s retrospective estimates of time elapsed during this treatment.
Materials and methods
This secondary analysis from three studies yielded a pooled sample of N=137 participants with breast, lung, or colon cancer. Each study employed a crossover design requiring two matched intravenous chemotherapy treatments, with participants randomly assigned to receive VR during one treatment. Regressions modeled the effect of demographic variables, diagnosis, and Piper Fatigue Scale and State Anxiety Inventory scores on the difference between actual and estimated time elapsed during chemotherapy with VR.
Results
In a forward regression model, three predictors (diagnosis, gender, and anxiety) explained a significant portion of the variability for altered time perception (F=5.06, p=0.0008). Diagnosis was the strongest predictor; individuals with breast and colon cancer perceived time passed more quickly.
Conclusions
VR is a noninvasive intervention that can make chemotherapy treatments more tolerable. Women with breast cancer are more likely and lung cancer patients less likely to experience altered time perception during VR (a possible indicator of effectiveness for this distraction intervention). Understanding factors that predict responses to interventions can help clinicians tailor coping strategies to meet each patient’s needs.
Keywords: Virtual reality, Time perception, Distraction, Chemotherapy, Cancer
Introduction
A major factor in the improvement of US 5-year cancer survival rates over the past three decades has been the development of increasingly effective cancer treatments, most of which now involve chemotherapy [11]. Chemotherapy has significantly extended life in patients with many forms of cancer, in some cases effecting cures, but these outcomes have been achieved at the cost of heavy collateral damage to patients from adverse side effects and impaired quality of life [7, 11, 38]. Despite considerable improvement in specificity and side effect profiles of chemotherapeutic regimens, prevalence of adverse treatment-related symptoms remains high [2, 8, 19, 26, 38].
Accordingly, although the expectations of patients vary, many experience considerable pretreatment anxiety [7, 16] and approach the first IV chemotherapy infusion cycle anticipating unpleasantness both during and after treatment [1, 2, 19, 38]. Anxiety and negative expectations are both significantly associated with worse treatment-related adverse effects, which in turn may trigger the development of conditioned responses to chemotherapy administration (such as anticipatory nausea/vomiting and needle phobia) or initiate vicious cycles of symptom exacerbation after subsequent treatments that diminish quality of life and increase physical and psychosocial distress [8, 9, 19, 26, 29, 46]. High levels of treatment-related distress increase the risk of noncompliance, decreased or delayed dosing, and interruption or discontinuance of chemotherapy [1, 16, 29], which can reduce the likelihood of remission or cure and jeopardize survival [15, 46]. In view of these considerations, early delivery of interventions to improve tolerability of initial chemotherapy sessions is a key to achieving better patient outcomes.
Time perception during chemotherapy: cognitive model
Occupation of treatment time has been identified as an important process-related concern of chemotherapy recipients [40], but patients’ experience of the passage of time during treatment has not been extensively explored. Patients’ perceptions of time can be framed in general terms using the pacemaker–accumulator (PA) model [4, 5, 12, 43]. This cognitive model, a dominant paradigm in time perception research, has been applied to studies of both prospective and retrospective duration estimation for intervals ranging from fractions of a second to many minutes [12, 17].
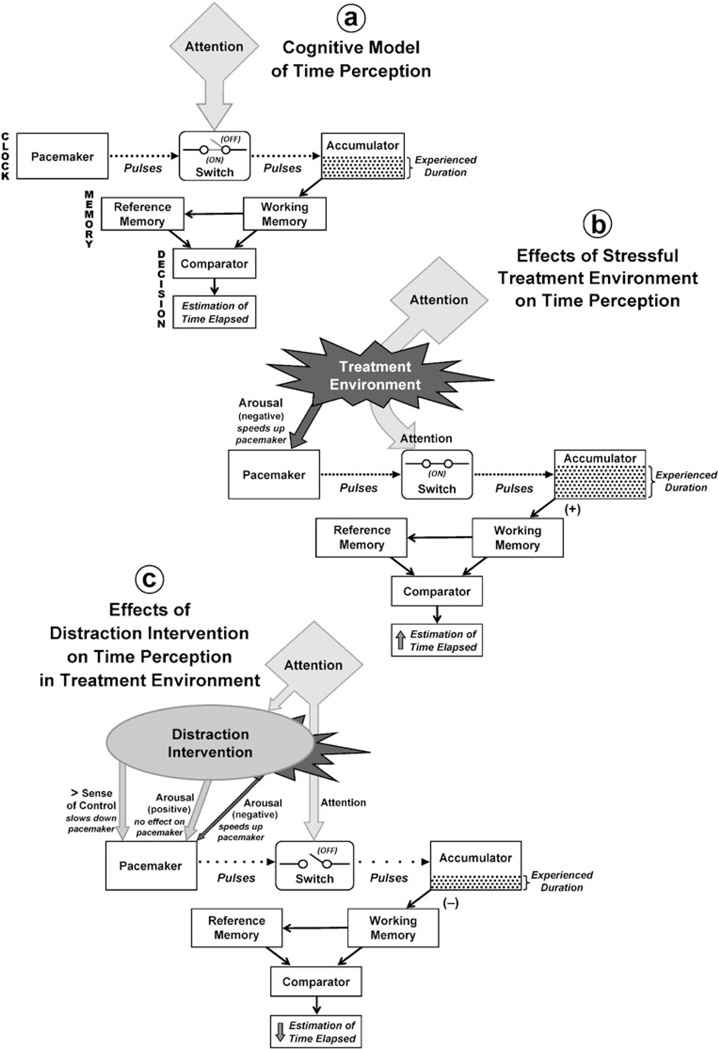
The PA model (Fig. 1a) includes three processing stages: clock, memory, and decision. The clock includes a pacemaker which emits regular pulses (representing discrete “packages” of time) and an accumulator which collects them, connected by an on/off switch controlling pulse transfer. When the interval starts, the switch turns on and pulses pass through to the accumulator. The switch turns off when the interval ends, and the total number of pulses collected in the accumulator represents its experienced duration. The memory stage includes working (short-term) and reference (long-term) memory. Working memory receives the accumulator output and packages it for transfer to reference memory (which adds it to duration reference data compiled from earlier experiences) and to the comparator. The comparator (the locus of the decision stage) compares the duration representation from working memory with significant durations previously stored in reference memory; the outcome of the comparison determines a behavioral response, such as the estimate given by an individual when asked to report the duration of an interval [4, 5, 12, 43].
