Abstract
Kinases play a key role in cellular signaling, and the overactivation or overexpression of these kinases has been linked to a variety of cancers. Tyrosine kinase inhibitors treat the mechanism of these cancers by targeting the specific kinases that are overactive. Some patients, however, do not respond to these inhibitors or develop resistance to these inhibitors during treatment. Additionally, even within cancers of the same tissue type, different kinases may be overactive in different patients. For example, some lung cancers overexpress epidermal growth factor receptor (EGFR) and respond to EGFR inhibitors, while other lung cancers do not overexpress EGFR and receive no benefit from this treatment. Even among patients exhibiting EGFR overexpression, some do not respond to EGFR kinase inhibitors because other kinases, such as Met kinase, are also overactivated. Here we describe a quantitative and specific multiplexed microfluidic assay using a hydrogel immobilized substrate for measuring the kinase activity of Met and Abl kinase from cancer cells. We immobilized kinase specific substrates into macroporous hydrogel micropillars in microchannels. These microchannels were incubated with 6 µl of a kinase reaction solution containing cancer cell lysate and measured kinase activity via fluorescence detection of a phosphotyrosine antibody. We showed that the assay can specifically measure the activity of both Met and Abl kinase within one microchannel with potential to measure the activity of as many as 5 kinases within one microchannel. The assay also detected Met kinase inhibition from lysates of cancer cells grown in the Met kinase inhibitor PHA665752.
Keywords: kinase activity, microfluidics, cancer diagnostic, kinase assay
Introduction
Tyrosine kinases are important cellular signaling proteins, regulating processes such as cell proliferation, cell survival, and angiogenesis [1]. Overactivation of tyrosine kinases may result from kinase overexpression or deregulation of kinase activity and often leads to cancer. For example, epidermal growth factor receptor (EGFR) and Met kinase are often overexpressed in non-small cell lung cancer (NSCLC) tumors [2]. In almost all cases of chronic myeloid leukemia (CML), Abl kinase has been mutated to the constitutively active Bcr-Abl kinase through a chromosomal translocation between chromosomes 9 and 22 [3].
Tyrosine kinase inhibitors (TKIs) offer a targeted approach for treating cancers caused by overactive kinases. Since the 2001 emergence of imatinib mesylate, which inhibited Bcr-Abl kinase and changed the 5 year survival rate for CML from 37.7% to 89% [4, 5], much effort has gone into finding new inhibitors and identifying kinases to target. Approximately one third of current protein pharmaceutical targets are kinases [6]. Unfortunately, many patients do not respond to these drugs. While a lack of response to TKIs may be because a particular kinase is not overactive in a patient, it may also be because multiple kinases are overactive in a patient. Amplification of Met kinase is thought to be the cause of TKI resistance in about 20% of NSCLC patients who acquire resistance to EGFR inhibitors during treatment [7]. Mutations in an overactive kinase that allow it to avoid binding by kinase inhibitors may also confer resistance [8]. Second generation TKIs, such as the Bcr-Abl kinase inhibitors dasatinib and nilotinib, have been developed to circumvent some resistance issues such as particular kinase mutations [9]. Since TKIs select for nonresponsive cancer cells, these cells accumulate over time so that some patients develop resistance to a TKI during treatment. While PCR and oligonucleotides arrays can assess gene transcript levels and immunocytochemistry can qualitatively assess protein expression levels, these methods provide insufficient information on kinase activity and the efficacy of particular inhibitors for a particular patient [10]. A primary culture of patient cancer cells grown with an inhibitor could potentially predict the effect of specific TKIs, but this process requires days or weeks. Assessment of tumor response to treatment also requires at least weeks of treatment [11]. No method exists to quickly determine which kinases are overactive in a patient and which of these TKIs will be effective for that patient. A method is also needed to quickly determine if a patient has become resistant to a particular inhibitor. As the number of targeted kinases and available kinase inhibitors continues to increase, there is also a need for diagnostics with multiplexed detection of kinase activity.
Hydrogels offer a variety of advantages for biological diagnostics and have been used to detect mRNA and proteins, recognize glycoproteins, and measure kinase activity [12–18]. The hydrated, 3-D environment within hydrogels allows proteins to remain in a more native and active conformation, and tunable parameters such as stiffness and porosity may be modified to best fit the needs of the application [19]. Hydrogels may also be chemically modified for covalent attachment of proteins or other molecules [15].
Microfluidic devices permit precise spatial and temporal control over flow and require only small sample and reagent volumes. In addition, microfluidic devices are amenable to automation and may have a larger dynamic range than traditional biochemical assays [20, 21]. Several groups have developed electrochemical-based microchannel systems to detect cancer biomarkers such as prostate specific antigen, α-fetoprotein, and carcinoembryonic antigen [20].
Here we report a quantitative and specific multiplexed microfluidic assay using a hydrogel immobilized substrate for measuring the kinase activity of Met kinase and Abl kinase from cancer cells. In this assay, we immobilized substrates for both Met and Abl kinase onto macroporous hydrogel micropillars within a microchannel. We then incubated cancer cell lysates within these microchannels and measured the phosphorylation of the kinase specific substrates through fluorescence detection of a phosphotyrosine antibody. Macroporous hydrogels provide improved macromolecular transport rates over non-macroporous hydrogels and increase detection sensitivity [13]. Since we used whole cell lysate, which eliminates kinase loss during purification, and an immobilized substrate, our assay provided quantitative information on the kinase activity in the cell population. This assay specifically measured the kinase activity of both Abl and Met kinase in cancer cells and shows potential to measure the activity of up to 5 kinases within one microchannel. We also detected inhibition of Met kinase activity from cells that have been grown in the Met kinase inhibitor, PHA665752, simulating cells from a patient currently receiving tyrosine kinase treatment.
Materials and Methods
Producing GST fusion proteins
The previously described Escherichia coli BL21 strains containing the pGEX-4T1 vector with inserted amino acid sequences for Gab1 residues 431 to 561, Crkl residues 120 to 303, or EGFR pathway substrate 15 (Eps15) residues 758 to 881 were used to produce the fusion proteins GST-Gab1, GST-Crkl, and GST-Eps15 [12, 14, 22]. An additional BL21 strain containing the pGEX-4T1 vector with the inserted sequence for tensin residues 1392 to 1672 was used to produce GST-tensin. To produce these proteins, BL21 cells were grown in 2×YT medium (16 g tryptone, 10 g yeast extract, 5 g NaCl in 1 L H2O) to an OD600 of 0.6. Protein production was induced using 1 mM isopropyl-β-d-thiogalactopyranoside for 4 hours at 37 °C. Cells were centrifuged for 20 minutes. The supernatant was removed, and cells were washed with cold PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.7 mM KH2PO4) and centrifuged as before. The supernatant was again removed and BPER II Bacterial Protein Extraction Reagent with cOmplete Protease Inhibitor Cocktail was used according to manufacturer’s instructions to lyse the cells. To avoid clogging the purification column, the viscosity of the solution was reduced by mild sonication. The sample was sonicated for 15 seconds, followed by 45 seconds rest, and the sonication procedure was repeated 4 additional times. The lysate was passed through an 18 gauge syringe needle and centrifuged for 20 minutes at 3720 × g, and the resulting supernatant was recovered. The viscosity of the solution was further reduced by passing through a 25 gauge syringe needle and centrifuging a final time. The substrates were purified with a GST affinity column according to the manufacturer’s instructions and concentrated using a 30 kDa molecular weight cut off filter. The protein concentration was determined using a BCA assay and the purified protein was then aliquoted and stored at −80 °C until needed.
