Abstract
Background
The Raloxifene Use for The Heart (RUTH) trial showed that raloxifene, a selective estrogen receptor modulator, had no overall effect on the incidence of coronary events in women with established coronary heart disease or coronary heart disease risk factors. We provide detailed results of the effect of raloxifene on coronary outcomes over time and for 24 subgroups (17 predefined, 7 post hoc).
Methods and Results
Postmenopausal women (n = 10 101; mean age, 67 years) were randomized to raloxifene 60 mg/d or placebo for a median of 5.6 years. Coronary outcomes were assessed by treatment group in women with coronary heart disease risk factors and those with established coronary heart disease. Raloxifene had no effect on the incidence of coronary events in any subgroup except in the case of a post hoc age subgroup analysis using age categories defined in the Women’s Health Initiative randomized trials. The effect of raloxifene on the incidence of coronary events differed significantly by age (interaction P = 0.0118). The incidence of coronary events in women <60 years of age was significantly lower in those assigned raloxifene (50 events) compared with placebo (84 events; hazard ratio, 0.59; 95% confidence interval, 0.41 to 0.83; P = 0.003; absolute risk reduction, 36 per 1000 women treated for 1 year). No difference was found between treatment groups in the incidence of coronary events in women ≥60 and <70 or ≥70 years of age.
Conclusions
In postmenopausal women at increased risk of coronary events, the overall lack of benefit of raloxifene was similar across the prespecified subgroups.
Keywords: coronary disease, hormones, raloxifene, randomized controlled trial, risk factors, women
Raloxifene, a benzothiophene selective estrogen receptor modulator, reduces the risk of vertebral fracture and the incidence of invasive breast cancer1–5 in postmenopausal women. Its effects on the arterial system include a decrease in total and low-density lipoprotein (LDL) cholesterol and levels of inflammatory markers.6,7 Raloxifene induces coronary artery and cardiac myocyte relaxation8,9 through stimulation of endothelial nitric oxide synthase10 and/or effects on plasma membrane ion channels.9,11 These actions could potentially translate into a reduction in the incidence of coronary heart disease (CHD) events in postmenopausal women.
In epidemiological studies, postmenopausal estrogen use has been associated with a reduction in the risk of CHD.12 This effect was believed to be mediated by the effect of estrogen on cardiovascular risk factors such as lipids and nonlipid factors.13–16 On the basis of these observations and the effects of raloxifene on cardiovascular risk factors, the Raloxifene Use for The Heart (RUTH) trial was designed to evaluate the effect of raloxifene on the incidence of coronary events in postmenopausal women with documented CHDnm (n = 5034) or CHD risk factors (n = 5067). RUTH was an international, multicenter, randomized, double-blind, placebo-controlled clinical trial that enrolled 10 101 post-menopausal women to receive either raloxifene 60 mg or placebo daily.17
Although randomized trials have failed to show a reduced risk of CHD events with hormone therapy (HT), this may reflect the timing of the initiation of therapy. HT and raloxifene may not be effective in older women. A similar lack of effect on cardiovascular events by statin therapy in elderly women has been demonstrated in one study.18 This may be due to changes in the underlying biological characteristics of the vessel wall and vascular responses to estrogen and related molecules in older, more atherosclerotic vessels.19 To investigate this hypothesis, we specifically evaluated, in a post hoc analysis, the effect of raloxifene on the incidence of coronary events in women <60 years of age in the RUTH trial and by discrete age at study entry in a manner similar to that reported in the Women’s Health Initiative and Nurses’ Health Study of postmenopausal women and HT.20–22
The overall findings of the RUTH trial have been reported.4 In the present report, we provide detailed results of the effect of raloxifene on coronary outcomes in women with CHD risk factors or established CHD and for 24 subgroups defined according to demographics, clinical characteristics, and selected biomarkers. We also report the effect of raloxifene on coronary outcomes over time.
Methods
Study Population, Recruitment, and Follow-Up
Between June 1998 and August 2000, 10 101 women were randomized at 177 sites in 26 countries. Eligible women were ≥55 years of age, ≥1 year postmenopausal, and at increased risk for CHD events or with established CHD.17 Participants were required to have a cardiovascular risk score of ≥4 according to a point system that took into account the presence of risk factors for myocardial infarction (MI) and coronary death identified through epidemiological study. Points were awarded as follows: established CHD = 4 points; arterial disease of the leg = 4 points; age of at least 70 years = 2 points; diabetes mellitus (self-reported diabetes mellitus and use of oral hypoglycemic medication or insulin or fasting serum glucose >140 mg/dL at visit 1) = 3 points; cigarette smoking (self-reported smoking of an average of ≥10 cigarettes a day over the 6 months before visit 1) = 1 point; hypertension (self-reported hypertension and use of antihypertensives or systolic blood pressure >160 mm Hg or diastolic blood pressure >95 mm Hg on at least 2 measurements) = 1 point; and hyperlipidemia (use of lipid-lowering medications, a fasting LDL cholesterol >160 mg/dL, or fasting high-density lipoprotein [HDL] cholesterol <45 mg/dL with fasting triglycerides >250 mg/dL) = 1 point.17 Further details of the trial design have been published.4,17,23
The primary end points of RUTH were a combined coronary end point, defined as coronary death, nonfatal MI, and hospitalized acute coronary syndrome (ACS) other than MI, whichever occurred first, and invasive breast cancer. Women were followed up for a minimum of 5 years after the last participant was enrolled. ECGs were recorded at baseline, at years 2 and 4, and at the final visit for the assessment of silent MI. Fasting biochemical and lipid assays were performed at baseline, at years 1 and 5, and at the final visit. Fibrinogen levels were measured in a subset of women enrolled in US centers. Throughout the study, the Executive Committee communicated to all investigators the need to implement the standards of cardiovascular care according to published guidelines.