Fig. 1.
The pacemaker– accumulator (PA ) cognitive model of time perception. a PA model in a neutral environment: Rate of pulse generation in pacemaker is constant and all pulses are collected in the accumulator, resulting in accurate perceptions of experienced duration and estimates of time elapsed. b PA model in a stressful treatment environment: Negative arousal increases rate of pulse generation in the pacemaker and full attention is focused on the passage of time during treatment, resulting in higher values of experienced duration and overestimates of time elapsed. c PA model showing effects of distraction intervention on time perception in a stressful treatment environment: Intervention increases the patient’s sense of control (reducing rate of pulse generation in the pacemaker) and substitutes positive arousal for most of the treatment-associated negative arousal shown in b, resulting in lower values of experienced duration and underestimates of time elapsed. Figures are derived from Wittman and Paulus [43] (p. 8) and Droit-Volet and Gil [12] (p. 1944) and incorporate concepts from Chaston and Kingstone [6], Humphreys and Buehner [20], and Wearden [41]
Figure 1a is an idealized representation of time perception in a neutral environment: The pacemaker generates pulses at a constant base rate, and sufficient attentional resources are allocated to time processing to keep the switch in the “on” position throughout an interval. These conditions permit uninterrupted pulse transfer to the accumulator, so that the total number of pulses collected increases linearly with elapsed time, and the system generates duration estimates that accurately represent reality [5, 43]. However, a patient “watching the drip to see if it’s near empty and watching the clock” [24] (p. 289) is not in an ideal situation; IV chemotherapy does not take place in a neutral environment. The treatment process can present unpleasant stimuli (e.g., pain from venipuncture or accessing a port; anticipatory nausea), and fear, sadness, or anxiety can arise when patients observe their own treatment or that of others [1, 9, 24, 45]. Patients experiencing pain, distress, and anxiety tend to perceive time as passing very slowly [36, 43, 44], and the PA model provides an explanation (Fig. 1b). Aversive stimuli distort time perception by promoting negative physiological arousal, which accelerates the rate of pulse generation in the pacemaker; they also focus patients’ attention on the passage of time during chemotherapy, which keeps the switch between pacemaker and accumulator in the “on” position [5, 12, 43] throughout treatment, allowing all generated pulses to reach the accumulator. The accumulator therefore collects more pulses during a treatment interval than during an equivalent period of time in a neutral environment, so the experienced duration of treatment exceeds its actual duration, yielding an overestimate of total time elapsed.
Distraction interventions that increase tolerability of initial treatment can decrease the salience of negative stimuli associated with chemotherapy [7, 31, 33], counteracting or even reversing distortions of time perception induced by those stimuli (Fig. 1c). Distraction overrides negative stimuli from the treatment environment and generates competing stimuli of positive emotional valence. These positive stimuli, like negative stimuli, can increase arousal; however, arousal by positive stimuli does not accelerate the pacemaker pulse generation rate [12]. Furthermore, distractions that increase patients’ sense of control and allow them to take action during treatment can actually decrease the rate of pulse generation because anticipation of intentional action and its consequences slows down the pacemaker [20].
Distraction interventions divert attention toward enjoyable non-temporal cognitive tasks and away from the passage of time [41]. Attention is a limited cognitive resource; a certain amount of it must be dedicated to time perception to keep the switch between pacemaker and accumulator in the “on” position so that all pulses generated in the pacemaker reach the accumulator [12, 43]. Distractions that divert enough attention from time perception to competing cognitive tasks can flip the switch to the “off” position during those tasks, causing some pulses to be lost [41]. This reduces the number of pulses collected in the accumulator during treatment, shortening its experienced duration [6, 12, 41], and ultimately leads to underestimation of time elapsed during treatment. Magnitude of underestimation is greatest when distractions involve highly complex tasks requiring considerable attentional engagement [6].
Distraction interventions for chemotherapy
Progressive relaxation, guided imagery, and cognitive distractions such as reading, humor, music, and movies have been used with some success to distract patients receiving chemotherapy [32, 45]. These interventions can counteract distortions of time perception that appear to slow the passage of time during chemotherapy by (a) decreasing arousal due to negative stimuli, preventing acceleration of pulse production in the pacemaker, and (b) diverting enough attention away from temporal processing to impair switch function and prevent transfer of some pulses to the accumulator (Fig. 1c). Relaxation and imagery allow patients to exercise some control over the treatment experience and may be able to slow pulse generation in the pacemaker so that time appears to pass more rapidly during treatment. However, these interventions can be effectively deployed only if patients learn and practice the requisite techniques before the first chemotherapy session, and some patients lack the capacity to master these techniques [32]. For all distraction interventions, successful implementation requires patients to consistently focus their attention on the distracter, ignoring input from the treatment environment, throughout a process which typically takes 45–90 min and may require several hours. Unfortunately, few patients can sustain full concentration for such durations [32].