Cell culture
NCI-H1975 (H1975) lung adenocarcinoma cells, IMR-90 lung fibroblast cells, K562 CML cells, and HL60 acute myeloid leukemia cells were obtained from the American Type Culture Collection. H1975, K562, and HL60 cells were grown in RPMI-1640 medium supplemented with 300 mg/L glutamine and 10% fetal bovine serum as well as 100 units/ml penicillin and 100 µg/ml streptomycin. IMR-90 cells were grown in MEM medium with 10% fetal bovine serum. For passaging and harvesting the adherent cultures, H1975 and IMR-90, cells were detached from the flask using trypsin-EDTA (0.25% trypsin, 1 mM EDTA). To harvest all cultures, the cells were removed from the flask and centrifuged to form a pellet. They were then resuspended and incubated for 20 minutes in mammalian cell lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 100 mM NaF, 10 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate, 1% Triton X-100, 10% glycerol, cOmplete Protease Inhibitor Cocktail and 1 mM PMSF. The cells were centrifuged for 10 minutes at 10,000 rpm at 4 °C. The supernatant was removed and stored at −80 °C until use, and the final protein concentration was determined using a BCA assay.
In vitro solution-phase kinase assay
Solution phase kinase assays were performed by incubating 0.2 µg/µl GST-Gab1 or GST-Crkl, 0.2 µg/µl cell lysate, and 0.2 mM ATP in 1× kinase reaction buffer for 1 hour at 37 °C. Kinase reaction buffer contains 50 mM Tris HCl, 10 mM MgCl2, 2.5 mM MnCl2, 100 µM EDTA, 1 µM DTT, 0.015% Brij 35, and 0.01% BSA.. After the reaction, samples were denatured using 3× denaturing buffer (0.29 M sucrose, 0.12 M Tris base, 1% sodium dodecyl sulfate, and 2% β-mercaptoethanol) and run on a 10% polyacrylamide gel. The gel was then transferred to a nitrocellulose membrane and blocked in 1% bovine serum albumin (BSA) in Tris Buffered Saline (10 mM Tris-HCl, 100 mM NaCl) with 0.1% Tween-20 (TBST). Phosphorylated GST-Gab1 or phosphorylated GST-Crkl was detected using 0.25 µg/ml 4G10 phosphotyrosine antibody (Millipore) followed by a goat α-mouse HRP-conjugated antibody (0.1 µg/ml) (Invitrogen). The secondary antibody was detected using enhanced chemiluminescence.
Microchannel fabrication
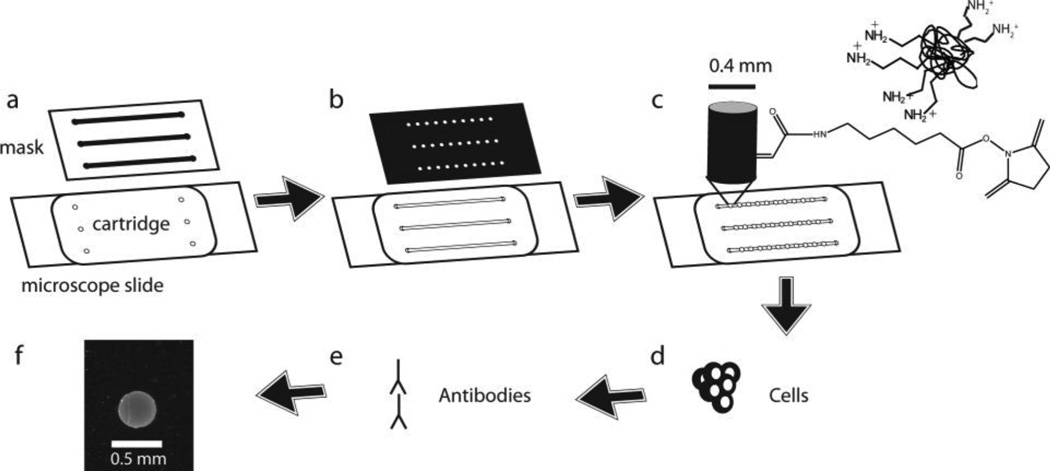
Microchannels were assembled as previously described [12, 13] and as shown in Figure 1. Briefly, we piranha-cleaned glass microscope slides and acrylic functionalized them with 2% (v/v) (3-acryloxypropyl)-trimethoxysilane. After placing a polycarbonate cartridge with 150 µM thick adhesive on the cleaned slides, we filled the slides with a prepolymer solution containing isobornyl acrylate. A transparency mask was placed over the cartridge and the solution was polymerized with 320–500 nm light at 5.9 mW/cm2 intensity for 13 seconds (EXFO OmniCure S1000) (Figure 1A). We rinsed the channels with ethanol and then polymerized the slides for another 13 seconds. The channels were then briefly incubated with Rain-X and then heated at 60 °C for 10 minutes to ensure the channels were dried.
Fig. 1.
Schematic of microchannel assay. a A polycarbonate cartridge is affixed onto a glass slide and filled with an isobornyl acrylate prepolymer solution. Microchannels are formed by UV polymerization of the solution through a transparency mask. b Microchannels are filled with a polyethylene glycol (PEG) diacrylate 700 solution containing PEG 3400. Another transparency mask is placed over the channels and macroporous hydrogel pillars are formed by UV polymerization. c Kinase substrates are covalently linked to the micropillars by covalent reaction of their primary amines with acryloyl-X, which is contained within the pillar. d Incubation of macroporous hydrogel micropillars with a kinase reaction solution results in phosphorylation of immobilized substrate protein. e Fluorescent antibodies are used to detect phosphorylated substrate. f Image of anti-phosphotyrosine/Alexa Fluor 488 immunofluorescent signal from macroporous hydrogel micropillar
Macroporous hydrogel micropillars were created in the microchannels as previously described 31 and as shown in Figure 1B. Channels were filled with a prepolymer solution containing MES buffer (0.1 M MES, 0.5 M NaCl, 0.05% Brij 35, pH 5.0) with 5% (v/v) polyethylene glycol (PEG) diacrylate 700, 20% (w/v) PEG 3400, 0.2 µg/µL Irgacure-2959, and 0.167% (w/v) 6-((acryloyl)amino)hexanoic acid, succinimidyl ester (acryloyl-X). Capillary action or a peristaltic pump was used to flow this solution and subsequent solutions through the microchannels. A transparency mask was placed over the slide and the micropillars were polymerized at 16.8 mW/cm2 intensity for 50 seconds.
After polymerization, the channels were washed briefly with cold PBSB (PBS with 0.05% Brij 35) and then a 4 mg/ml solution of a kinase substrate protein in PBSB was incubated in the channels in a humidity chamber for 1 hour at room temperature. The macroporous hydrogel micropillars contained acryloyl-X, which reacted with the exposed primary amines of the substrate protein and covalently linked the proteins to the macroporous hydrogel micropillars (Figure 1C). The channels were washed with PBSB, and the reaction was quenched for 20 minutes with freshly made 0.5 M hydroxylamine in PBSB at pH 8.0 to 8.5. The solution was then briefly washed with TBST and incubated for 30 minutes in 1% BSA in TBST.
