Abstract
Complement receptor 1 (CR1) expressed on the surface of phagocytic cells binds complement-bound IC playing an important role in the clearance of circulating immunecomplexes (IC). This receptor is critical to prevent accumulation of IC, which can contribute to inflammatory pathology. Accumulation of circulating IC is frequently observed during malaria, although the factors contributing to this accumulation are not clearly understood.
We have observed that the surface expression of CR1 on monocyte/macrophages and B cells is strongly reduced in mice infected with Plasmodium yoelii, a rodent malaria model. Monocyte/macrophages from these infected mice present a specific inhibition of complement-mediated internalization of IC caused by the decreased CR1 expression. Accordingly, mice show accumulation of circulating IC and deposition of IC in the kidneys that inversely correlates with the decrease in CR1 surface expression. Our results indicate that malaria induces a significant decrease on surface CR1 expression in the monocyte/macrophage population that results in deficient internalization of IC by monocyte/macrophages.
To determine whether this phenomenon is found in human malaria patients, we have analyzed 92 patients infected with either P. falciparum (22) or P. vivax (70), the most prevalent human malaria parasites. The levels of surface CR1 on peripheral monocyte/macrophages and B cells of these patients show a significant decrease compared to uninfected control individuals in the same area. We propose that this decrease in CR1 plays an essential role in impaired IC clearance during malaria.
INTRODUCTION
Malaria is one of the most prevalent parasitic diseases in the world, causing more than 700,000 deaths each year, mostly in children in Sub-Saharan Africa (1). Similarly to other infectious diseases, malaria induces the formation of immunecomplexes (IC), which are detected in peripheral blood during infection (2, 3). IC have an inflammatory effect in the immune system, which is mediated by the Fc receptors that are present in most hematopoietic cells (4). On the other hand, mononuclear phagocytes have a protective role against IC-mediated inflammation by removing circulating IC, which is required to avoid over-stimulation of the system (5). The efficient handling of these complexes by the cells of the mononuclear phagocyte system contribute to their clearance, decreasing their deposition on other tissue sites such as renal glomeruli (6). Most of the IC uptake in the body takes place in the liver and the spleen (7), where complement receptor 1 (CR1 or CD35) is an important mediator in the clearance of IC (8). In addition to IC clearance, CR1 has an anti-inflammatory effect that is mediated by the inactivation of C3b and C4b, which attenuates complement amplification (9).
Different receptors recognize IC in different ways: Fc receptors bind directly to the immunoglobulin part of the IC, but CR1 recognizes complement factors that are bound to the IC, such as the C opsonins C4b, C3b, iC3b and C1q (5). Therefore, the presence of a functional complement system is required for efficient clearance of IC (10).
CR1 is expressed in macrophages, B cells, neutrophils and follicular dendritic cells in mice (11), but in humans it is also expressed in erythrocytes, where it contributes to the clearance of IC transferring them to macrophages for degradation (12). On the contrary, mouse erythrocytes do not express CR1, but a close homologue called Crry. This protein cannot act as C3 receptor and consequently does not contribute to IC clearance (13). Therefore, mice constitute an optimal model to study the role of CR1 in IC clearance mediated by phagocytes, since in humans it is difficult to differentiate between erythrocyte-mediated and macrophage-mediated CR1 clearance.
CR1 in the surface of human erythrocytes is also a receptor for P. falciparum invasion (14) and mediates adhesion of infected erythrocytes to uninfected ones (15), a phenomenon called rosetting, which is associated with cerebral malaria. Polymorphisms associated with low CR1 expression on erythrocytes are highest in the malaria-endemic regions of Asia and are believed to confer protection against severe malaria (16, 17). It is important to note that these mechanisms do not play a role in the mouse model, since CR1 is not expressed in erythrocytes. Another point to consider is that the Cr1 gene produces two splice variants, CR1 and CR2, however mouse monocyte/macrophages express very low levels of CR2 (18). Here we have focused on CR1 expressed on monocyte/macrophages and B cells, which had not been studied before in the context of malaria.
