Abstract
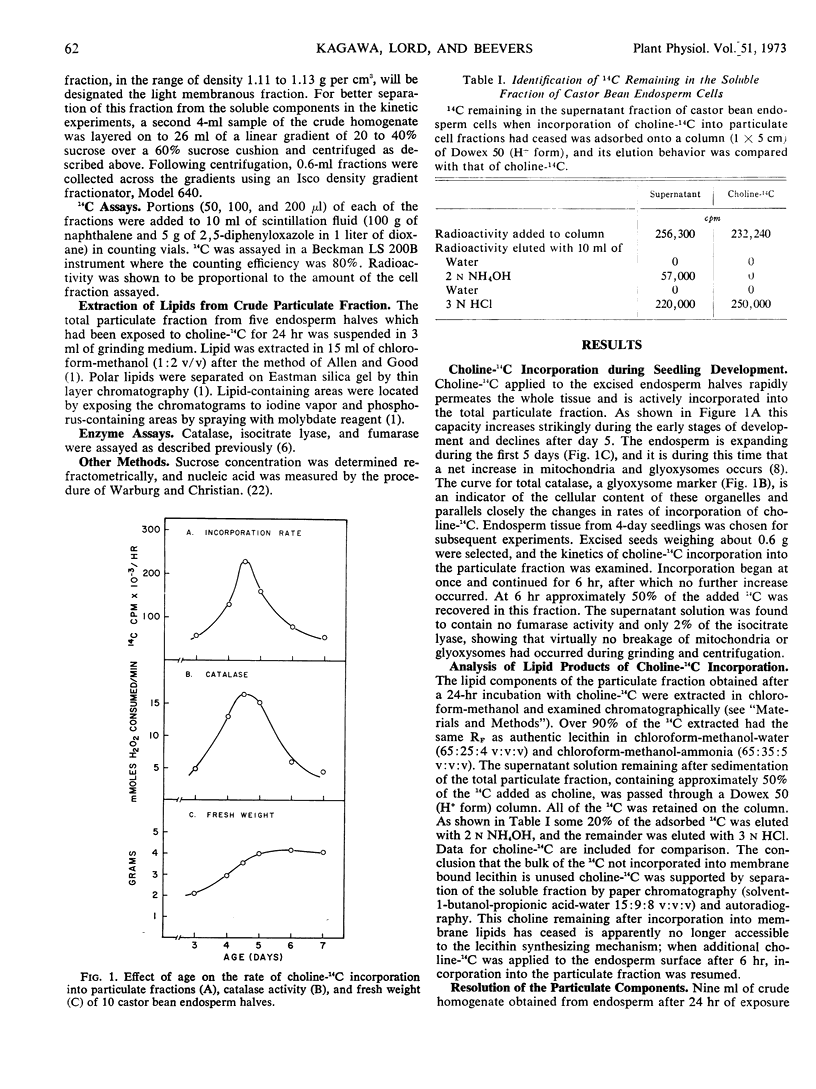
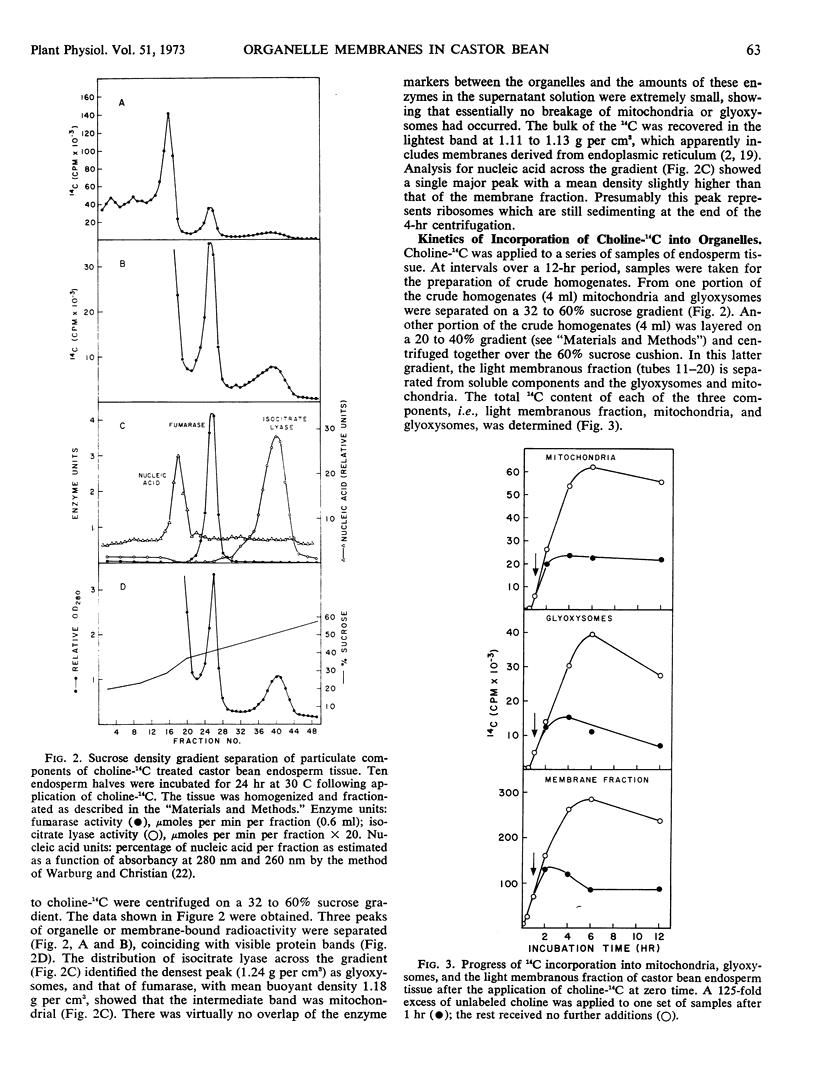
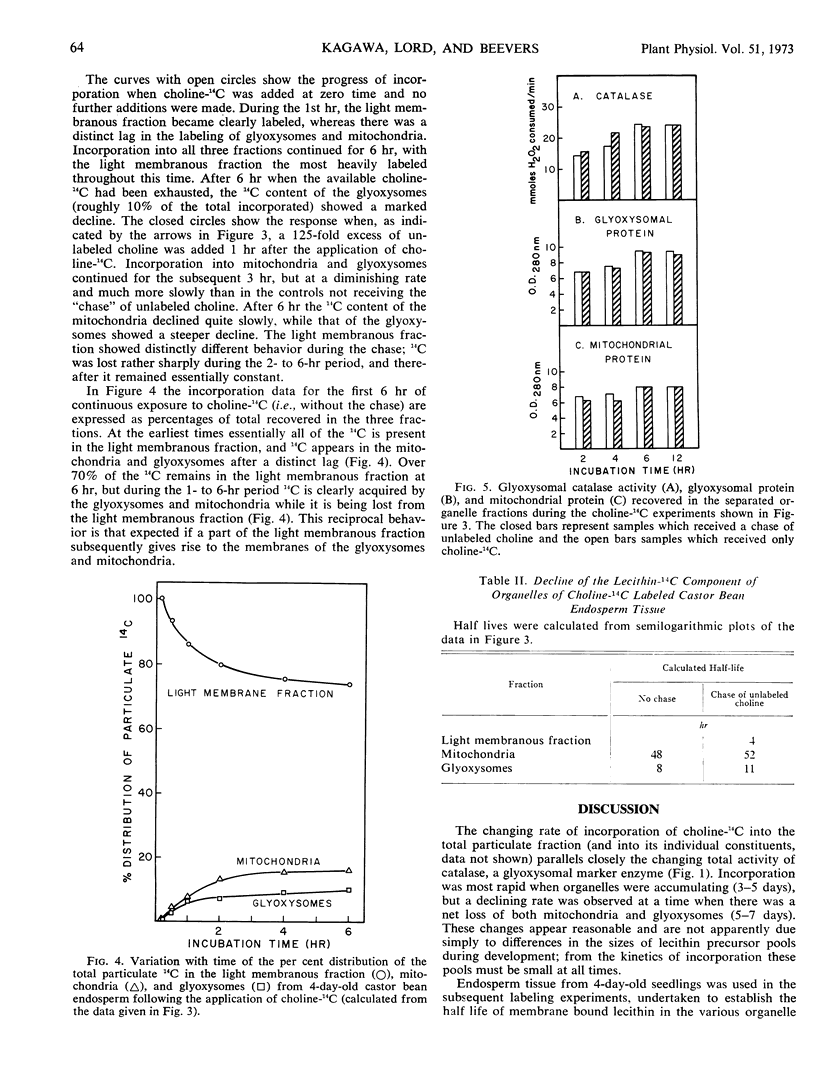
The origin and turnover of organelle membranes in castor bean (Ricinus communis L. var. Hale) endosperm was examined using choline-14C as a phospholipid precursor. On sucrose gradients three major particulate fractions were separated; a light membranous fraction (density 1.11-1.13 gram per cm3), the mitochondria (1.18 gram per cm3), and the glyoxysomes (1.24 gram per cm3). Choline-14C was readily incorporated into lecithin in all three particulate fractions, but the light membranous fraction became labeled first. Incorporation continued into all three fractions for 6 hours, at which time the available choline-14C had been completely used. Subsequently, 14C was lost from the three components at distinctly different rates. When an excess of unlabeled choline was added after 1 hour (pulse-chase experiment), incorporation of choline-14C into glyoxysomes and mitochondria continued for three hours, but at a diminishing rate. This was followed by a period in which the 14C content of the mitochondria declined at a rate