Microchannel kinase assay
An immobilized phase kinase reaction was performed in the microchannels by incubating approximately 6 µl of a reaction solution containing kinase buffer, 0.2 mM ATP, and lysate from 16,600 cells (6 µg total protein) in each channel for 1 hour at 37 °C (Figure 1D). To fill the microchannels, a drop of solution was placed on the inlet side of the channel and allowed to flow into the channel. In this step and in further steps in the assay, a peristaltic pump was used to pull solution through the channel at a rate of up to 0.64 ml/min. Met kinase buffer (50 mM HEPES, 5 mM MgCl2, 5 mM MnCl2 0.02% BSA, and 0.25 mM DTT at pH 7.5) was used when only Met, EGFR, or focal adhesion kinase (FAK) kinase activity was being measured, and kinase buffer was used when Abl kinase activity was being measured. When EGFR kinase activity was measured, 10 µg/ml of EGF was also included in the reaction solution. After the reaction, the channels were washed, blocked, and incubated with 2 µg/mL 4G10 mouse α-phosphotyrosine antibody and 6 µg/mL rabbit α -GST antibody (Invitrogen) as previously described [12]. After washing again, the channels were incubated with a secondary antibody solution containing Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 donkey anti-rabbit (Invitrogen) at 10 µg/mL overnight at 4 °C. After washing again for 1 hour in TBST, the channels were fluorescently imaged with an inverted epifluorescence microscope (Olympus IX70) with an attached monochrome CCD digital camera. The channels were imaged for fluorescent signals resulting either from the presence of GST or the presence of phosphorylated tyrosines (Figures 1E and 1F).
Growth of cells in kinase inhibitor
H1975 cells were detached using trypsin and centrifuged to form a pellet. Cells were then resuspended in growth media and counted with a hemocytometer. An appropriate volume of cell containing media and fresh media was mixed such that the solution contained 230,000 cells/ml. Each well of a 24 well plate received 495 µl of this solution so that each well contained 114,000 cells. 5µl of either DMSO or an inhibitor solution dissolved in DMSO containing PHA665752, erlotinib, or both PHA665752 and erlotinib was added to each of the wells. On days 1, 3, and 5 after plating, cell counts were recorded for one well at each of the inhibitor concentrations. The cells were then centrifuged and lysed as previously described for cell harvesting.
Substrate immobilization for multiplexed kinase detection
To immobilize proteins in an alternating GST-Gab1 and no substrate pattern, we first polymerized the two hydrogel pillars on the outlet end of the microchannel. These pillars were then washed with PBSB and incubated with 4 mg/ml GST-Gab1 in PBSB for 1 hour. The reaction was quenched with 0.5 M hydroxylamine inserted from the outlet end of the channel and in a small enough volume as to not reach the inlet end of the channel. After 20 minutes, the quenching solution was removed and the channels were washed with PBSB. The two hydrogel pillars adjacent to the previously polymerized pillars were polymerized, washed with PBSB, and immediately quenched as just described. This procedure continued until all 5 sets of hydrogel pillars had been polymerized and sets 1, 3, and 5 had been incubated with a GST-Gab1 solution. The channels were then washed with TBST followed by 1% BSA in TBST and the reaction proceeded as previously described.
To immobilize GST-Crkl and GST-Gab1 proteins in the microchannels, we first polymerized the four hydrogel pillars on the outlet side of the microchannel, washed these pillars with PBSB, and incubated them with 4 mg/ml GST-Crkl. The reaction was quenched with 0.5 M hydroxylamine inserted from the outlet end of the channel and in a small enough volume as to not reach the inlet end of the channel. After 20 minutes, the quenching solution was removed and the channels were washed with PBSB. Next, the 4 hydrogel pillars on the inlet side of the slide were polymerized, washed with PBSB, and incubated with GST-Gab1. The pillars were washed with PBSB, briefly quenched, and washed again with PBSB. The final two pillars in the middle of the channel were then polymerized and the whole channel was quenched for 20 minutes with 0.5 M hydroxylamine. The channels were then washed with TBST followed by 1% BSA in TBST and the reaction proceeded as previously described.
Immunodepletion of kinases
H1975 cell lysate was depleted of FAK by incubating the lysate overnight at 4 °C with FAK MAB2156 (0.03 mg/ml) (Millipore) followed by incubation for 2 hours at room temperature using Protein G agarose (final concentration 22.5% Protein G resin). The immunodepletion of H1975 lysate was then continued using Pierce’s immunoprecipitation procedure for Protein G agarose. H1975 cell lysate was depleted of EGFR kinase in a similar method using EGFR rabbit polyclonal antibody (0.03 mg/ml) (Santa Cruz Biotechnology).
Data analysis
Fluorescence signal intensity was analyzed for brightness of the hydrogel pillar using Matlab as previously described [12]. For the microchannel data, we normalized the data in each experiment. We then averaged the normalized signals of each pillar from 3 independent experiments. To determine significance among samples, we used an ANOVA single factor test and defined significance to be a 95% confidence level unless otherwise noted.
Results
Immobilization of substrate on specific pillars within microchannels
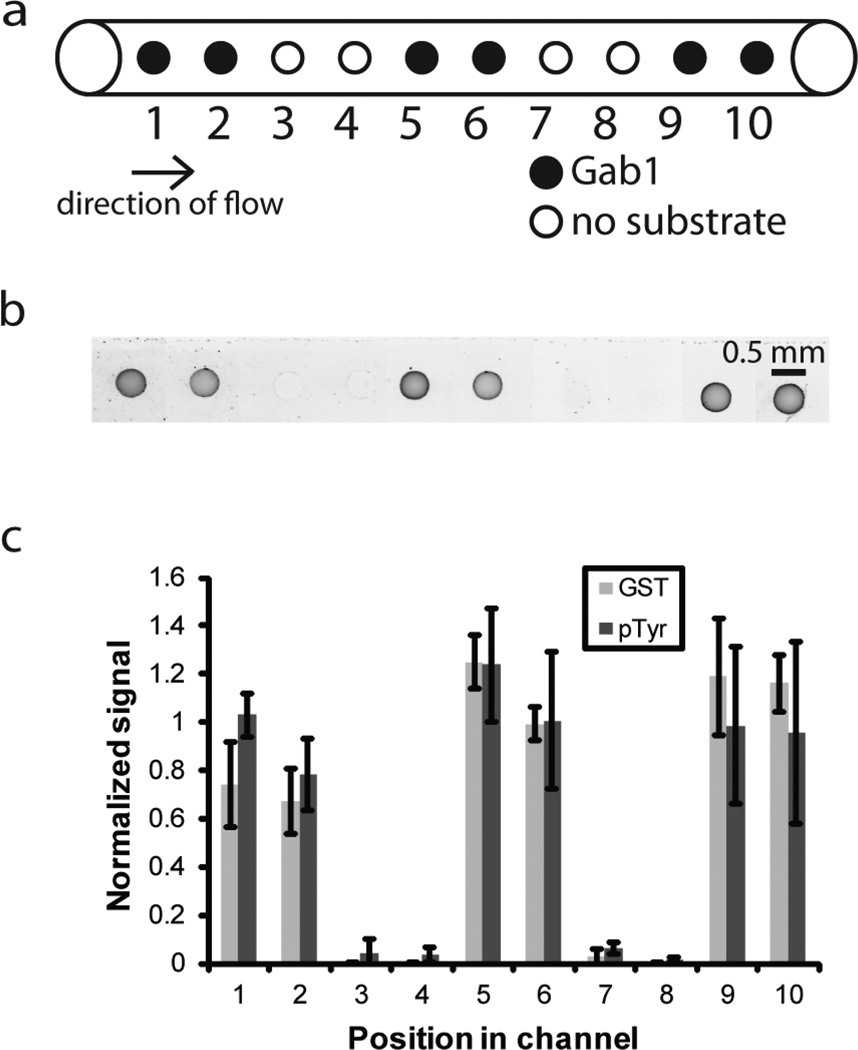
In order to create a multiplexed microchannel assay, we began by immobilizing either GST-Gab1 or no substrate in alternating sets of 2 hydrogel pillars, as shown in Figure 2A. Gab1 is a Grb2 binding protein that interacts with Met kinase via a proline rich domain and has previously been identified as a substrate suitable for detecting Met kinase activity [12, 23]. High expression and activity levels of the receptor tyrosine kinase Met have been linked to a poor prognosis in NSCLC and Met kinase has been shown to cause resistance to EGFR inhibitors in some NSCLC patients [24].