Virtual reality distraction interventions
In this context, virtual reality (VR) is a promising intervention for chemotherapy recipients. VR is an interactive technology providing multimodal sensory input that allows users to participate actively in an immersive computer-generated environment [42]. A head-mounted display (HMD) generates visual and auditory stimuli. It can reproduce and enhance the distractive qualities of guided imagery for patients who cannot visualize successfully [42]. The HMD also effectively blocks competing external stimuli, withdrawing patients from “the anxiety-inducing sights and sounds of the ‘sick patient’ environment” [22] (p. 261) of the clinic. VR also provides kinesthetic stimulation as users navigate the virtual environment with a computer mouse or joystick [23, 42].
VR therapy modulates attentional and emotional processes and reduces pain-related brain activity [18]. It is an effective analgesic for patients with burn injuries, providing better pain relief for them than distraction with movies or video games because the HMD enhances the subjective experience of immersion and physical location in the virtual world (presence) while minimizing input from the medical environment [27, 35]. VR distraction intervention can also reduce acute pain, distress, and anxiety in patients undergoing other painful medical and dental procedures [23, 27, 35, 42] and relieve symptom distress in both adult and pediatric chemotherapy patients, with high satisfaction reported in all age groups [31–34].
The limited literature on time perception during VR immersion suggests that VR can make time seem to pass more quickly. VR distraction permitted healthy adults [22] and children [10] to tolerate experimentally generated ischemic pain for significantly longer periods of time. Subjective responses by pediatric patients in trials of VR distraction intervention during chemotherapy indicated that time seemed to pass more quickly when VR was used [34]. Seven of ten adults receiving VR therapy during dental treatment underestimated time elapsed [42]. Sixteen older women with breast cancer receiving VR distraction during chemotherapy made estimates of treatment duration that were significantly shorter than the actual elapsed time [31], and this finding has been replicated in a larger, more diverse patient sample [32].
Meta-analyses of time perception research indicate that age and gender may affect accuracy of time estimation [3], raising the possibility that demographic factors affect duration judgments made by patients receiving therapy while immersed in VR, although these issues have not yet been addressed.
Clinical factors such as anxiety and distress are also known to affect time perception and have been associated with overestimation of interval duration in various samples [12, 43] including cancer patients [44]. There is also some evidence that fatigue can also distort time perception. Duration estimates lengthened significantly as fatigue increased in one study of sleep-deprived healthy volunteers, but fatigue had little effect on the length of estimates in two similar studies [25]. Effects of fatigue on time perception have not yet been explored in patients with cancer, although fatigue is one of the most frequently reported side effects of the treatments used to combat their disease[19].
In this context, the effects of VR intervention on time perception might be expected to differ among patients with different demographic and clinical characteristics. In addition, there are reports of differences in symptom patterns among cancer diagnoses [13, 16], raising the possibility of diagnosis-specific differences in patient responses to VR intervention during chemotherapy.
Study objectives
This study was designed to:
Explore the influence of age, gender, state anxiety, fatigue, and diagnosis on time perception in cancer patients receiving intravenous chemotherapy with a VR distraction intervention within the framework of the PA cognitive model of time perception and
predict the effects of these variables on the difference between the actual time elapsed while patients received chemotherapy while immersed in a VR environment and their retrospective estimates of elapsed time.
Materials and methods
This is a secondary analysis of pooled data collected from three trials evaluating effectiveness of VR in reducing chemotherapy-related symptom distress in cancer patients. Study participants were recruited from Comprehensive Cancer Centers at Duke University [31, 32] and Case Western Reserve University [33]. Each study was approved by the human subjects review board and protocol review committee of the participating cancer center. Informed consent was obtained from all participants.
Sample
Inclusion criteria for participation in all studies were: first diagnosis of breast, colon, or lung cancer; age≥18 years; planned treatment included at least two matched IV chemotherapy cycles; able to read and write in English and give informed consent. Exclusion criteria for study participation were: any clinical evidence of primary or metastatic disease to the brain and any history of either motion sickness or seizures. The pooled sample of participants from the three studies yielded a sample of N=137 for analysis. Of these, 66.4% were diagnosed with breast cancer, 20.5% with lung cancer, and 13.1% with colon cancer.
Procedures
Each study employed a crossover design requiring two matched intravenous chemotherapy treatments. Participants were randomly assigned to receive VR distraction intervention during the first or second treatment and standard care with no distraction during the alternate treatment. The VR intervention, delivered using commercially available HMDs,1 was provided during the entire period of IV infusion, including delivery of pre-medications, antiemetics, and chemotherapeutic agents. The researcher installed the HMD on the participant’s head as IV infusion started and removed the HMD as soon as the infusion process was completed. Patients selected an initial VR scenario from a menu of multiple options and were free to switch scenarios at any point during the treatment period [30, 31]. Results supported the premise that VR as a distraction intervention helped mitigate chemotherapy-related symptoms [33]. Additional details of subject recruitment, data collection methods, VR scenarios, and outcomes are reported elsewhere [31–33].
