Abstract
Parasitic neglected diseases are in dire need of new drugs either to replace old drugs rendered ineffective because of resistance development, to cover clinical needs that had never been addressed or to tackle other associated problems of existing drugs such as high cost, difficult administration, restricted coverage or toxicity. The availability of transgenic parasites expressing reporter genes facilitates the discovery of new drugs through high throughput screenings, but also by allowing rapid screening in animal models of disease. Taking advantage of these, we propose an alternative pathway of drug development for neglected diseases, going from high throughput screening directly into in vivo testing of the top ranked compounds selected by medicinal chemistry. Rapid assessment animal models allow for identification of compounds with bona fide activity in vivo early in the development chain, constituting a solid basis for further development and saving valuable time and resources.
New tools in drug development for parasitic diseases
The development of drugs for parasitic neglected diseases is constrained by the lack of adequate levels of funding and difficulties in the coordination between academic scientists, who frequently discover drug candidates, and pharmaceutical companies, which develop them into drugs for potential clinical use. Any improvements in the process of early drug development would have positive repercussions in the discovery pipeline by lowering the cost of identification of lead candidate compounds that would be ready for clinical development.
Recent technological developments in the area of drug development are now being applied to neglected diseases and should result in a significant increase in the numbers of lead compounds reaching the clinical stage of the development chain in the future years. Two of the most important recent innovations in the area are the development of high throughput screening (HTS) protocols and the production of transgenic parasites with reporter genes that are used in many of these HTS protocols and also allow for direct quantification of infection in vivo (Figure 1). These innovations cannot be applied yet to all neglected diseases, but there are a growing number of pathogens amenable to these types of studies.
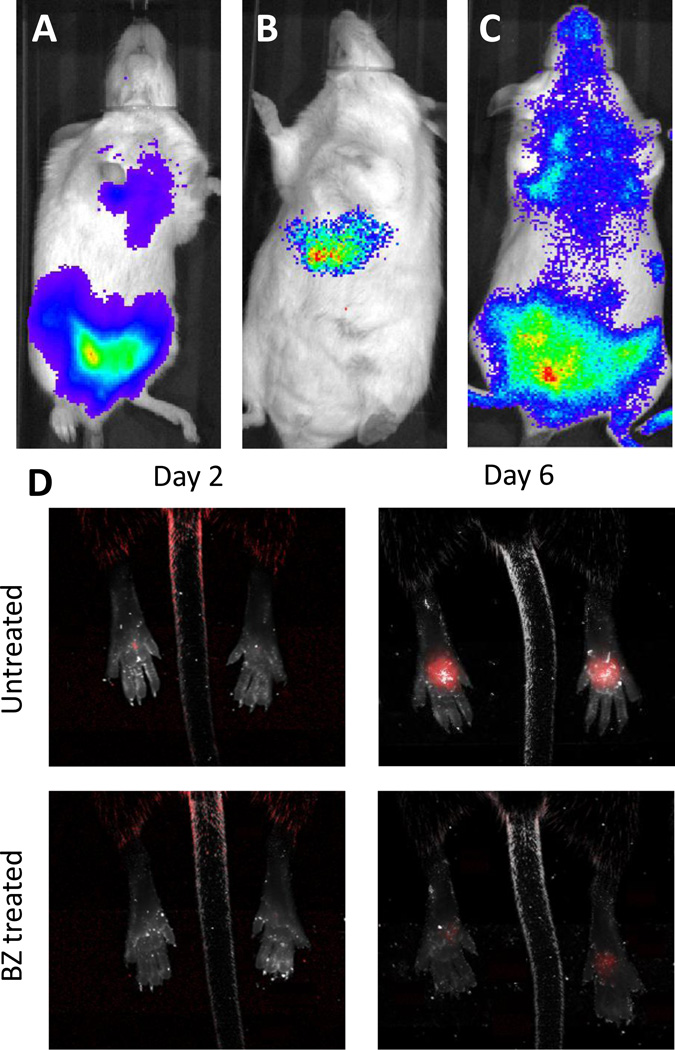
Figure 1. Transgenic parasites facilitate monitoring of mice infections.
Mice infected with Trypanosoma cruzi expressing luciferase (6) (a), Plasmodium berghei expressing luciferase (10) during liver stage (b) and blood stage (c) infections. The luminescent signal is quantified by the imager (IVIS lumina), and it is proportional to parasite load during infection. (d)
The effect of benznidazole (BZ) treatment (100 mg/kg i.p. on days 2 and 3 post-infection) on the expansion of tandem tomato (TdT) fluorescent protein-expressing T. cruzi in the footpads of mice is shown.