Fig. 2.
Immobilization of kinase substrate in alternating sets of pillars in microchannels. GST-Gab1 was immobilized at alternating sets of micropillars in a microchannel. No substrate was immobilized on the micropillars that did not contain GST-Gab1. a Schematic showing micropillars with and without immobilized GST-Gab1 in a microchannel. b Antiphosphotyrosine/Alexa Fluor 488 immunofluorescent images of macroporous hydrogel micropillars containing immobilized GST-Gab1 in the microchannel incubated with H1975 lysate. Image color is inverted. c Anti-GST/Alexa-Fluor 594 (GST) and anti-phosphotyrosine/Alexa Fluor 488 (pTyr) signal intensity of macroporous hydrogel micropillars containing GST-Gab1 or no substrate and incubated with H1975 lysate. Position in channel corresponds to the position of the spot starting from the inlet side of the channel. Signal intensity is the average gray value of the micropillar minus the average gray value of the background in the area immediately surrounding the micropillar. Three experiments were performed and the results of each experiment were normalized by the average value for the micropillars containing GST-Gab1 in that experiment. Error bars = standard deviation
Briefly, we created microchannels on a microscope slide by photopolymerization of an isobornyl acrylate solution. We then formed two PEG diacrylate hydrogel pillars containing acryloyl-X on the outlet side of the channel and incubated these channels with GST-Gab1, allowing primary amines of GST-Gab1 to covalently react with acryloyl-X. We continued forming sets of hydrogel pillars with either GST-Gab1 or no substrate until we created 10 pillars in the channel (Figure 2A). This approach allowed us to measure the amount of protein immobilized at each pillar by detecting fluorescent signal with an α-GST antibody.
The lung adenocarcinoma cell line, H1975, is known to overexpress Met kinase [25]. We then incubated 6 µg of H1975 cell lysate, corresponding to 16,600 cells, in a kinase reaction solution in the channels to measure phosphorylation of the immobilized GST-Gab1. As shown in Figure 2B–C, there was a significant difference in GST signal between the hydrogel pillars with immobilized GST-Gab1 and the hydrogel pillars with no immobilized substrate. Figure 2B and 2C also show that the immobilized substrate could still be phosphorylated by H1975 lysate even after multiple rounds of pillar immobilization and that there was no significant difference in phosphorylation based on pillar position.
Solution phase detection of kinase activity of Abl and Met kinase in cancer lysates
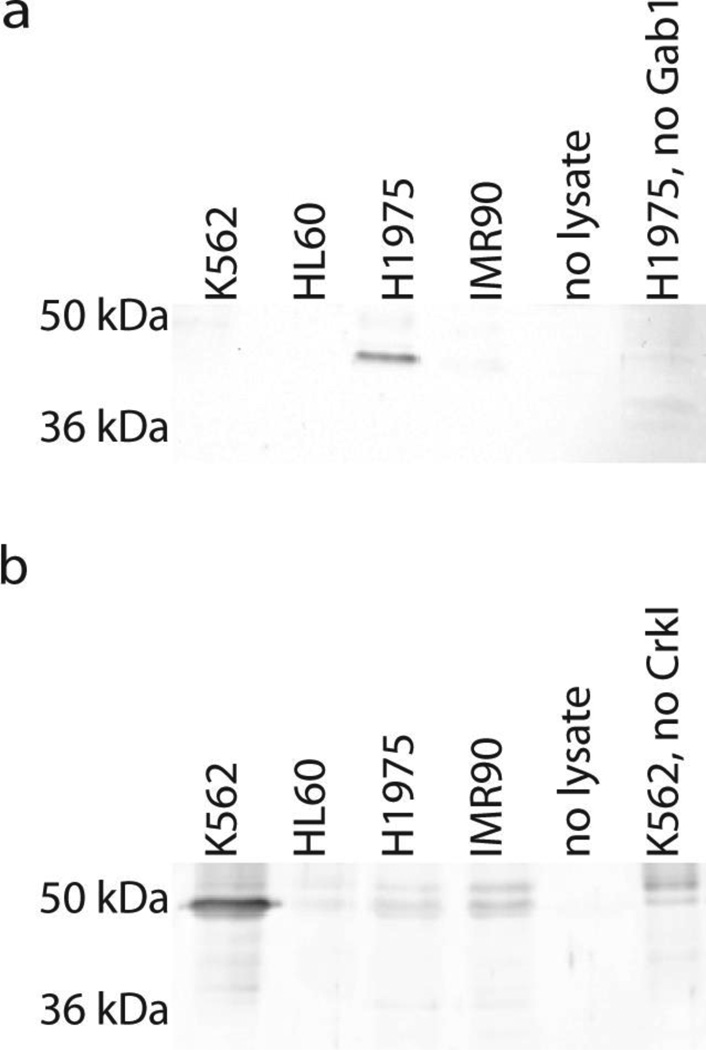
We next extended the assay to simultaneously detect the kinase activity of both Met and Abl kinases. We chose 4 different cell lysates in which to measure the activity of these kinases. K562 is a CML cell line and has previously been shown to possess high Bcr-Abl activity [13]. The HL60 cell line was derived from an acute promyelocytic leukemia patient and does not express Bcr-Abl [26]. We also used H1975 cells and the non-cancerous IMR-90 fetal lung fibroblast cells. To verify the differences in Met and Abl kinase activity in each of these cell lines, we performed solution phase kinase reactions with each of these cell lines with either GST-Gab1 or GST-Crkl. Previous kinase reactions for Abl and Met kinase activity had used different buffers for each kinase, with one notable difference being the presence of MnCl2 in the Met kinase reaction buffer [12, 13]. Since neither Met nor Abl kinase activity was detected in a kinase reaction performed in the buffer for the other kinase (not shown), we added 2.5 mM MnCl2 to the Abl kinase buffer. Using this buffer we observed strong phosphorylation of GST-Gab1 only by H1975 lysate (Figure 3A). We also observed much stronger phosphorylation of GST-Crkl by the K562 lysate than by any other lysates (Figure 3B). These results demonstrate specific detection of Met and Abl activity in a common kinase reaction buffer and provide a comparison for the immobilized kinase assay.