Although complement activation and IC formation are prominent features of malaria infection, the role of complement regulatory proteins and IC in this infection remains unclear. In this work, we have studied the role of CR1 on the surface of monocyte/macrophages in IC clearance during malaria infection. Using a rodent malaria model, P. yoelii, we found that CR1 expression on the surface of macrophages is strongly reduced during malaria infection. Monocyte/macrophages of P. yoelii-infected mice have reduced complement-mediated capacity to internalize IC caused by the decrease in CR1 levels, which probably contributes to the observed accumulation of circulating IC. Analysis of peripheral blood samples from human malaria patients in Iquitos, Peru, also present decreased levels of CR1 expression in the surface of monocyte/macrophages. Taken together, our results indicate that malaria induces a significant decrease on surface CR1 expression in the monocyte/macrophage population that results in deficient internalization of IC by monocyte/macrophages.
MATERIALS AND METHODS
Ethics Statement
This study has human subjects approval from the Internal Review Boards from both the New York University School of Medicine and the Peruvian National Institute of Health. All studies involving human subjects were conducted in accordance with the guidelines of the World Medical Association's Declaration of Helsinki. All individuals and/or their legal guardians gave written informed consent. All personal information was removed from the files before handing this information to the laboratory team, in accordance with the Health Insurance Portability and Accountability Act.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of New York University School of Medicine, which is fully accredited by the Association For Assessment and Accreditation Of Laboratory Animal Care International (AAALAC).
Mice, Parasites and Infections
Female Swiss Webster mice were purchased from the National Institutes of Health and Jackson Laboratories. Nonlethal strain P. yoelii 17XNL-infected erythrocytes were harvested by cardiac puncture of infected, anesthetized Swiss Webster mice before the peak in parasitemia. Erythrocytes were washed twice with PBS and separated from white blood cells by centrifugation at 2000 g for 3 minutes. Erythrocytes were then spun on an Accudenz (Accurate Chemical & Scientific Corporation) gradient to isolate schizonts- and late trophozoite-stage infected erythrocytes. The collected infected erythrocytes were washed and resuspended in PBS. To start blood-stage infections, Swiss Webster mice were injected intraperitoneally with 106 infected erythrocytes per mouse resuspended in PBS. To evaluate parasitemia, thin blood smears were made by bleeding mice from a nick in the tail. Smears were stained with KaryoMAX Giemsa (Gibco), and a minimum of 500 erythrocytes per smear were counted.
Histological and serological analysis
Histological examination of kidneys was done on H&E-stained paraffin sections. For immunofluorescence microscopy, 6-µm frozen kidney sections were processed and stained with FITC goat anti-mouse IgG (Biolegend). For each condition, ten images with constant time of acquisition and fluorescent light intensity were obtained randomly with a 40× objective lens in each of two different sections processed in parallel. Metamorph imaging software was used to determine the average fluorescence intensity in each image. Mice anti-C1q and anti-Merozoite Surface Protein-1 (MSP-1) Ab in serum were detected by ELISA. Plates were coated with anti-C1q (Abcam) or recombinant P. yoelii MSP-1 (obtained through MR4 (MRA-48) deposited by DC Kaslow) followed by incubation with serum and development with anti-mouse IgG labeled with horseradish peroxidase. Since C1q only binds to IC and not to individual antibodies, development with anti-IgG results in specific detection of C1q bound to IC. For human anti-C1q a modification of kit (Hycult, Biotech) was used. Development was performed with anti-human IgG labeled with horseradish peroxidase to detect only C1q bound to IC.
J774 macrophage incubation with IC
106/ml J774 cells were incubated with either IC (10 µg/ml) purified by dialysis from serum of P. yoelii infected mice at day 10 post infection or LPS (10 µg/ml) in the presence of mouse control serum (1:1 serum/culture medium) for 30 min at 37°C, before analysis of surface markers by FACs.
Cell separation
For mice cells
Single cell suspensions were obtained by mechanical disruption of spleen or liver through a cell strainer and then osmotically lysing erythrocytes by incubation in an ammonium chloride/potassium hydrogen carbonate buffer. Peritoneal cells were extracted by aspiration and peripheral blood processed as detailed for humans below. All spleen cell preparations were resuspended in PBS with 3% fetal bovine serum (FBS; Gibco), and kept on ice.