Measures
Participants provided demographic information (age, gender, and race/ethnicity) and diagnosis on a questionnaire administered before the first chemotherapy treatment. Anxiety and fatigue were measured pretest and posttest for both VR distraction and standard care treatments.
Anxiety was assessed with the State-Trait Anxiety Inventory for Adults (STAI) [36] which measures transitory anxiety states including those associated with stressful procedures. The STAI can be completed in 8–12 min. Respondents rate each of 20 items on a 1–4 Likert-type scale; half of the items are reverse-scored. Weighted item scores are summed to obtain the total score (range, 20–80; high values indicate greater anxiety). Validity and reliability of the STAI are well established [37]; the standardized Cronbach’s α for this sample was 0.93.
Fatigue was assessed using the Revised Piper Fatigue Scale (PFS) which measures multiple dimensions of subjective fatigue. The PFS can be completed in 5–8 min. It includes 22 items scaled 0–10 (higher scores indicate greater fatigue); total score is the mean of all item scores [28]. Standardized Cronbach’s α for the PFS in this sample of cancer patients was 0.97.
The researcher recorded the time elapsed during each patient’s chemotherapy treatment session with VR intervention without alerting the patient that this measurement was being taken. The starting point for time measurement was the placement of the virtual reality HMD on the patient’s head as infusion began; the end point was the removal of the HMD as infusion ended (waiting time in the chemotherapy suite and time required for IV/port access were not included). Thus, for the purposes of this study, the acual time elapsed during the chemotherapy session with VR intervention is defined as the full duration of IV infusion— including time required to deliver pre-medications and antiemetics as well as all chemotherapeutic agents—as measured by the researcher.
Patients were not informed beforehand that they would be asked any questions about the passage of time during chemotherapy. Instead, as the HMD was being removed at the end of the treatment session, the researcher asked the patient to retrospectively estimate the amount of time that had elapsed (in minutes) while the patient was using the virtual reality intervention. The estimate provided by the patient in response to this request is defined as the estimated time elapsed.
Analysis
Each participant’s estimate of the number of minutes elapsed during the chemotherapy session with VR distraction was subtracted from the actual number of minutes elapsed as recorded by the researcher for that treatment session. This time difference variable (which takes a positive value when participants underestimate time elapsed and a negative value when participants overestimate time elapsed) was the dependent variable in all models. Independent variables of interest included age, gender, diagnosis (breast, colon, or lung cancer), and total pretest scores on anxiety and fatigue measures administered before the chemotherapy session with VR distraction. Data were analyzed with SAS® version 9.1.3. After initial screening of data for errors and outliers, descriptive statistics were calculated for all variables.
Ordinary least squares (OLS) multiple regressions using forward, backward, and stepwise selection techniques were used to evaluate effects of age, gender (0 = female, 1 = male), cancer diagnosis, anxiety (STAI total), and fatigue (PFS total) on the dependent variable (difference between the actual and estimated time elapsed). Cancer diagnosis was recoded by constructing dummy variables “Breast” (coded as 1 in patients with breast cancer, 0 otherwise) and “Colon” (coded as 1 in patients with colon cancer, 0 otherwise). Each cancer diagnosis was thus represented by a unique pair of Breast and Colon dummy variables: breast cancer (1,0); colon cancer (0,1); lung cancer (0,0).
Independent variables were entered into the models as follows:
Forward selection: Enter variable with the highest F value first; add subsequent variables one by one until the model includes every variable with F significant at p<0.50.
Backward elimination: Start with the model including all independent variables; delete variables one by one until all variables remaining in the model have F significant at p<0.10.
Stepwise selection: Enter variable with the highest F value first; set F significant at p<0.15 as threshold value for adding subsequent variables to the model and retaining variables in later steps.
Results
Descriptive statistics
Sample demographics are presented in Table 1. Actual time elapsed during chemotherapy treatment sessions with VR intervention as recorded by the researcher averaged 63 min for the pooled sample. Mean values of this variable for patients with breast, colon, and lung cancer were 62, 76, and 57 min, respectively, with a wide range of values (from 15–20 min to 3+ h) recorded within each of the three diagnosis groups (Table 2).
Table 1.
Demographic and clinical characteristics of pooled sample (N=137)
| Variable | N (%) | Mean (SD) | Range |
|---|---|---|---|
| Age (years) | 137 | 52.4 (SD 10.8) | 27–78 |
| Gender | |||
| Male | 25 (18.3%) | ||
| Female | 112 (81.7%) | ||
| Race/Ethnicity | |||
| African American | 12 (8.8%) | ||
| Caucasian | 119 (86.9%) | ||
| Other | 6 (4.3%) | ||
| Cancer diagnosis | |||
| Breast | 91 (66.4%) | ||
| Colon | 18 (13.1%) | ||
| Lung | 28 (20.5%) | ||
| Anxiety (STAI total score) | 137 | 40.9 (SD 12.2) | 20–69 |
| Fatigue (Revised PFS total score) | 137 | 2.3 (SD 1.9) | 0–7.4 |
STAI Spielberger State-Trait Anxiety Inventory, PFS Piper Fatigue Scale
Table 2.