Fig. 3.
Solution phase detection of Met and Abl kinase activity in cell lysates. a Western blot analysis for the detection of tyrosine phosphorylated GST-Gab1 after solution phase in vitro kinase reactions with lysates from K562, HL60, H1975, and IMR-90 cells. b Western blot analysis for the detection of tyrosine phosphorylated GST-Crkl after solution phase in vitro kinase reactions with lysates from K562, HL60, H1975, and IMR-90 cells
Multiplexed detection of Abl and Met kinase activity in microchannels
To prepare microchannels for multiplexed detection of Abl and Met kinase, we photopolymerized 4 micropillars on the outlet side of the microchannel and incubated these pillars with either GST-Crkl or GST-Gab1 (Figure 4A). We carried out replicates of the experiment with GST-Crkl immobilized on the outlet side or with GST-Gab1 immobilized on the outlet side to verify that the position of the substrate in the channel did not affect the relative phosphorylation by different lysates. Next we polymerized 4 micropillars on the inlet side of the microchannel and incubated these pillars with the remaining substrate. Finally, we polymerized two pillars in the center of the channel but did not incubate these pillars with any substrate. We then incubated the channels with one of the four cell lysates. Similarly to the solution phase results, H1975 phosphorylated GST-Gab1 and K562 phosphorylated GST-Crkl to a significantly greater extent than the other lysates (Figures 4B and 4C).
Fig. 4.
Multiplexed detection of Met and Abl kinase activity in the presence and absence of Met and Abl kinase inhibitors. a Schematic of the arrangement of micropillars immobilized with GST-Gab1, GST-Crkl, or no substrate. The position of GST-Gab1 and GST-Crkl was switched in one replicate of the experiments. b Anti-phosphotyrosine/Alexa Fluor 488 immunofluorescent images of macroporous hydrogel micropillars containing immobilized GST-Gab1 and incubated with K562, HL60, H1975, or IMR-90 lysates. During some of the incubations, the Met kinase inhibitor PHA665752 or the Abl kinase inhibitor imatinib mesylate was also included. c Antiphosphotyrosine/Alexa Fluor 488 immunofluorescent images of macroporous hydrogel micropillars containing immobilized GST-Crkl and incubated with K562, HL60, H1975, or IMR-90 lysates. During some of the incubations, the Met kinase inhibitor PHA665752 or the Abl kinase inhibitor imatinib mesylate was also included. All microchannels contained 4 macroporous hydrogel micropillars with immobilized GST-Gab1, 4 macroporous hydrogel micropillars with immobilized GST-Crkl, and 2 macroporous hydrogel micropillars with no substrate. Signal intensity is the average gray value of the micropillar minus the average gray value of the background in the area immediately surrounding the micropillar. For each graph, three experiments were performed and the values for each experiment were normalized by the average value for the no inhibitor sample in that experiment. Error bars=SD
To test whether the substrate phosphorylation was the direct result of Met kinase or Abl kinase activity in the cell lysates, we also included PHA665752, a Met kinase inhibitor, or imatinib mesylate, an Abl kinase inhibitor, in some of the reactions [27, 28]. Met kinase inhibition by PHA665752 significantly diminished phosphorylation of the Gab1 substrates by H1975 lysates but not other lysates, indicating that the elevated Gab1 phosphorylation was the result of Met kinase activity in H1975 cells (Figure 4B). Similarly, imatinib mesylate decreased phosphorylation of immobilized Crkl by K562 cell lysate (Figure 4C). These results suggest that a multiplexed kinase assay has potential predictive value for determining the activity and specificity of a kinase inhibitor in a particular patient.
Inhibition of cell growth by Met kinase inhibitors
Hydrogel-immobilized substrates also have potential to be used as a tool to monitor the effects of TKI therapies on kinase activity. To simulate this situation, we cultured cells in the presence of TKIs. We first examined the effect of growing H1975 cells in the presence of the Met kinase inhibitor PHA665752. As shown in Figure 5A, we grew cells in 5 concentrations of PHA665752 varying from 0.1 µM to 10 µM as well as control cells in the absence of inhibitor. Cells were then counted and harvested on days 1, 3, or 5 after plating and the kinase assay was performed on these cells to measure Met kinase activity. Figure 5B shows that the cells grown in the highest concentrations of PHA665752 showed slightly decreased expansion as compared to cells grown in cell culture medium without PHA665752. To determine if this growth inhibition was significant, we calculated the specific growth rate at each of the growth conditions using Equation 1
| (1) |
where X is the number of cells at a specific day, X0 is the initial number of cells, µ is the specific growth rate (days −1), and t is time (days). We then plotted ln (X/X0) versus t, and fit a linear regression line through the data to calculate growth rate. As shown in Table 1, there was significant (p<0.05) growth inhibition when cells were cultured in 3 µM or 10 µM PHA665752. We also observed a significant decrease in the Met kinase activity of cells grown in higher concentrations of PHA665752, as shown in Figure 5C. Since previous literature has reported a synergistic effect between Met and EGFR TKIs in some lung cancer cells, including H1975 cells [2], we next decided to grow H1975 cells in PHA665752 and erlotinib, an EGFR inhibitor. We maintained the concentration of erlotinib constant at 3 µM and again varied the concentration of PHA665752 from 0.1 µM to 10 µM. In this experiment, while we detected a statistically significant decrease in cell growth rate with only erlotinib, we observed an even greater decrease in growth rate when PHA665752 was also included at a concentration of 3 µM or 10 µM, consistent with the notion that both Met and EGFR regulate H1975 cell proliferation (Figure 5D and Table 2).
Fig. 5.
Growth of H1975 cells in Met kinase inhibitor PHA665752. a Schematic of cell growth conditions. H1975 cells were grown in a 24 well plate in no inhibitor or in varying concentrations of PHA665752 and harvested 1, 3, or 5 days after plating. b Number of H1975 cells per well after 1, 3, or 5 days of growth in a 24 well plate in varying concentrations of PHA665752. c Antiphosphotyrosine/Alexa Fluor 488 immunofluorescent images of macroporous hydrogel micropillars containing immobilized GST-Gab1 and incubated with cells that had been grown in varying concentrations of PHA665752 for 1, 3, or 5 days. Signal intensity is the average gray value of the micropillar minus the average gray value of the background in the area immediately surrounding the micropillar. d Number of H1975 cells per well after 1, 3, or 5 days of growth in a 24 well plate in varying concentrations of Erlotinib and PHA665752. For each graph, three experiments were performed and the values for each experiment were normalized by the average value for the channel that did not contain a kinase inhibitor in that experiment. Error bars=SD
Table 1.