High Throughput Screenings
HTS of chemical compounds, both for specific molecular targets and for whole pathogens, have been performed for several neglected parasitic diseases, providing a significant number of novel candidate compounds with inhibitory activity (1–4). In this area, academic institutions and pharmaceutical companies have made available several lists of HTS hits with inhibitory activity against different parasites in various online data repositories, such as the Collaborative Drug Discovery (CDD) website (www.collaborativedrug.com) and ChEMBL-Neglected Tropical Diseases archive (www.ebi.ac.uk/chemblntd). The NIH, through the Molecular Libraries Program (mli.nih.gov/mli) has also contributed to the effort of performing HTS campaigns for parasitic neglected diseases in collaboration with academic scientists and have made the results publicly available through PubChem (pubchem.ncbi.nlm.nih.gov). As a result, there is already a considerable quantity of publicly available data identifying compounds with activity against specific parasites, and this resource is expected to continue to increase. Given this new resource, the challenge for drug discovery in these diseases is now changing from the identification of active compounds to the adequate selection of those hits that should be targeted for further development.
Transgenic parasites
Transgenic pathogens not only simplify HTS campaigns using whole parasites as targets (‘phenotypic screens‘), but also provide fundamental advantages for drug testing in animal models. The generation of fluorescent and luminescent pathogens, together with the development of sensitive imaging instruments, allow for direct visualization of the infection in the living animal. The advantages to these methods compared to the traditional quantification of pathogens in samples extracted from the animals include less labor, higher reproducibility and sensitivity and the ability to serially monitor a single set of animals over time rather than sampling different animals for each time-point/determination. Furthermore, these transgenic parasite lines are readily available to all investigators, and the screening methods are sufficiently simple as to be easily established in any laboratory that has access to the appropriate imaging equipment. For those who lack this access, service centers have been developed (e.g. http://cores.med.nyu.edu/cores-and-shared-resources/anti-infectives-screening-core, which offers in vitro and in mouse screening services for Plasmodium, Trypanosoma cruzi, Trypanosoma brucei and Leishmania). These resources should facilitate the transition from hit to lead that frequently delays the progression along the development pipeline.
The current protocol for drug development
The availability of technologies provides an opportunity to reshape the drug discovery pipeline. The standard process of going from HTS hits to lead compounds for preclinical studies involves a number of distinct levels of investigations: synthetic and medicinal chemistry, pharmacokinetics (PK) and biological assays. Iterative cycles between these disciplines allow for the selection of drug candidates that have strong in vitro activity against the pathogen and adequate characteristics for delivery and activity in the body, such as intestinal absorption, stability in serum or solubility. Generally it is only after this process of optimization that a candidate drug will advance into testing in animal models (usually mice), wherein anti-pathogen activity and toxicity are directly assessed.
This model of drug development is followed with small variations almost unanimously, both in academic and pharmaceutical laboratories, and has provided numerous successful drugs that are currently in clinical use. Animal testing prior to this substantial period of optimization is generally considered to be too risky, expensive and time-consuming. However, despite adequate optimization of PK parameters in vitro, a high proportion of candidate drugs that showed strong activity in vitro fail when tested in animals. When this happens, researchers frequently have to go back and start the optimization process from the beginning with the same or with a new compound (Figure 2).