Time elapsed during chemotherapy treatment sessions with VR intervention, for pooled sample and by cancer diagnosis
| Sample/diagnosis | Time elapsed (min) | Mean (SD) | Range |
|---|---|---|---|
| Pooled sample (N=137) | Actual time (A) | 62.9 (SD 33.1) | 15–202 |
| Estimated time (E) | 45.4 (SD 35.2) | 5–300 | |
| Time difference (A – E) | 17.5 (SD 24.5) | −98 to 110 | |
| CA diagnosis: breast (N=91) | Actual time (A) | 62.3 (SD 30.0) | 15–200 |
| Estimated time (E) | 39.3 (SD 24.3) | 5–120 | |
| Time difference (A – E) | 23.0 (SD 22.1) | −45 to 110 | |
| CA diagnosis: colon (N=18) | Actual time (A) | 75.8 (SD 42.4) | 20–180 |
| Estimated time (E) | 63.9 (SD 36.1) | 20–150 | |
| Time difference (A – E) | 11.9 (SD 24.5) | −40 to 45 | |
| CA diagnosis: lung (N=28) | Actual time (A) | 56.9 (SD 35.3) | 15–202 |
| Estimated time (E) | 53.4 (SD 55.2) | 10–300 | |
| Time difference (A – E) | 3.5 (SD 26.2) | −98 to 35 |
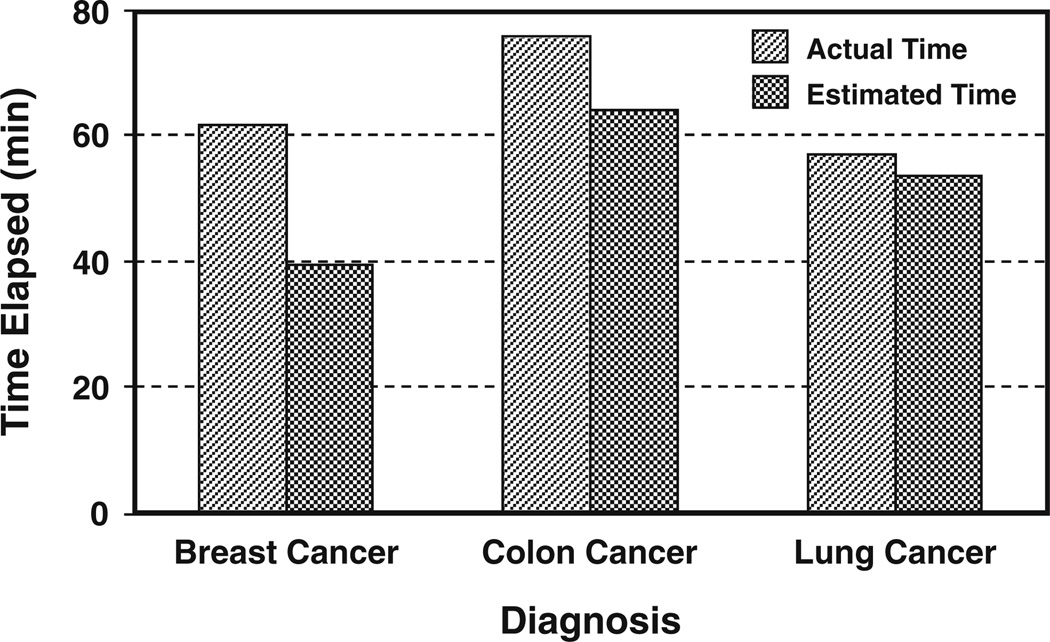
Most participants underestimated the duration of VR intervention during chemotherapy sessions, but the magnitude of differences between actual time elapsed and estimated time elapsed varied by cancer diagnosis (Table 2). On average, patients with breast cancer underestimated the duration of the chemotherapy treatment session with VR immersion by 23 min, colon cancer patients underestimated it by 12 min, and lung cancer patients underestimated it by <4 min (Fig. 2).
Fig. 2.
Actual time elapsed during chemotherapy treatment with VR immersion versus patient estimates of time elapsed during the treatment: mean values for patients with cancer of the breast (N=91), colon (N=18), and lung (N=28)
Regressions
In regressions of the time difference variable (actual minus estimated time elapsed during chemotherapy with VR immersion) on the independent variables, no collinearity or influential observations were detected. F values for all models were significant at p≤0.001, but each included a different set of independent variables (Table 3). All three models included both cancer diagnosis variables; two included gender, one included anxiety, and none included age or fatigue. Adjusted R2 was highest (0.134) in the forward selection model, with cancer diagnoses, gender, and anxiety explaining ~13% of variation in the time difference variable. In all models, diagnoses of breast and colon cancer predicted greater values of this variable and the signs of the coefficients were positive, indicating that patients with these diagnoses tended to underestimate treatment time. Breast cancer effects were significant at p<0.01 in all models; colon cancer effects were significant at trend level (0.05 < p < 0.10) in forward and stepwise models. The negative sign of the gender coefficient in forward and stepwise models suggests that males might overestimate rather than underestimate treatment time, but this effect was non-significant. The effect of anxiety was slight in the one model that included this variable.
Table 3.