End-Point Ascertainment
The Coronary Primary End Point Committee comprised 10 independent cardiologists, none of whom were employees of Eli Lilly and Co. This committee, which was blinded to treatment assignment, adjudicated all investigator-reported events of coronary death, non-fatal MI, or hospitalized ACS other than MI.
An event was adjudicated as an MI if at least one of the following criteria was present: (1) ischemic symptoms and cardiac enzyme levels >2 times the upper limit of normal with or without new or equivocal changes on ECG, (2) new Q waves with or without ischemic symptoms (ie, including silent MI) or cardiac enzyme levels >2 times the upper limit of normal, and (3) new Q waves or markedly abnormal levels of cardiac enzymes after invasive coronary procedures. Hospitalized ACS was defined as hospitalization for or the development during hospitalization of one of the following: cardiac symptoms with new ST-T changes on ECG or cardiac enzyme levels above the upper limit of normal but <2 times the upper limit of normal or troponin above the upper limit of normal. An event was adjudicated as coronary death if it was due to acute MI, a coronary artery procedure, or heart failure in the presence of coronary artery disease or if death was sudden or unwitnessed.
Myocardial revascularization was defined as coronary artery bypass graft or catheter-based coronary revascularization, whereas noncoronary arterial revascularization was defined as carotid and lower-extremity revascularization. All events were required to be documented by a procedure report or an equivalent document.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Statistical Analysis
Baseline characteristics were compared between treatment groups and between women with CHD risk factors and women with established CHD through the use of 1-way ANOVA for continuous variables and χ2 tests for categorical variables.
Outcomes analyses followed the intention-to-treat principle and were based on independently adjudicated components of the primary coronary end point. A log-rank test was used to compare the incidence of coronary events between treatment groups. Statistical significance for treatment group differences in the incidence of coronary events was tested at the 2-sided level of α = 0.0423, obtained from an α spending rule that accounted for the multiplicity of the coronary end point and the 3 planned interim analyses. For all other analyses, statistical significance for treatment effect was tested at α = 0.05 for a nominal probability value. In RUTH, the power calculations were based on assumptions of a 20.0% relative reduction in the incidence of coronary events and a 58.5% relative reduction in the incidence of invasive breast cancer (the other primary outcome in RUTH).
For all analyses, unadjusted Cox proportional-hazards regression models were used to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for the primary and secondary coronary outcomes. Time to event was defined as the number of days between randomization and the first diagnosis of a coronary event. Participants not experiencing the event were censored at the last date, at which time study information was collected, or their date of death.
Secondary analyses compared the rate of coronary events between treatment groups in an “as-treated” population defined as women who were at least 70% adherent to the study treatment on the basis of the pill count. Coronary and revascularization outcomes were assessed separately for women with CHD risk factors and those with established CHD through the use of Cox proportional-hazards models.
Prespecified (n = 17; race, body mass index, prior MI, prior angina with documented CHD, prior coronary artery bypass graft, lower-extremity arterial disease, cardiovascular risk score, current smoker, diabetes mellitus, hypertension, hyperlipidemia, statin use, aspirin use, β-blocker use, calcium channel blocker use, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, diuretic use) and post hoc (n = 7; Women’s Health Initiative–specified age, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides groups; geographical region of the world; years since menopause) exploratory subgroup analyses were performed to assess the consistency of the effect of treatment with raloxifene on CHD risk; interaction effects were tested at the 0.10 significance level using 1- or 2-df tests for the interaction terms in Cox proportional-hazards regression models. In the post hoc age subgroup analysis, the prespecified age cuts for the age subgroup analysis (≤65, >65 and <70, and ≥70 years of age) were changed to match those in the Women’s Health Initiative randomized trials.24 No adjustments for multiple testing were made for these subgroup analyses.
To further explore the effect of raloxifene on the incidence of coronary events by age, a post hoc Cox proportional-hazards regression model was fitted with age as a continuous variable. The log (hazard) was modeled as a quadratic function of age, with age as a continuous variable and terms for treatment and treatment interactions with age and age.2 HRs and 95% CIs were calculated at discrete ages (for each discrete year from 54 to 70 years of age and then every 2 years thereafter). Baseline laboratory values and percentage change from baseline to 1 year were analyzed with the use of an unadjusted ranked 1-way ANOVA. All statistical analyses were performed with SAS software version 8.2 (SAS Institute, Inc, Cary, NC).