expected, if the half life of lecithin in the membrane were about 50 hours and that of the glyoxysomes 10 hours. These values are close to those calculated from the experiments in which no chase was used. The labeling in the light membrane fraction behaved differently from that of the mitochondria and glyoxysomes following the chase of unlabeled choline. Incorporation continued for only 1 additional hour, and then the 14C content declined sharply in the subsequent 4 hours. The early kinetics and subsequent interrelationships are those expected if the lecithin in the membranes of mitochondria and glyoxysomes originates in components of the light membrane fraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castelfranco P. A., Tang W. J., Bolar M. L. Membrane transformations in aging potato tuber slices. Plant Physiol. 1971 Dec;48(6):795–800. doi: 10.1104/pp.48.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. M. Glyoxysomes in megagamethophyte of germinating ponderosa pine seeds. Plant Physiol. 1970 Sep;46(3):475–482. doi: 10.1104/pp.46.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormone-controlled synthesis of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1631–1633. doi: 10.1073/pnas.68.7.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. C., Taylor J. M. Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol. 1970 Oct;46(4):538–542. doi: 10.1104/pp.46.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Longo C. P. The development of glyoxysomes in maize scutellum: changes in morphology and enzyme compartmentation. Plant Physiol. 1970 Oct;46(4):599–604. doi: 10.1104/pp.46.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer H. H., Totten C. Studies on seeds: v. Microbodies, glyoxysomes, and ricinosomes of castor bean endosperm. Plant Physiol. 1970 Dec;46(6):794–799. doi: 10.1104/pp.46.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Nyquist S., Rivera E. Lecithin Biosynthetic Enzymes of Onion Stem and the Distribution of Phosphorylcholine-Cytidyl Transferase among Cell Fractions. Plant Physiol. 1970 Jun;45(6):800–804. doi: 10.1104/pp.45.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Beevers H. Gluconeogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967 Nov;42(11):1587–1595. doi: 10.1104/pp.42.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]