Results of forward, backward, and stepwise OLS multiple regressions of the difference between actual and predicted elapsed time during chemotherapy with VR immersion on demographic and clinical independent variables
| Regression method | Independent variables included in model | β | SE | F | Prob>F | Model R2 |
|---|---|---|---|---|---|---|
| Forward selection | Breast cancer | 15.25 | 5.81 | 6.89 | 0.0097 | |
| Colon cancer | 13.09 | 7.59 | 2.98 | 0.0868 | ||
| Male gender | −11.98 | 7.46 | 2.57 | 0.1112 | ||
| Anxiety (STAI) | −0.20 | 0.16 | 1.54 | 0.2163 | ||
| Model characteristics | df=(4,131) | 5.06 | 0.0008 | 0.134 | ||
| Backward selection | Breast cancer | 19.62 | 5.06 | 15.04 | 0.0002 | |
| Colon cancer | 8.39 | 7.06 | 1.41 | 0.2372 | ||
| Model characteristics | df=(2,133) | 8.14 | 0.0005 | 0.109 | ||
| Stepwise selection | Breast cancer | 15.29 | 5.82 | 6.89 | 0.0097 | |
| Colon cancer | 12.63 | 7.59 | 2.77 | 0.0986 | ||
| Male gender | −11.02 | 7.44 | 2.20 | 0.1408 | ||
| Model characteristics | df=(3,132) | 6.20 | 0.0006 | 0.124 | ||
Discussion
This analysis is the first to examine factors influencing time perception in patients using VR distraction during chemotherapy. Retrospective estimates of time in chemotherapy with VR immersion averaged 45 min, a 28% underestimate of actual time elapsed (mean=63 min). Comparable underestimates (21–31%) have been reported in retrospective estimation by adolescents playing video games for a standardized 24-min period [39].
Age was not a significant predictor of differences in time perception in this study. However, comparability of our sample (mean age 52.4 years, range 27–78) to the literature (which focuses on contrasts between young and old adults and maturation effects in children) [3, 12] may be limited. Gender did not significantly affect time perception in this analysis, but the low proportion of men to women in our sample limits the generalizability of this finding [39]. Earlier research on anxiety and time perception [43, 44] indicated that individuals with lower anxiety levels tend to perceive time as passing more quickly. Anxiety met the criteria for inclusion in the model that explained the largest amount of variance (forward selection), and the direction of the anxiety effect on the time difference variable in this model (Table 3) was consistent with what would be expected from the literature. However, the magnitude of this anxiety effect was too small to be significant. The relatively low baseline level of anxiety in this sample may have limited detectability of an anxiety effect on time perception during VR distraction. Baseline measurements of fatigue were also relatively low in this sample, a factor which may have contributed to the absence of significant fatigue effects on time perception in this study.
Other study limitations include the sample bias toward women and the preponderance of breast cancer diagnoses, as well as the retrospective design which did not allow us to assess the effects on time perception of variables which can influence cognition (e.g., physiological variables such as oxygen saturation, body temperature, and electrolyte balance, depression, and medication effects) but for which measurements were not available.
Of the factors considered in the model, cancer diagnosis was the best predictor of altered time perception during VR immersion (R2=0.109). Alteration was greatest in breast cancer patients who perceived treatment time as passing the most rapidly. The model controlled for gender, so this difference in time perception is most likely attributable to diagnosis rather than gender. Lung cancer patients showed minimal alteration in time perception; the mean difference between their estimates and actual time in VR immersion was only 3.5 min. Some studies [13, 16] have reported greater severity physical and/or psychological symptoms in patients with lung cancer when compared to those with other cancer diagnoses. With respect to the PA model, more severe symptoms may preferentially direct patients’ attention to negative stimuli (accelerating pulse generation in the pacemaker) and inhibit their ability to immerse themselves in VR (increasing awareness of the passage of time during chemotherapy). Such patients would thus be less likely to underestimate (and more likely to overestimate) time elapsed during treatment. Baseline symptom severity data available from one of the studies in this aggregate sample suggested that individuals with lung cancer were more likely to self-report pain and breathlessness than those with breast or colon cancer. However, sample size in this study was too small to detect statistically significant differences in these symptoms among diagnosis groups, particularly in the context of the relatively low levels of baseline symptom severity reported by most participants.
This study is the first to suggest that diagnosis predicts altered time perception in patients receiving VR during chemotherapy. The regression models explained a relatively low proportion of the variance, so further research using prospective experimental designs should explore: (a) the effects of additional factors (e.g., symptom severity) on diagnosis-related differences in interval estimation and (b) distortions of time perception during VR immersion in more heterogeneous age groups and among patients with varying degrees of anxiety.
Some researchers have hypothesized that therapeutic effectiveness of VR is based on a sense of presence in the environment [10, 30, 42], an elusive concept that has hitherto been difficult to measure. Further research on the relationship between patients’ estimates of time elapsed in VR and its effectiveness as a distracter should explore the validity of altered time perception as an alternative measure of presence.
Previous studies have shown that VR during chemotherapy treatment is generally enjoyable and well received by patients [31–34]. Patients who estimate the duration of chemotherapy as shorter than actual time elapsed are most likely to be satisfied with this treatment. This study indicates that factors such as diagnosis and anxiety could impact time estimation mechanisms. Given the study findings, this distraction intervention may be better suited for patients who are less symptomatic, such as breast and colon cancer patients.
Clinicians should not assume that all patients will become distracted and experience altered time perception while using VR. Not all patients welcome distraction during unpleasant medical treatments; some may prefer the sense of control of observing clinic routines, and others may appreciate “low-tech” distractions [14, 21]. Still others may require the immersive VR environment to change their focus of attention. VR distraction may considerably improve the ability of this group to tolerate and adhere to chemotherapy. An implication for practice is that understanding the factors that predict patient responses to VR and other interventions can help clinicians develop a “tool box” of strategies for coping with chemotherapy that can be tailored to meet the specific needs of each patient.