Results
Baseline Characteristics
Overall, treatment groups were similar with respect to baseline characteristics except for a slightly higher cardiovascular risk score in the raloxifene group (placebo, 7.8; raloxifene, 7.9; P = 0.03)4 and a higher proportion of women with coronary artery bypass surgery in the raloxifene group (placebo, 15.5%; raloxifene, 17.3%; P = 0.015).
Of the 10 101 participants, 5067 women (50.2%) had CHD risk factors (placebo, 2561; raloxifene, 2506), and 5034 women (49.8%) had established CHD (placebo, 2496; raloxifene, 2538). The baseline characteristics for women with CHD risk factors and those with established CHD are presented in Table 1. Within each subpopulation, the baseline characteristics were similar between treatment groups except that among women with CHD a higher prevalence of smoking was found in women assigned placebo and a higher mean cardiovascular risk score and reported history of coronary artery bypass surgery in women assigned raloxifene (see Table 1 footnote). Most baseline characteristics differed significantly between women with CHD and those with CHD risk factors; women with CHD risk factors had a higher body mass index; reported a higher prevalence of smoking, diabetes mellitus, and hypertension; reported lower use of statins and aspirin; and had higher total cholesterol and LDL cholesterol levels (Table 1) than women with established CHD.
Table 1.
Baseline Characteristics of All Women Randomized in RUTH With CHD Risk Factors or With Established CHD
| Characteristic | With CHD Risk Factors (n = 5067)
|
Established CHD (n = 5034)
|
P* | ||
|---|---|---|---|---|---|
| Placebo (n = 2561) | Raloxifene (n = 2506) | Placebo (n = 2496) | Raloxifene (n = 2538) | ||
| Mean age, y | 67.50 ± 6.81 | 67.27 ± 6.76 | 67.47 ± 6.54 | 67.65 ± 6.48 | 0.181 |
| Age range, y, % | 0.013 | ||||
| <60 | 17.26 | 18.52 | 16.11 | 14.26 | … |
| 60–69 | 42.09 | 42.62 | 46.79 | 48.07 | … |
| ≥70 | 40.65 | 38.87 | 37.10 | 37.67 | … |
| Mean body mass index, kg/m2 | 29.26 ± 5.41 | 29.40 ± 5.49 | 28.16 ± 4.71 | 28.27 ± 4.78 | <0.001 |
| Current smoker, % | 17.18 | 17.72 | 8.37† | 6.42 | <0.001 |
| Mean time postmenopausal, y | 19.29 ± 8.08 | 18.93 ± 8.86 | 19.69 ± 8.51 | 19.70 ± 8.76 | <0.001 |
| Diabetes mellitus, % | 64.65 | 64.38 | 26.39 | 27.11 | <0.001 |
| Hypertension, % | 85.04 | 85.08 | 70.42 | 70.79 | <0.001 |
| Hyperlipidemia, % | 66.38 | 65.84 | 80.93 | 80.58 | <0.001 |
| Prior MI, % | 0 | 0 | 58.81 | 58.39 | |
| Prior CABG, % | 0 | 0 | 31.37† | 34.32 | |
| Prior PCI, % | 0 | 0 | 43.78 | 43.94 | |
| Lower-extremity arterial disease, % | 13.39 | 13.57 | 7.90 | 8.00 | <0.001 |
| Prior angina pectoris with documented CHD | 0 | 0 | 65.63 | 67.10 | |
| Mean CV risk score | 5.3 ± 1.49 | 5.28 ± 1.44 | 10.26 ± 3.70† | 10.52 ± 3.93 | <0.001 |
| Baseline medication use, % | |||||
| Statins | 30.93 | 30.29 | 62.86 | 63.95 | <0.001 |
| Antihypertensive | 85.63 | 86.11 | 93.31 | 92.95 | <0.001 |
| Nitrates | 18.20 | 17.76 | 49.84 | 48.98 | <0.001 |
| Warfarin | 2.54 | 2.71 | 5.21 | 6.07 | <0.001 |
| Aspirin | 32.33 | 32.20 | 81.61 | 80.34 | <0.001 |
| Nonaspirin antiplatelet agent | 1.64 | 2.23 | 4.01 | 3.94 | <0.001 |
| Oral hypoglycemic agent | 49.28 | 49.28 | 17.99 | 18.95 | <0.001 |
| Insulin | 19.45 | 18.99 | 9.13 | 8.59 | <0.001 |
| Abnormal ECG | 31.92 | 30.99 | 50.98 | 49.21 | <0.001 |
| Total cholesterol, mmol/L | 5.82 | 5.79 | 5.52 | 5.48 | <0.001 |
| LDL cholesterol, mmol/L | 3.24 | 3.24 | 3.07 | 3.05 | <0.001 |
| HDL cholesterol, mmol/L | 1.37 | 1.37 | 1.35 | 1.34 | 0.006 |
| Non-HDL cholesterol, mmol/L | 4.44 | 4.42 | 4.16 | 4.14 | <0.001 |
| Triglycerides, mmol/L | 1.85 | 1.84 | 1.74 | 1.74 | <0.001 |
| Fibrinogen, mmol/L | 1.05 | 1.03 | 1.04 | 1.06 | 0.441 |
CABG indicates coronary artery bypass graft; PCI, percutaneous coronary intervention; and CV, cardiovascular.