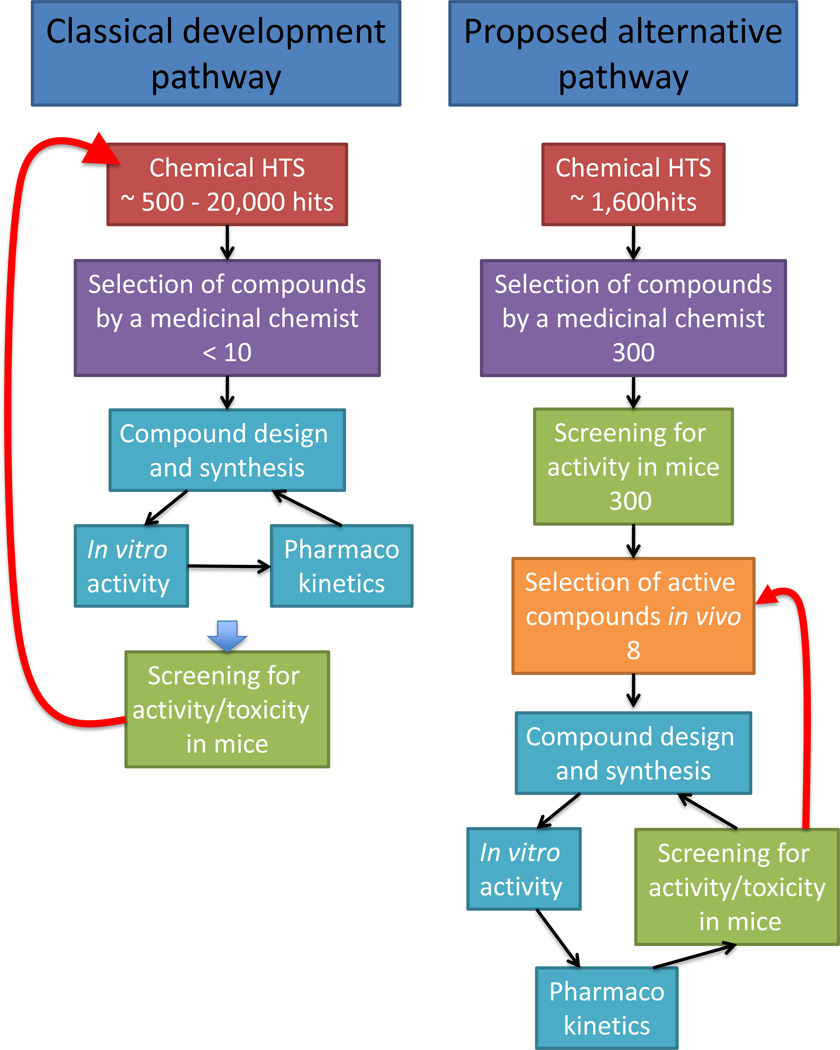
Figure 2. Drug development pathways.
In the classical pathway for preclinical drug development, any failure in the last step when testing activity in mice results in the need to re-start development of new compounds from the beginning (red arrow). Our proposed alternative pathway would create a solid base of compounds with activity in mice which, in case of failure in the late steps of development, would facilitate alternative compounds already at a high level in the development chain. The number of compounds cited for each step are based on previous HTS and unpublished observations from our laboratories.
An alternative pathway for drug development in neglected diseases
Taking into consideration (i) the limited resources available for the development of urgently needed treatments for neglected diseases, (ii) the plentiful number of candidates identified in HTS campaigns, (iii) the time and effort required for the further optimization of each candidate prior to (often failed) in vivo testing, and (iv) the availability of the new technologies noted above, we propose modifications of this pathway of drug development to identify new lead compounds for neglected parasitic diseases. Specifically, we propose two steps prior to extensive PK optimization studies: first, the grouping and ranking of HTS hits to select a subset with the best ’drug-like‘ properties and predicted low cost of production, followed by in vivo testing of the top ranked compounds in rapid assessment models. Experienced chemists can relatively easily reduce the thousands of compounds from large HTS efforts into several hundred non-redundant chemical structures. New in vivo tests that use low numbers of mice and relatively small amounts of compounds can provide efficacy data on hundreds of compounds within a few weeks (5), allowing for a rapid selection of lead compounds that have confirmed in vivo activity and no overt toxicity. Thus, very quickly and with minimal resources, the ’avalanche‘ of data from HTS can be reduced to a relative few compounds that can then be directed into PK studies and iterative cycles of optimization.
Applying this approach to an HTS campaign performed for T. cruzi infection of host cells (Pubchem AIDs: 1885; 2010; 2044), the ~1 600 low toxicity/high potency hits provided were reduced to ~300 by three independent medicinal chemists and then to eight in vivo active compounds within only a few months ((6) and Park, et al., unpublished). This selected group of chemical structures with activity in vivo constitutes a solid base for further development in the pre-clinical and clinical phases.
This approach of early screening in animals would certainly miss compounds with activity in vitro that after PK optimization might prove active in vivo, thus possibly removing promising scaffolds from consideration before they have been fully evaluated. However, since each HTS yields very high numbers of hits, it seems wiser to invest the development efforts in screening a higher number of hits to select the most suitable for activity in vivo, rather than to focus only on a few compounds that may turn out not to be suitable for use in vivo in the end. A more classical approach to optimization of particularly attractive structures can still be pursued and/or analogues of these in vivo inactive compounds could be rapidly screened in vitro to identify those with both anti-parasite activity and the required PK properties.