Acknowledgments
Data used in these analyses were from studies funded by the American Cancer Society, the Oncology Nursing Foundation (through an unrestricted grant from Ortho Biotech Products, L.P.), NINR 1 P20 NR07795-01 (PI: E. Clipp), and Duke University Medical Center.
Footnotes
Sony PC Glasstron PLM-S700 or iO Display Systems Inc. i-Glasses SVGA 3D.
Author disclosures The authors of this manuscript have no conflicts of interest to disclose. The authors have no financial relationship with the organizations that sponsored the research. The corresponding/ primary author has full control of all the primary data and agrees to allow the journal to review the data if requested.
Contributor Information
Susan M. Schneider, Email: susan.schneider@duke.edu, Duke University School of Nursing, DUMC 3322, Durham, NC 27710, USA.
Cassandra K. Kisby, Duke University School of Medicine, Durham, NC 27710, USA
Elizabeth P. Flint, Duke University School of Nursing, DUMC 3322, Durham, NC 27710, USA
References
- 1.Abetz L, Coombs JH, Keininger DL, Earle CC, Wade C, Bury-Maynard D, Copley-Merriman K, Hsu M-A. Development of the cancer therapy satisfaction questionnaire: item generation and content validity testing. Value Health. 2005;8(Suppl 1):S41–S53. doi: 10.1111/j.1524-4733.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 2.Bell K. ‘If it almost kills you that means it’s working!’ Cultural models of chemotherapy expressed in a cancer support group. Soc Sci Med. 2009;68(1):169–176. doi: 10.1016/j.socscimed.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Brañas-Garza P, Espinosa-Fernández L, Serrano-del-Rosal R. Effects of gender and age on retrospective time judgements. Time Soc. 2007;16(1):99–118. [Google Scholar]
- 4.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 5.Burle B, Casini L. Dissociation between activation and attention effects in time estimation: implications for internal clock models. J Exp Psychol Hum Percept Perform. 2001;27(1):195–205. doi: 10.1037//0096-1523.27.1.195. [DOI] [PubMed] [Google Scholar]
- 6.Chaston A, Kingstone A. Time estimation: the effect of cortically mediated attention. Brain Cogn. 2004;55(2):286–289. doi: 10.1016/j.bandc.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Chernecky C. Temporal differences in coping, mood, and stress with chemotherapy. Cancer Nurs. 1999;22(4):266–276. doi: 10.1097/00002820-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 9.Cox AC, Fallowfield LJ. After going through chemotherapy I can’t see another needle. Eur J Oncol Nurs. 2007;11(1):43–48. doi: 10.1016/j.ejon.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Dahlquist LM, Weiss KE, Clendaniel LD, Law EF, Ackerman CS, McKenna KD. Effects of videogame distraction using a virtual reality type head-mounted display helmet on cold pressor pain in children. J Pediatr Psychol. 2009;34(5):574–584. doi: 10.1093/jpepsy/jsn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeVita VT, Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 12.Droit-Volet S, Gil S. The time–emotion paradox. Philos Trans R Soc Lond B Biol Sci. 2009;364(1525):1943–1953. doi: 10.1098/rstb.2009.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer DJ, Villines D, Kim YO, Epstein JB, Wilkie DJ. Anxiety, depression, and pain: differences by primary cancer. Support Care Cancer. 2010 doi: 10.1007/s00520-009-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon J, Zimand E, Pickering M, Rothbaum BO, Hodges L. A pilot and feasibility study of virtual reality as a distraction for children with cancer. J Am Acad Child Adolesc Psychiatr. 2004;43(10):1243–1249. doi: 10.1097/01.chi.0000135621.23145.05. [DOI] [PubMed] [Google Scholar]
- 15.Goldspiel BR. Chemotherapy dose density in early-stage breast cancer and non-Hodgkin’s lymphoma. Pharmacotherapy. 2004;24(10):1347–1357. doi: 10.1592/phco.24.14.1347.43154. [DOI] [PubMed] [Google Scholar]
- 16.Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. J Psychosom Res. 2008;65(6):549–552. doi: 10.1016/j.jpsychores.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grondin S, Plourde M. Judging multi-minute intervals retrospectively. Q J Exp Psychol. 2007;60(9):1303–1312. doi: 10.1080/17470210600988976. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman HG, Richards TL, Bills AR, Van Oostrom T, Magula J, Seibel EJ, Sharar SR. Using FMRI to study the neural correlates of virtual reality analgesia. CNS Spectr. 2006;11(1):45–51. doi: 10.1017/s1092852900024202. [DOI] [PubMed] [Google Scholar]
- 19.Hofman M, Morrow GR, Roscoe JA, Hickok JT, Mustian KM, Moore DF, Wade JL, Fitch TR. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center-community clinical oncology program study of 938 patients from community practices. Cancer. 2004;101(4):851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys GR, Buehner MJ. Magnitude estimation reveals temporal binding at super-second intervals. J Exp Psychol Hum Percept Perform. 2009;35(5):1542–1549. doi: 10.1037/a0014492. [DOI] [PubMed] [Google Scholar]
- 21.Kwekkeboom KL. Music versus distraction for procedural pain and anxiety in patients with cancer. Oncol Nurs Forum. 2003;30(3):433–440. doi: 10.1188/03.ONF.433-440. [DOI] [PubMed] [Google Scholar]