P values refer to differences between subgroups (with CHD risk factors versus with established CHD), with treatment groups combined within each subgroup.
Significant differences between placebo and raloxifene treatment groups.
The median duration of follow-up was 5.6 years for both treatment groups. Overall, 71% of women in the placebo group and 70% in the raloxifene group took at least 70% of assigned medication and were classified as adherent to treatment (P = 0.62).
Coronary Events
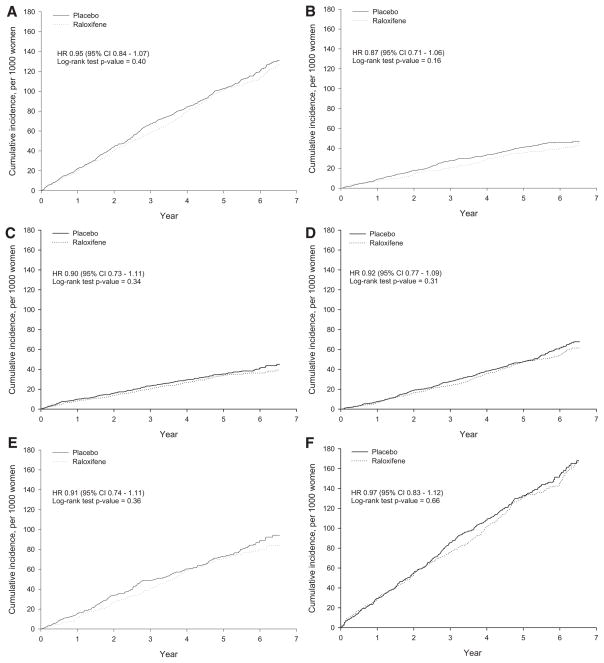
No effect of raloxifene was found on incidence of all coronary events (coronary death, nonfatal MI, or hospitalized ACS other than MI) or coronary death, nonfatal MI, and hospitalized ACS individually (Figure 1). No early increase was observed in the incidence of coronary events with raloxifene compared with placebo (Figure 1), and the incidence of coronary events was numerically lower than with placebo in each individual year of follow-up. Women with a history of CHD had a numerically higher incidence of coronary events than those with CHD risk factors (annualized incidence, 2.81% versus 1.54%), but treatment with raloxifene had no effect on the incidence of coronary events in either population (Table 2).
Figure 1.
Cumulative incidence for (A) primary coronary outcome, (B) nonfatal MI (including silent MI), (C) hospitalized ACS (HACS) other than MI for the entire cohort (n = 10 101), and (D) coronary death. Cumulative incidence for the primary coronary outcome for (E) women with CHD risk factors (n = 5067) and (F) women with established CHD (n = 5034).
Table 2.
Incidence and HR for Coronary Events and Revascularizations for Women With Risk Factors for CHD or Established CHD
| End Point | With CHD Risk Factors
|
With Established CHD
|
||||
|---|---|---|---|---|---|---|
| Events, n (Annualized Rate, %)
|
HR (95% CI) | Events, n (Annualized Rate, %)
|
HR (95% CI) | |||
| Placebo (n = 2561) | Raloxifene (n = 2506) | Placebo (n = 2496) | Raloxifene (n = 2538) | |||
| Primary coronary point* | 200 (1.54) | 182 (1.40) | 0.91 (0.74–1.11) | 353 (2.81) | 351 (2.72) | 0.97 (0.83–1.12) |
| Nonfatal MI or HACS | 112 (0.86) | 89 (0.68) | 0.80 (0.60–1.05) | 248 (1.98) | 237 (1.84) | 0.93 (0.78–1.11) |
| Nonfatal MI | 71 (0.54) | 54 (0.41) | 0.76 (0.53–1.09) | 137 (1.06) | 129 (0.97) | 0.92 (0.72–1.17) |
| HACS | 53 (0.40) | 40 (0.30) | 0.76 (0.50–1.14) | 132 (1.03) | 129 (0.98) | 0.95 (0.75–1.21) |
| Coronary death | 112 (0.84) | 104 (0.79) | 0.93 (0.71–1.22) | 162 (1.23) | 151 (1.12) | 0.91 (0.73–1.13) |
| Acute MI | 15 (0.11) | 12 (0.09) | 0.81 (0.38–1.72) | 30 (0.23) | 21 (0.16) | 0.68 (0.39–1.19) |
| Sudden death | 62 (0.47) | 54 (0.41) | 0.87 (0.61–1.26) | 75 (0.57) | 74 (0.55) | 0.96 (0.70–1.33) |
| Unwitnessed death | 3 (0.02) | 2 (0.02) | 0.67 (0.11–4.00) | 1 (0.01) | 1 (0.01) | NA |
| Heart failure + CAD history | 21 (0.16) | 21 (0.16) | 1.00 (0.55–1.83) | 38 (0.29) | 36 (0.27) | 0.92 (0.59–1.46) |
| CAD procedure related | 3 (0.02) | 6 (0.05) | 2.01 (0.50–8.03) | 8 (0.06) | 3 (0.02) | 0.37 (0.10–1.38) |
| Coronary cause unavailable | 8 (0.06) | 9 (0.07) | 1.12 (0.43–2.91) | 10 (0.08) | 16 (0.12) | 1.56 (0.71–3.43) |
| All revascularizations† | 199 (1.56) | 189 (1.48) | 0.95 (0.78–1.16) | 416 (3.49) | 422 (3.44) | 0.99 (0.86–1.13) |
| Coronary revascularization | 133 (1.03) | 121 (0.93) | 0.91 (0.71–1.16) | 334 (2.75) | 338 (2.70) | 0.98 (0.85–1.14) |
| PCI | 77 (0.59) | 77 (0.59) | 1.00 (0.73–1.38) | 244 (1.97) | 243 (1.89) | 0.96 (0.81–1.15) |
| CABG | 60 (0.46) | 45 (0.34) | 0.75 (0.51–1.10) | 104 (0.81) | 104 (0.79) | 0.98 (0.74–1.28) |
| Other | 0 (0.00) | 1 (0.01) | NA | 3 (0.02) | 2 (0.02) | 0.65 (0.11–3.89) |
HACS indicates hospitalized ACS; CAD, coronary artery disease; PCI, percutaneous coronary intervention; and CABG, coronary artery bypass graft.