Specific growth rate of H1975 cells grown in Met kinase inhibitor PHA665752
| PHA665752 (µM) | 0 | 0.1 | 0.3 | 1 | 3 | 10 |
| Specific growth rate (days −1) | 0.205* | 0.214 | 0.203 | 0.188 | 0.166* | 0.157 |
| Standard deviation | 0.018 | 0.026 | 0.021 | 0.002 | 0.015 | 0.021 |
Specific growth rates were calculated by fitting a linear regression line to the data for each growth condition based on Equation 1. Specific growth rates were calculated for each of three experiments were then averaged together.
denotes statistical significance at 95% confidence
Table 2.
Specific growth rate of H1975 cells grown in EGFR kinase inhibitor erlotinib and Met kinase inhibitor PHA665752
| Erlotinib (µM) | 0 | 3 | 3 | 3 | 3 | 3 |
| PHA665752 (µM) | 0 | 0 | 0.1 | 1 | 3 | 10 |
| Specific growth rate (days −1) | 0.223* | 0.183* | 0.173 | 0.170 | 0.138* | 0.010 |
| Standard deviation | 0.002 | 0.014 | 0.026 | 0.018 | 0.020 | 0.026 |
Specific growth rates were calculated by fitting a linear regression line to the data for each growth condition based on Equation 1. Specific growth rates were calculated for each of three experiments and then averaged together.
denotes statistical significance at 95% confidence
FAK and EGFR activity in microchannels
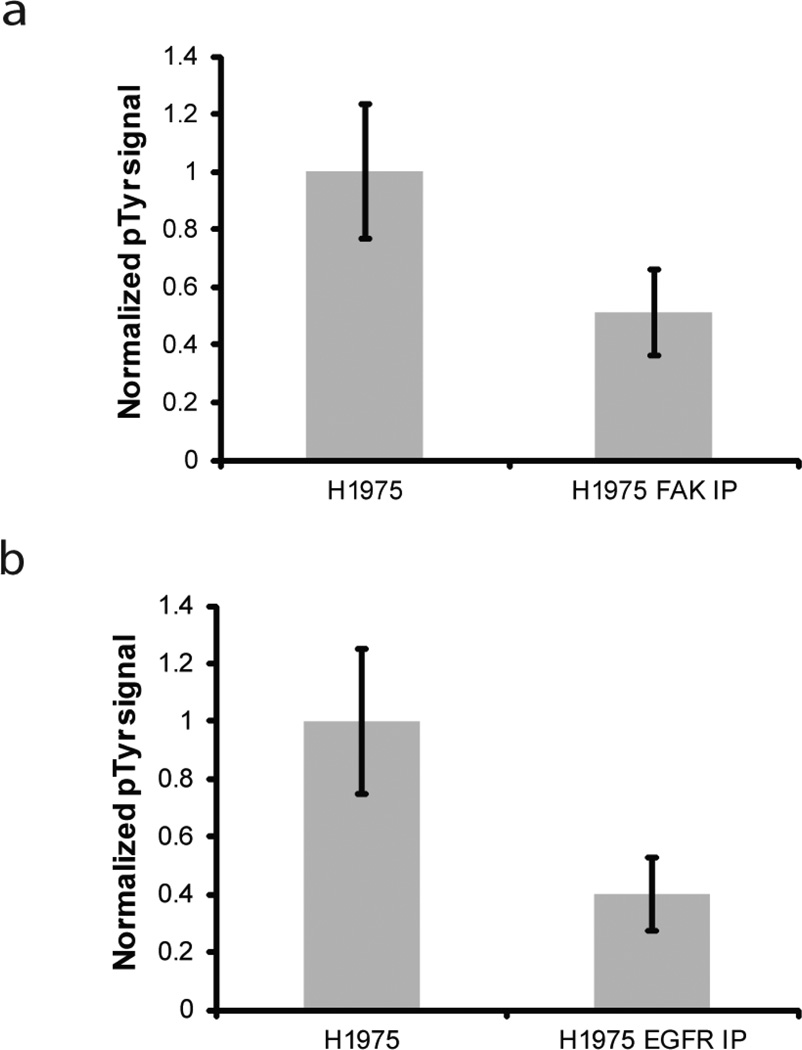
About 50 genes known to be related to cancer encode for protein kinases and 150 kinase targeted drugs are in development for a variety of kinases [6]. Design of personalized cancer therapies may benefit from monitoring a large subset of these cancer-associated kinases. In our initial experiments alternating the immobilization of Gab1 and no substrate, we showed that up to 5 sets of substrates could be immobilized per channel, with one duplicate for each substrate. As a proof-of-concept for multiplexing kinase assay quantification, we investigated two additional tyrosine kinases, FAK and EGFR, in addition to Met and Abl. FAK is a non-receptor tyrosine kinase associated with angiogenesis and up-regulated in some breast, colon, thyroid, prostate, oral, neck, lung and ovarian cancers[1]. EGFR is a receptor tyrosine kinase in the ErbB family and has been linked to several cancers, including glioblastoma, head and neck cancer, NSCLC, breast cancer, colorectal cancer, and pancreatic cancer [29]. FAK is known to phosphorylate tensin on tyrosine residues [30, 31] and a previous immobilized phase kinase assay identified Eps15 as a phosphorylation substrate that could be used for measuring the kinase activity of EGFR [14]. H1975 lysate is known to overexpress both EGFR and FAK [25]. To determine if the kinase assay could specifically measure EGFR and FAK activity, we immunodepleted H1975 lysate of either EGFR or FAK. We then used our immobilized phase kinase assay to measure phosphorylation of either GST-tensin or GST-Eps15 by these lysates. As shown in Figure 6A–B, we detected decreased phosphorylation of GST-tensin and GST-Eps15 when the lysate was depleted of FAK or EGFR, respectively, showing that the assay could detect kinase activity of these two kinases.
Fig. 6.
Specific detection of FAK and EGFR kinase activity in microchannels. a Antiphosphotyrosine/Alexa Fluor 488 signal intensity of macroporous hydrogel micropillars containing GST-tensin for H1975 lysate and FAK depleted H1975 lysate. b Antiphosphotyrosine/Alexa Fluor 488 signal intensity of macroporous hydrogel micropillars containing GST-Eps15 for H1975 lysate and EGFR depleted H1975 lysate. Signal intensity is the average gray value of the micropillar minus the average gray value of the background in the area immediately surrounding the micropillar. Three experiments were performed and the results of each experiment were normalized by the average value for H1975 lysate in that experiment. Error bars = standard deviation. Difference in phosphorylation signal was significant at 95% confidence level for both a and b
Discussion
Multiplexed detection provides an opportunity to assay for multiple kinases more quickly and with smaller sample sizes than would be needed to assay for each component separately. Kinase inhibitors are available for an increasing number of kinases, with FDA approved inhibitors for at least 10 kinases available in 2012 [6], and there are frequently multiple kinases which may be overactive in a particular type of cancer. It is therefore often desirable to examine the activity of multiple kinases in a particular patient to determine which kinase inhibitors may be effective for that patient. Previous work using the microchannel system described in this paper has shown that the Met kinase activity of as few as 150 H1975 cells can be detected in a single microchannel as determined by dilution of cell lysate and detection with 95% confidence of phosphorylation signal above the signal levels obtained with no lysate [12]. By immobilizing the substrates for two kinases into a single microchannel, we have demonstrated the ability to measure the activity of two kinases without increasing the sample size. Furthermore, by alternating immobilization of Gab1 and no substrate, we have demonstrated a possibility of measuring the activity of up to 5 kinases in a single microchannel, potentially further decreasing the sample size needed per kinase. In contrast, previous assays have required at least several hundred to several thousand cells to measure the activity of one kinase [13, 32]. Multiplexed detection also decreases the assay time per kinase. While it requires about 12 hours to perform the assay on 12 samples for one kinase, only an additional 2.5 hours are required for immobilizing a second substrate onto the slide to detect a second kinase. Since new microchannels and micropillars must be fabricated for each use and labor costs are expected to be the most expensive component of this assay, this is a significant cost reduction per kinase tested.