- 22.Magora F, Cohen S, Shochina M, Dayan E. Virtual reality immersion method of distraction to control experimental ischemic pain. Isr Med Assoc J. 2006;8(4):261–265. [PubMed] [Google Scholar]
- 23.Mahrer NE, Gold JI. The use of virtual reality for pain control: a review. Curr Pain Headache Rep. 2009;13(2):100–109. doi: 10.1007/s11916-009-0019-8. [DOI] [PubMed] [Google Scholar]
- 24.McIlfatrick S, Sullivan K, McKenna H, Parahoo K. Patients’ experiences of having chemotherapy in a day hospital setting. J Adv Nurs. 2007;59(3):264–273. doi: 10.1111/j.1365-2648.2007.04324.x. [DOI] [PubMed] [Google Scholar]
- 25.Miró E, Cano MC, Espinosa-Fernández L, Buela-Casal G. Time estimation during prolonged sleep deprivation and its relation to activation measures. Hum Factors. 2003;45(1):148–159. doi: 10.1518/hfes.45.1.148.27227. [DOI] [PubMed] [Google Scholar]
- 26.Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C. A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer. 2008;16(2):201–208. doi: 10.1007/s00520-007-0343-7. [DOI] [PubMed] [Google Scholar]
- 27.Morris LD, Louw QA, Grimmer-Somers K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. Clin J Pain. 2009;25(9):815–826. doi: 10.1097/AJP.0b013e3181aaa909. [DOI] [PubMed] [Google Scholar]
- 28.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised piper fatigue scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 29.Roscoe JA, Morrow GR, Colagiuri B, Heckler CE, Pudlo BD, Colman L, Hoelzer K, Jacobs A. Insight in the prediction of chemotherapy-induced nausea. Support Care Cancer. 2010 doi: 10.1007/s00520-009-0723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider SM. A series of studies exploring the use of virtual reality for chemotherapy treatments. Oncol Nurs Forum. 2007;34(1):182–183. doi: 10.1188/07.ONF.39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneider SM, Ellis M, Coombs WT, Shonkwiler EL, Folsom LC. Virtual reality intervention for older women with breast cancer. Cyberpsychol Behav. 2003;6(3):301–307. doi: 10.1089/109493103322011605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider SM, Hood LE. Virtual reality: a distraction intervention for chemotherapy. Oncol Nurs Forum. 2007;34(1):39–46. doi: 10.1188/07.ONF.39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider SM, Prince-Paul M, Allen MJ, Silverman P, Talaba D. Virtual reality as a distraction intervention for women receiving chemotherapy. Oncol Nurs Forum. 2004;31(1):81–88. doi: 10.1188/04.ONF.81-88. [DOI] [PubMed] [Google Scholar]
- 34.Schneider SM, Workman ML. Virtual reality as a distraction intervention for older children receiving chemotherapy. Pediatr Nurs. 2000;26(6):593–597. [PubMed] [Google Scholar]
- 35.Sharar SR, Miller W, Teeley A, Soltani M, Hoffman HG, Jensen MP, Patterson DR. Applications of virtual reality for pain management in burn-injured patients. Expert Rev Neurother. 2008;8(11):1667–1674. doi: 10.1586/14737175.8.11.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somov PG. Time perception as a measure of pain intensity and pain type. J Back Musculoskelet Rehabil. 2000;14(3):111–121. [Google Scholar]
- 37.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1983. [Google Scholar]
- 38.Thuné-Boyle ICV, Myers LB, Newman SP. The role of illness beliefs, treatment beliefs, and perceived severity of symptoms in explaining distress in cancer patients during chemotherapy treatment. Behav Med. 2006;32(1):19–29. doi: 10.3200/BMED.32.1.19-29. [DOI] [PubMed] [Google Scholar]
- 39.Tobin S, Grondin S. Video games and the perception of very long durations by adolescents. Comput Hum Behav. 2009;25(2):554–559. [Google Scholar]
- 40.Verdura GS, Alaimo S, Borzì R, Fazio M, Scavo V. Trying to listen: needs of patients treated with chemotherapy. Support Palliat Cancer Care. 2005;2(1):15–20. [Google Scholar]
- 41.Wearden JH. The perception of time: basic research and some potential links to the study of language. Lang Learn. 2008;58:149–171. [Google Scholar]
- 42.Wiederhold MD, Wiederhold BK. Virtual reality and interactive simulation for pain distraction. Pain Med. 2007;8(Suppl 3):S182–S188. [Google Scholar]
- 43.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12(1):7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Wittmann M, Vollmer T, Schweiger C, Hiddemann W. The relation between the experience of time and psychological distress in patients with hematological malignancies. Palliat Support Care. 2006;4(4):357–363. doi: 10.1017/s1478951506060469. [DOI] [PubMed] [Google Scholar]
- 45.Yoo HJ, Ahn SH, Kim SB, Kim WK, Han OS. Efficacy of progressive muscle relaxation training and guided imagery in reducing chemotherapy side effects in patients with breast cancer and in improving their quality of life. Support Care Cancer. 2005;13(10):826–833. doi: 10.1007/s00520-005-0806-7. [DOI] [PubMed] [Google Scholar]
- 46.Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, von der Maase H. Anticipatory nausea: the role of individual differences related to sensory perception and auto-nomic reactivity. Ann Behav Med. 2007;33(1):69–79. doi: 10.1207/s15324796abm3301_8. [DOI] [PubMed] [Google Scholar]