Primary coronary point comprised coronary death, nonfatal MI, or hospitalized ACS other than MI. For any participant with multiple coronary events, each event was counted in each subcategory separately, but participants were counted only once in the primary coronary point analysis.
All revascularizations comprised coronary and noncoronary revascularizations.
Among both women with CHD risk factors and those with established CHD, no difference was found between treatment groups in the incidence of coronary events overall or nonfatal MI, hospitalized ACS, coronary death, and revascularization individually (Table 2). The effect of raloxifene on the incidence of coronary events did not differ between women with CHD risk factors and those with established CHD (interaction P = 0.64). The results of as-treated analyses were similar to those of the intention-to-treat analyses for the primary coronary outcome (HR for the comparison of the raloxifene group with the placebo group, 0.96; 95% CI, 0.83 to 1.12; P = 0.61) and its individual components (P > 0.05 for each comparison).
Coronary Events by Subgroup
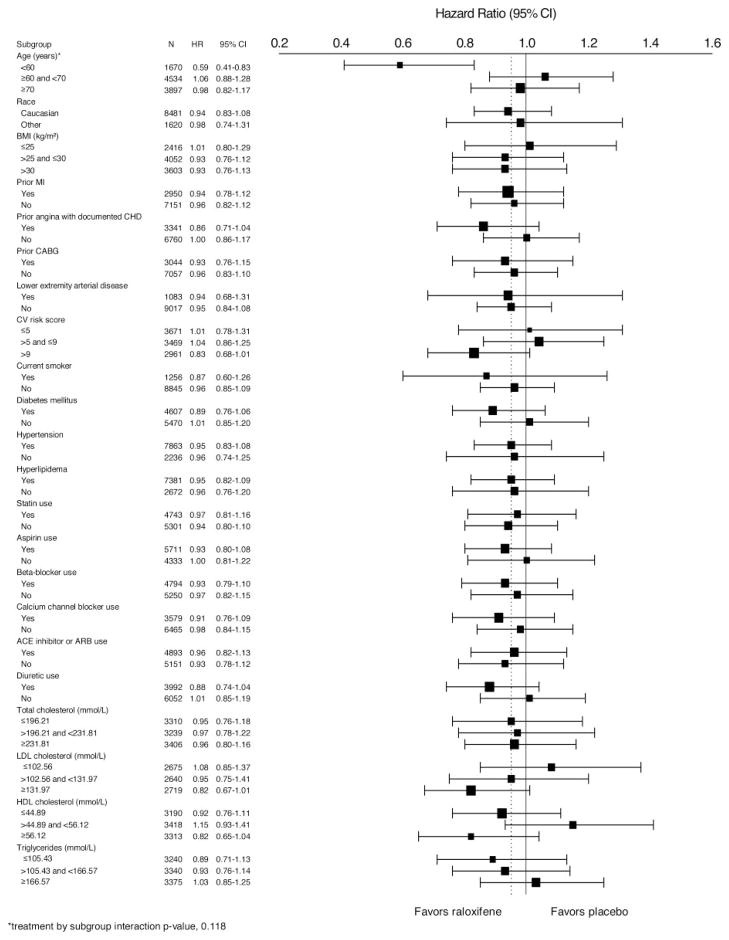
The effect of raloxifene on the incidence of coronary events among subgroups of participants is shown in Figure 2. The effect of raloxifene on the incidence of coronary events was similar across subgroups (interaction P > 0.1), except in the case of the age subgroup analysis (interaction P = 0.0118).
Figure 2.
Effect of raloxifene on the primary coronary events by subgroup. HRs and 95% CIs are shown. BMI indicates body mass index; CABG, coronary artery bypass graft; CV, cardiovascular; ACE, angiotensin-converting enzyme; and ARB, angiotensin receptor blocker.