Several aspects of this assay need to be further developed for reliable multiplexed detection of additional kinases. While the signal intensity of 10 micropillars may be averaged together to generate a kinase activity profile for one kinase, this value falls to 4 micropillars per kinase when two kinase substrates are immobilized and 2 pillars are used as no substrate controls. For detection of 5 or even 10 kinases per channel, only 1 or 2 micropillars are available for detecting the activity of each kinase so that accurate signal intensity from each micropillar becomes critical and variations between microchannels or micropillars are less tolerable. While we demonstrate the reproducibility of this assay by detecting specific activity of two kinases with 95% confidence over 3 independent replicates, a more consistent microchannel fabrication method may be needed for detection of additional kinases. Hot embossing of thermoplastics has been used to create microchannels ranging from tens of micrometers to millimeters and offers potential for rapid and high fidelity fabrication of microchannels [33]. Another challenge of creating a multiplexed assay is identifying appropriate substrates for detection of each kinase. These substrates must be specifically phosphorylated by only one tyrosine kinase in the cell lysate. While a thorough literature search will likely reveal potential substrate candidates, in vitro verification of the sensitivity and specificity of each of these substrates will be necessary.
While the use of kinase inhibitors has revolutionized the way certain cancers are treated, choosing an effective kinase inhibitor or set of kinase inhibitors for a particular patient is still a challenge. For example, only some patients with NSCLC exhibit overactivity of EGFR kinase [34]. Additionally, only about 75% of patients with overactive EGFR will respond to the EGFR inhibitors erlotinib or gefitinib [34]. In some cases, this resistance is caused by an additional mutation in the EGFR kinase [35]. In other cases, however, the resistance results from overactivity of another kinase, such as Met kinase, and could potentially be treated by a combination of both Met and EGFR kinase inhibitors [2]. A predictive method is needed to determine which of these inhibitors or combinations of inhibitors a patient would be most likely to respond to. In this assay, we included the kinase inhibitors PHA665752 and imatinib mesylate during the kinase reaction step of the assay. Inhibition of substrate phosphorylation showed that PHA665752 was successful at inhibiting Met kinase in H1975 cells and that imatinib mesylate successfully inhibited Bcr-Abl kinase in K562 cells. Use of these inhibitors during the kinase reaction step for reactions including patient samples could show whether a particular patient is susceptible or resistant to a particular kinase before a patient even begins receiving the inhibitor.
While our results showed the substrate-immobilized hydrogels could be used to measure the activity of FAK and EGFR kinase, the fluorescent signal was low even for H1975 lysate that had not been depleted of FAK or EGFR. Although the number of cells used in this experiment, 16,600 cells per microchannel, would commonly be available from a clinical sample, such as a fine needle aspirate, we would prefer to use fewer cells so that some of the sample can be saved for other purposes or in case the sample also contained a large number of non-cancerous cells [36]. Sensitivity limitations may also present a challenge for assaying actual patient samples in which FAK or EGFR activity may be present at lesser yet still significant levels. These results indicate that further optimization of parameters such as reaction time, substrate concentration, and buffer is likely needed for reliable detection of these kinases.
We also investigated the effect of kinase inhibitors during cell growth. Since resistance to inhibitors often develops during the course of treatment [35], it is important to measure changes in kinase activity in patients who are currently taking a kinase inhibitor. By growing cells in PHA665752 and then measuring Met kinase activity in these cells, we showed that kinase activity during cell growth, or potentially in a patient tumor, could be assessed with this assay. Likewise, we expect that resistance to inhibitors through kinase mutation would also be observed as increased Met kinase activity. Monitoring kinase activity from a patient treated with a kinase inhibitor could also provide information on the mechanism of the resistance. If a patient has developed resistance due to another overactive kinase in the tumor, the TKI would still be effective at inhibiting the activity of a particular kinase, but not at inhibiting cell growth, similar to H1975 cells grown only in PHA665752. Mutations in the kinase targeted by TKIs that prevented its inhibition would appear as increased phosphorylation of the hydrogel pillars. This information could be valuable in helping clinicians to choose second generation TKIs targeting the same kinase or to choose additional TKIs targeting other kinases.
When inhibitor resistance is caused by the over-activation of multiple kinases in cancer cells [35], a method is needed to determine which combination of inhibitors may be effective even when an inadequate effect on cell growth is seen with one inhibitor. Our data show that although both Met inhibition by PHA665752 and EGFR inhibition by erlotinib were needed for the greatest inhibition of H1975 cell growth, Met kinase activity and susceptibility of this activity to PHA665752 could be detected with this assay even when the cells were grown only in PHA665752. By detecting the kinase activity and inhibition of particular kinases and inhibitors even when effects on cell growth are small, this assay has potential to predict combinations of inhibitors that may be effective for a particular patient. This information could be useful for patients who do not adequately respond to Met or EGFR inhibition alone, but may respond to a combination of inhibitors.
Conclusions
In this work, we developed a multiplexed kinase activity assay to detect the activity of Met and Abl kinases. We showed that as many as 5 different sets of duplicate substrate spots can be immobilized per channel offering the possibility of detecting up to 5 kinases in a single channel. We also showed that this assay could detect Met kinase inhibition in cells grown in the Met kinase inhibitor PHA665752. This system has potential to be developed into a clinical diagnostic to determine which kinases are overactive in a particular patient and to predict to which inhibitors of those kinases a particular patient may respond. This device may also potentially be used to continue to monitor patients during kinase inhibitor treatment to quickly detect resistance to kinase inhibitors.
Acknowledgements
This work was supported by the NIH/NIGMS Grant 1R01GM074691, a doctoral fellowship from the NIH Biotechnology Training Program (Grant 5T32 GM-08349), and the NSF Graduate Research Fellowship Program (Grant DGE-0718123).