Of course this proposed approach requires that investigators have an in vivo screening model that is affordable and which rapidly and faithfully reflects treatment efficacy and potential for cure. Our models in T. cruzi using luminescent and fluorescent parasite lines meet these requirements and have high throughput (>60 compounds per week using minimal staff). Also, since these assays are directly monitoring parasite growth in vivo – which in the case of T. cruzi only takes place inside host cells , we are certain that compounds are directed against the appropriate parasite life stage and that they are able to enter host cells. Additionally, since anti-parasitic drug discovery is increasingly dependent on in vitro phenotypic screens, which select compounds with unknown targets, this alternative approach addresses very early the risk that the target might be not essential for survival in vivo.
The rapid in vivo screening models require relatively small amounts of compound (6, 7), although this could be an issue for compounds that are not commercially available or for which resynthesis is difficult.
The possibility of applying this approach for drug development in other parasitic diseases seems high. Transgenic parasites expressing luciferase are already available for several species and can be used effectively for drug discovery in mice models: Leishmania (8), T. brucei (9) and Plasmodium, both in blood stage (10) and in liver stage (11, 12). As in the case of T. cruzi (13), the luminescence signal was found to correlate reliably with parasitemia for all infections, a characteristic that is required for drug development. In addition, visualizing whole animal infections with luciferase-expressing parasites, as opposed to quantifying circulating parasites in the blood, provides a more reliable determination on whether a particular drug is able to clear the parasite for specific organs in the body where it accumulates, as observed with T. brucei (9) and Plasmodium blood stage (14) infections.
Concluding remarks
Developing similar models in other parasite systems and then moving compounds quickly into these in vivo efficacy models will allow a more rapid identification of compounds with bona fide drug potential and is a cheaper and more efficient way to proceed in the drug development chain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gamo FJ, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465(7296):305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 2.Guiguemde WA, et al. Chemical genetics of Plasmodium falciparum. Nature. 2010;465(7296):311–315. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharlow ER, et al. Identification of potent chemotypes targeting Leishmania major using a high-throughput, low-stringency, computationally enhanced, small molecule screen. PLoS Negl Trop Dis. 2009;3(11):e540. doi: 10.1371/journal.pntd.0000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simeonov A, et al. Quantitative high-throughput screen identifies inhibitors of the Schistosoma mansoni redox cascade. PLoS Negl Trop Dis. 2008;2(1):e127. doi: 10.1371/journal.pntd.0000127. (in eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canavaci AM, et al. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS neglected tropical diseases. 2010;4(7):e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andriani G, Chessler AD, Courtemanche G, Burleigh BA, Rodriguez A. Activity In Vivo of Anti-Trypanosoma cruzi Compounds Selected from a High Throughput Screening. PLoS Negl Trop Dis. 2011;5(8):e1298. doi: 10.1371/journal.pntd.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustamante JM, Tarleton RL. Methodological advances in drug discovery for Chagas disease. Expert Opin Drug Discov. 2011;6(6):653–661. doi: 10.1517/17460441.2011.573782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang T, Goyard S, Lebastard M, Milon G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cellular microbiology. 2005;7(3):383–392. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 9.Claes F, et al. Bioluminescent imaging of Trypanosoma brucei shows preferential testis dissemination which may hamper drug efficacy in sleeping sickness. PLoS neglected tropical diseases. 2009;3(7):e486. doi: 10.1371/journal.pntd.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke-Fayard B, et al. Simple and sensitive antimalarial drug screening in vitro and in vivo using transgenic luciferase expressing Plasmodium berghei parasites. Int J Parasitol. 2008;38(14):1651–1662. doi: 10.1016/j.ijpara.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Mwakingwe A, et al. Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J Infect Dis. 2009;200(9):1470–1478. doi: 10.1086/606115. [DOI] [PubMed] [Google Scholar]
- 12.Ploemen IH, et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One. 2009;4(11):e7881. doi: 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyland KV, Asfaw SH, Olson CL, Daniels MD, Engman DM. Bioluminescent imaging of Trypanosoma cruzi infection. Int J Parasitol. 2008;38(12):1391–1400. doi: 10.1016/j.ijpara.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke-Fayard B, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A. 2005;102(32):11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]