In women <60 years of age, the incidence of coronary events was significantly lower in those assigned to raloxifene compared with placebo (HR, 0.59; 95% CI, 0.41 to 0.83; P = 0.003). In women ≥60 and <70 or ≥70 years of age, no significant difference was found in the incidence of coronary events between treatment groups (HR, 1.06; 95% CI, 0.88 to 1.28; and HR, 0.98; 95% CI, 0.82 to 1.17, respectively). Using the predefined age subgroup cutoffs did not yield a significant interaction (P = 0.287), and the incidence of coronary events was similar across treatment groups in each age cohort (≤65 years of age: HR, 0.84; 95% CI, 0.68 to 1.04; >65 and <70 years of age: HR, 1.09; 95% CI, 0.85 to 1.40; ≥70 years of age: HR, 0.97; 95% CI, 0.81 to 1.16).
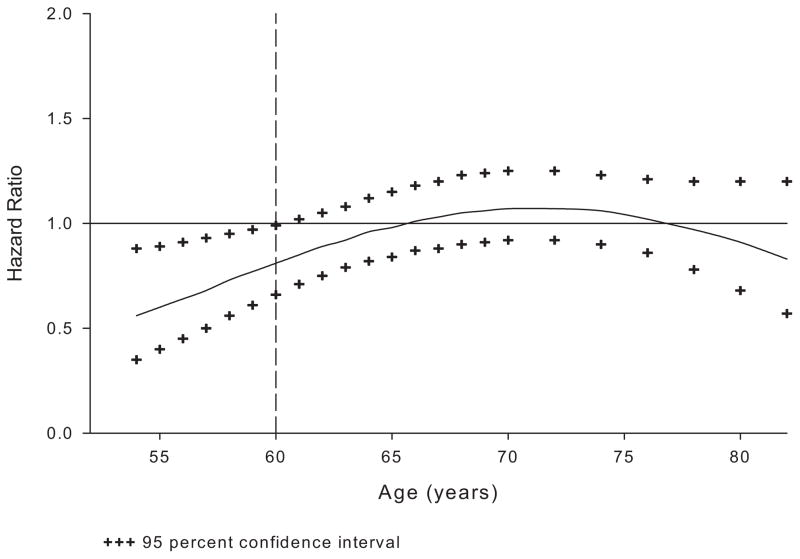
To further explore the effect of raloxifene on the incidence of coronary events by age, the incidence of coronary events was compared between treatment groups, with age as a continuous variable in linear and quadratic terms, along with their interactions with treatment in the model. The HR was then estimated in 1-year increments from 54 to 70 years of age and in 2-year increments from 70 to 92 years of age (Figure 3). The incidence of coronary events in the raloxifene group was significantly lower than in the placebo group only for women <60 years of age.
Figure 3.
Effect of raloxifene on the incidence of the primary coronary end point (coronary death, nonfatal MI, or hospitalized ACS other than MI, whichever occurred first) by age. The vertical line at 60 years of age was based on the post hoc analysis to match the Women’s Health Initiative age categories.
In a post hoc analysis of the Women’s Health Initiative studies, a nonsignificant trend was found toward a benefit of HT on CHD risk in women who initiated it closer to menopause. We therefore analyzed our RUTH data in a similar way, by years since menopause, and found that raloxifene had no significant effect on the incidence of coronary events in any subgroup (<10 years postmenopausal: HR, 0.94; 95% CI, 0.64 to 1.37; ≥10 and ≤20 years postmenopausal: HR, 1.02; 95% CI, 0.84 to 1.25; >20 years postmenopausal: HR, 0.92; 95% CI, 0.78 to 1.08; interaction P = 0.68). In women <60 years of age, the mean length of time since menopause was 9.9 years compared with 19.4 years for the overall RUTH population.
Coronary Events by Region and Changes in Levels of Cardiometabolic Risk Factors and Blood Pressure
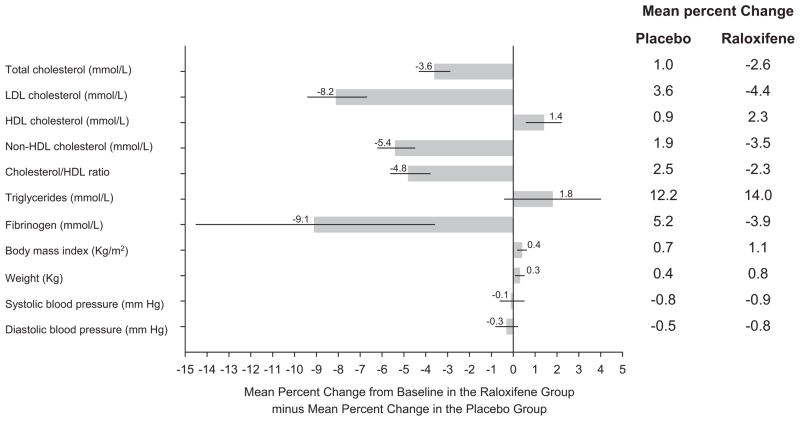
The effect of raloxifene on the incidence of coronary events did not differ significantly by region of the world (data not shown; interaction P = 0.78). Compared with placebo, women assigned to raloxifene had greater increases in HDL cholesterol (1.4%), body mass index (0.4%), and weight (0.3%) and greater reductions in LDL cholesterol (−8.1%), non-HDL lipoprotein levels (−5.4%), the ratio of cholesterol to HDL (−4.8%), and fibrinogen levels (−9.1%) (Figure 4). No significant differences were observed between treatment groups for systolic or diastolic blood pressure.