Abbreviations
- BSA
Bovine serum albumin
- CML
Chronic myeloid leukemia
- EGFR
Epidermal growth factor receptor
- Eps15
EGFR pathway substrate 15
- FAK
Focal adhesion kinase
- NSCLC
Non-small cell lung cancer
- PBS
Phosphate buffered saline
- PBSB
PBS with 0.05% Brij 35
- PEG
Polyethylene glycol
- TBST
Tris buffered saline with 0.1% Tween 20
- TKI
Tyrosine kinase inhibitor
References
- 1.Lechertier T, Hodivala-Dilke K. Focal adhesion kinase and tumour angiogenesis. J Pathol. 2012;226:404–412. doi: 10.1002/path.3018. [DOI] [PubMed] [Google Scholar]
- 2.Tang Z, Du R, Jiang S, Wu C, Barkauskas DS, Richey J, Molter J, Lam M, Flask C, Gerson S, Dowlati A, Liu L, Lee Z, Halmos B, Wang Y, Kern JA, Ma PC. Dual MET-EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant lung cancer. Br J Cancer. 2008;99:911–922. doi: 10.1038/sj.bjc.6604559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87:1037–1045. doi: 10.1002/ajh.23282. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Homer MJ, editors. Cancer Survival Among Adults - US SEER Program, 1988-2001 - SEER Publications. National Cancer Institute, SEER Program; 2007. [Google Scholar]
- 5.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MWN, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Fabbro D, Cowan-Jacob SW, Möbitz H, Martiny-Baron G. Targeting cancer with small-molecular-weight kinase inhibitors. Methods Mol Biol. 2012;795:1–34. doi: 10.1007/978-1-61779-337-0_1. [DOI] [PubMed] [Google Scholar]
- 7.Ma C, Wei S, Song Y. T790M and acquired resistance of EGFR TKI: a literature review of clinical reports. J Thoracic Dis. 2011;3:10–18. doi: 10.3978/j.issn.2072-1439.2010.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, Pan Y, Wang L, de Stanchina E, Shien K, Aoe K, Toyooka S, Kiura K, Fernandez-Cuesta L, Fidias P, Yang JCH, Miller VA, Riely GJ, Kris MG, Engelman JA, Vnencak-Jones CL, Dias-Santagata D, Ladanyi M, Pao W. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A. 2012;109:E2127–E2133. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilhot F, Roy L, Tomowiak C. Current treatment strategies in chronic myeloid leukemia. Curr Opin Hematol. 2012;19:102–109. doi: 10.1097/MOH.0b013e32834ff610. [DOI] [PubMed] [Google Scholar]
- 10.Galanina N, Bossuyt V, Harris LN. Molecular predictors of response to therapy for breast cancer. Cancer journal (Sudbury, Mass.) 2011;17:96–103. doi: 10.1097/PPO.0b013e318212dee3. [DOI] [PubMed] [Google Scholar]
- 11.Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, Phelps ME, Weber WA, Czernin J, Allen-Auerbach MS. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2011;52:1684–1689. doi: 10.2967/jnumed.111.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers AD, Liu B, Lee AG, Palecek SP. Macroporous hydrogel micropillars for quantifying Met kinase activity in cancer cell lysates. Analyst. 2012;137:4052–4061. doi: 10.1039/c2an35464k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AG, Beebe DJ, Palecek SP. Quantification of kinase activity in cell lysates via photopatterned macroporous poly(ethylene glycol) hydrogel arrays in microfluidic channels. Biomed microdevices. 2012;14:247–257. doi: 10.1007/s10544-011-9602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh G, Lee AG, Palecek SP. Hydrogel-based protein array for quantifying epidermal growth factor receptor activity in cell lysates. Anal Biochem. 2009;393:205–214. doi: 10.1016/j.ab.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi NW, Kim J, Chapin SC, Duong T, Donohue E, Pandey P, Broom W, Hill WA, Doyle PS. Multiplexed detection of mRNA using porosity-tuned hydrogel microparticles. Anal Chem. 2012;84:9370–9378. doi: 10.1021/ac302128u. [DOI] [PubMed] [Google Scholar]
- 16.Hall B, Jones L, Forrest JA. Measuring the kinetics and activity of adsorbed proteins: In vitro lysozyme deposited onto hydrogel contact lenses over short time periods. J Biomed Mater Res A. 2013;101:755–764. doi: 10.1002/jbm.a.34357. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Leulmi RF, Juncker D. Hydrogel droplet microarrays with trapped antibody-functionalized beads for multiplexed protein analysis. Lab Chip. 2011;11:528–534. doi: 10.1039/c0lc00291g. [DOI] [PubMed] [Google Scholar]
- 18.Miyata T, Hayashi T, Kuriu Y, Uragami T. Responsive behavior of tumormarker- imprinted hydrogels using macromolecular cross-linkers. J Mol Recognit. 2012;25:336–343. doi: 10.1002/jmr.2190. [DOI] [PubMed] [Google Scholar]
- 19.Aurand ER, Lampe KJ, Bjugstad KB. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci Res. 2012;72:199–213. doi: 10.1016/j.neures.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogojevic D, Chamberlain MD, Barbulovic-Nad I, Wheeler A R. A digital microfluidic method for multiplexed cell-based apoptosis assays. Lab Chip. 2012;12:627–634. doi: 10.1039/c2lc20893h. [DOI] [PubMed] [Google Scholar]
- 22.Brueggemeier SB, Wu D, Kron SJ, Palecek SP. Protein-acrylamide copolymer hydrogels for array-based detection of tyrosine kinase activity from cell lysates. Biomacromolecules. 6:2765–2775. doi: 10.1021/bm050257v. [DOI] [PubMed] [Google Scholar]
- 23.Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- 24.Sierra JR, Tsao M-S. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol. 2011;3:S21–S35. doi: 10.1177/1758834011422557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 27.Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, Lipson KE, Ramphal J, Do S, Cui JJ, Cherrington JM, Mendel DB. A Selective Small Molecule Inhibitor of c-Met Kinase Inhibits c-Met-Dependent Phenotypes in Vitro and Exhibits Cytoreductive Antitumor Activity in Vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 28.Druker BJ. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol Med. 2002;8:S14–S18. doi: 10.1016/s1471-4914(02)02305-5. [DOI] [PubMed] [Google Scholar]
- 29.Gan HK, Burgess AW, Clayton AHA, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 30.Petch L, Bockholt S, Bouton A, Parsons J, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J. Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 31.Zhu T. Growth Hormone Stimulates the Tyrosine Phosphorylation and Association of p125 Focal Adhesion Kinase (FAK) with JAK2. FAK is not required for STAT-mediated transcription. J Biol Chem. 1998;273:10682–10689. doi: 10.1074/jbc.273.17.10682. [DOI] [PubMed] [Google Scholar]
- 32.Fang C, Wang Y, Vu NT, Lin WY, Hsieh YT, Rubbi L, Phelps ME, Müschen M, Kim YM, Chatziioannou AF, Tseng HR, Graeber TG. Integrated microfluidic and imaging platform for a kinase activity radioassay to analyze minute patient cancer samples. Cancer Res. 2010;70:8299–8308. doi: 10.1158/0008-5472.CAN-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greener J, Li W, Ren J, Voicu D, Pakharenko V, Tang T, Kumacheva E. Rapid, cost-efficient fabrication of microfluidic reactors in thermoplastic polymers by combining photolithography and hot embossing. Lab Chip. 2010;10:522–524. doi: 10.1039/b918834g. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Chang A. Molecular predictors of EGFR-TKI sensitivity in advanced non-small cell lung cancer. Int J Med Sci. 2008;5:209–217. doi: 10.7150/ijms.5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liotta LA, Espina V, Mehta AI, Calvert V, Rosenblatt K, Geho D, Munson PJ, Young L, Wulfkuhle J, Petricoin EF. Protein microarrays: meeting analytical challenges for clinical applications. Cancer cell. 2003;3:317–325. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]