Figure 4.
Differences in mean percentage change in cardiometabolic risk factors and blood pressure from baseline in the raloxifene vs placebo group. Horizontal lines represent the 95% CIs. The differences between groups were significant (P < 0.05) for total cholesterol, LDL, HDL, non-HDL cholesterol, ratio of cholesterol to HDL, fibrinogen, body mass index, and weight. The data on the right indicate the mean percent changes in the parameters for the placebo and raloxifene groups.
Discussion
In these analyses of the RUTH trial data, raloxifene did not increase or decrease the incidence of coronary events overall in postmenopausal women with CHD risk factors or in those with established CHD and did not cause early CHD harm at any age. The incidence of coronary events was significantly lower in postmenopausal women <60 years of age assigned raloxifene compared with placebo. Raloxifene had no effect on the incidence of coronary events in any other subgroup. Of note, the annualized CHD event rate in RUTH was similar to that in a large international study of subjects (men and women) with established arterial disease or multiple risk factors for atherothrombosis.25
At baseline, women with CHD risk factors had a higher body mass index; reported a higher prevalence of smoking, diabetes mellitus, and hypertension and a lower use of statins and aspirin; and had higher total cholesterol and LDL cholesterol levels (Table 1) than women with established CHD. This difference was likely created by the eligibility criteria. Women with CHD were automatically eligible, whereas those without CHD were required to have a certain risk factor score to be eligible.17
No evidence was found of an early increase in coronary events with raloxifene in the RUTH trial. This finding is in contrast to results of some studies of HT, which have shown an early increased risk of CHD outcomes associated with HT use.20,26–28 One potential reason may be that treatment with oral HT results in a proinflammatory response that could result in destabilization of susceptible atherosclerotic plaques.29 In contrast to oral HT, raloxifene does not increase levels of proinflammatory markers such as C-reactive protein,30 perhaps partly explaining the lack of early CHD harm with raloxifene.
In RUTH, raloxifene had a relatively minor effect on lipids. Lack of a larger effect of raloxifene on lipid levels may have occurred because 66% of women in the raloxifene group and 68% in the placebo group in RUTH used statins during the trial (unpublished data).
The age subgroup findings suggest a possible cardioprotective action of raloxifene in women ≤60 years of age enrolled in RUTH. Although this was a post hoc analysis, it has biological plausibility based on the results of the Women’s Health Initiative and Nurses’ Health Study with estrogen therapy20–22 and animal studies of HT.19 It is also of note that the risk reductions with estrogen therapy in the Women’s Health Initiative20 study and with raloxifene in RUTH in women <60 years of age are similar, namely 0.62 (95% CI, 0.33 to 1.16) and 0.59 (95% CI, 0.41 to 0.83), respectively. Recent results from the observational Nurses’ Health Study also support the possibility that timing of postmenopausal estrogen use initiation in relation to age might influence its effect on the incidence of coronary events.21,31 A nonsignificant trend toward a benefit of HT on coronary outcomes depending on years since menopause has been reported.23 We did not find that the effect of raloxifene was dependent on years since menopause.
These coronary effects of raloxifene should not be considered in isolation of the recognized effects of raloxifene on other body systems. Raloxifene reduces the risk of vertebral fracture1 and invasive breast cancer.2–5 Raloxifene also is associated with an increased risk for venous thromboembolism and, in RUTH, was associated with an increased incidence of death from stroke but not overall stroke or mortality.4
Conclusions
Treatment with raloxifene for a median of 5.6 years had no overall effect on coronary risk among postmenopausal women with established CHD or at increased risk for coronary disease and did not appear to cause early coronary harm. This finding is at best hypothesis generating. Likewise, similar to trends in trials of HT, raloxifene may decrease the risk of coronary events in younger postmenopausal women. Individual patient potential benefits and risks should be considered in the final decision as to whether to prescribe raloxifene to a postmenopausal woman.
CLINICAL PERSPECTIVE.
Raloxifene is a selective estrogen receptor modulator that reduces the risk of vertebral fracture and the incidence of invasive breast cancer in postmenopausal women. The Raloxifene Use for The Heart (RUTH) trial of 10 101 women showed that raloxifene had no overall effect on the incidence of coronary events in women with established coronary heart disease or coronary heart disease risk factors. In this further analysis, we provide detailed results of the effect of raloxifene on coronary outcomes over time and for 24 subgroups (17 predefined, 7 post hoc). The effect of raloxifene was similar across the prespecified subgroups. Therefore, we further analyzed the effect based on a post hoc age subgroup analysis using age categories defined in the Women’s Health Initiative randomized trials in an attempt to test the “younger woman hypothesis” for hormone therapy response. The effect of raloxifene on the incidence of coronary events significantly differed by age (interaction P = 0.0118). The incidence of coronary events in women <60 years of age was significantly lower in those assigned raloxifene (50 events) compared with placebo (84 events; hazard ratio, 0.59; 95% confidence interval, 0.41 to 0.83; P = 0.003; absolute risk reduction, 36 per 1000 women treated for 1 year). No difference was found in the incidence of coronary events in women ≥60 and <70 or ≥70 years of age. Our hypothesis-generating data support the view that the cardiovascular response to hormones and hormone-related drugs may vary, depending on the age of the recipient.
Acknowledgments
We thank the RUTH investigators and the women who enrolled in the study; Roberta Secrest, PhD, for scientific review; and Melinda Rance for help preparing the tables and figures.
Source of Funding
This study was sponsored by Eli Lilly and Co, Indianapolis, Ind.
Footnotes
Clinical trial registration information—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00190593.
Disclosures
Drs Collins, Barrett-Connor, Mosca, Grady, Kornitzer, and Wenger received fees from Eli Lilly as members of the Executive Committee of the RUTH study. Drs Geiger, Dowsett, Effron, and Amewou-Atisso are employees and stockholders of Eli Lilly.
References
- 1.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial: Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 2.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial: Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 3.Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 5.Grady D, Cauley JA, Geiger MJ, Kornitzer M, Mosca L, Collins P, Wenger NK, Song J, Mershon J, Barrett-Connor E. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100:854–861. doi: 10.1093/jnci/djn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal RS, Baranowski B, Dowsett SA. Cardiovascular effects of raloxifene: the arterial and venous systems. Am Heart J. 2004;147:783–789. doi: 10.1016/j.ahj.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, Shah AS, Anderson PW. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- 8.Liew R, Stagg MA, MacLeod KT, Collins P. Raloxifene acutely suppresses ventricular myocyte contractility through inhibition of the L-type calcium current. Br J Pharmacol. 2004;142:89–96. doi: 10.1038/sj.bjp.0705736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figtree GA, Lu Y-Q, Webb CM, Collins P. Raloxifene acutely relaxes rabbit coronary arteries in vitro by an estrogen receptor– and nitric oxide–dependent mechanism. Circulation. 1999;100:1095–1101. doi: 10.1161/01.cir.100.10.1095. [DOI] [PubMed] [Google Scholar]
- 10.Simoncini T, Genazzani AR, Liao JK. Nongenomic mechanisms of endothelial nitric oxide synthase activation by the selective estrogen receptor modulator raloxifene. Circulation. 2002;105:1368–1373. doi: 10.1161/hc1102.105267. [DOI] [PubMed] [Google Scholar]
- 11.Leung FP, Yao X, Lau CW, Ko WH, Lu L, Huang Y. Raloxifene relaxes rat intrarenal arteries by inhibiting Ca2+ influx. Am J Physiol Renal Physiol. 2005;289:F137–F144. doi: 10.1152/ajprenal.00353.2004. [DOI] [PubMed] [Google Scholar]
- 12.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 13.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-Up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 14.Eaker E, Castelli W. Coronary heart disease and its risk factors among women in the Framingham study. In: Eaker E, Packard B, Wenger N, Clarkson T, Tyroler H, editors. Coronary Heart Disease in Women. New York, NY: Haymarket Doyma; 1987. pp. 122–132. [Google Scholar]
- 15.Stevenson JC, Crook D, Godsland IF, Collins P, Whitehead MI. Hormone replacement therapy and the cardiovascular system nonlipid effects. Drugs. 1994;47(suppl 2):35–41. doi: 10.2165/00003495-199400472-00007. [DOI] [PubMed] [Google Scholar]
- 16.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial: the Writing Group for the PEPI Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 17.Mosca L, Barrett-Connor E, Wenger NK, Collins P, Grady D, Kornitzer M, Moscarelli E, Paul S, Wright TJ, Helterbrand JD, Anderson PW. Design and methods of the Raloxifene Use for the Heart (RUTH) study. Am J Cardiol. 2001;88:392–395. doi: 10.1016/s0002-9149(01)01685-x. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, MacFarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 19.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 21.Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: the role of time since menopause and age at hormone initiation. J Womens Health (Larchmt) 2006;15:35–44. doi: 10.1089/jwh.2006.15.35. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 23.Wenger NK, Barrett-Connor E, Collins P, Grady D, Kornitzer M, Mosca L, Sashegyi A, Baygani SK, Anderson PW, Moscarelli E. Baseline characteristics of participants in the Raloxifene Use for the Heart (RUTH) trial. Am J Cardiol. 2002;90:1204–1210. doi: 10.1016/s0002-9149(02)02835-7. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 25.Steg PG, Bhatt DL, Wilson PW, D’Agostino R, Sr, Ohman EM, Rother J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 26.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–612. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 27.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J for the Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 28.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 29.Rosenfeld ME, Kauser K, Martin-McNulty B, Polinsky P, Schwartz SM, Rubanyi GM. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002;164:251–259. doi: 10.1016/s0021-9150(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 30.Walsh BW, Paul S, Wild RA, Dean RA, Tracy RP, Cox DA, Anderson PW. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2000;85:214–218. doi: 10.1210/jcem.85.1.6326. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, Manson JE, Grodstein F, Stampfer MJ, Colditz GA. Re: “combined postmenopausal hormone therapy and cardiovascular disease: toward resolving the discrepancy between observational studies and the Women’s Health Initiative clinical trial”. Am J Epidemiol. 2006;163:1067–1068. doi: 10.1093/aje/kwj156. Comment. [DOI] [PubMed] [Google Scholar]