For human peripheral blood
Blood was incubated for 1 h with Fix/Lyse Solution, BioLegend buffer at room temperature and centrifuged at 600 × g 5 minutes before staining.
Flow Cytometry
All flow cytometry was performed on a FACS Calibur (Becton Dickinson) and analyzed with either CellQuest (Becton Dickinson) or FlowJo (TreeStar). All antibodies for FACS were purchased from Biolegend or BD Biosciences.
Expression of PE F4/80, CD40 and MHC-II was analyzed on J774 macrophages stained with PE anti-F4/80 (BM8), PerCP anti-CD40 (3-23) and FITC anti-I-Ad (39-10-8)
Expression of CR1 was analyzed in mouse monocyte/macrophages CD11b+F4/80+ (stained with FITC anti-CD11b (M1/70), PerCP anti-F4/80 (16-10A1) and PE anti-CD21/CD35 (CR2/CR1) (7E9) (Biolegend) and B cells stained with FITC-B220 (30-F11) and PerCP anti-CD19 (6D5). The anti-CD21/CD-35 recognizes both CR2 and CR1; however, mouse monocyte/macrophages express very low levels of CR2 (18). For transfer experiments, splenocytes form control mice were labeled with DDAO (1 µg/ml, 15 min at 37°C). 3 ×106 splenocytes were injected i.v. into eight recipient mice and four of them were infected with P. yoelii one day later. Ten days after infection, the levels of CR1 were analyzed on monocyte/macrophages that were identified in recipient mice as cells positive for CD11b, F4/80 and DDAO. To measure the effect of inflammatory stimuli on CR1 expression in vivo, mice were treated with commercial FITC-labeled IC anti-OVA (4.5 mg/kg; Fc BURST Green, Invitrogen), LPS (10 mg/kg) or both, every 3 days during 10 days before analysis of CR1 expression on macrophages.
Expression of CR1 was analyzed in different populations of human PBMCL: monocyte/macrophages (CD16+ CD10−.); B cells (B220+, CD19+) and neutrophils (CD10+) cells and CD35 expression was also analyzed on them. PBMC were stained with either PE anti-human CD35, PerCP anti-human CD16, FITC anti-human CD10 or PE anti-human CD35 (E11), PerCP anti-human CD45 (HI30), FITC anti-human CD19 (HIB19), BioLegend. Data are expressed as percentage of CR1hi cells in each cell population.
Phagocytosis quantification
Mouse splenocytes from control, P. yoelii-infected mice (day 10 of infection) or mice injected with anti-CR1, were incubated for 30 min at 37°C with commercial FITC-labeled IC anti-OVA (Fc BURST Green, Invitrogen at 120µg/ml) pre-incubated or not with uninfected mouse serum for 30 min at 4°C to allow for complement binding. Alternatively, splenocytes were incubated with control or P. yoelii-infected erythrocytes stained with DDAO (Invitrogen) for 1 h at 37°C. Cells were transferred to ice before staining with PerCP anti- CD11b and FITC anti- F4/80 and FACs analysis, as described above.
Injection of blocking anti-CR1 antibody, IC and LPS
Groups of three mice were injected i.v. with 300 mg/mouse of either anti-CR1 antibody (Abcam 7G9 Rat IgG) or control Rat IgG (Equitech-bio, Inc). To study the effects on phagocytosis of IC, five days after inoculation, splenic monocyte/macrophages were analyzed for surface CR1 expression and phagocytosis of IC. To study deposition of IC in the kidneys, five days after anti-CR1 treatment, mice were inoculated with commercial IC (Fc BURST Green, Invitrogen at 4.5 mg/kg) and ten days later kidneys were analyzed for IC deposition. To study the role of inflammation and IC in CR1 expression levels, groups of three mice were injected i.v. with commercial IC (Fc BURST Green, Invitrogen at 4.5 mg/kg), LPS (10 mg/kg) or both, every 3 days during 10 days before analysis of CR1 expression on macrophages.
Field study site
The human samples were from participants in the Malaria Immunology and Genetics in the Amazon (MIGIA) field study, including participants enrolled from February 2008 to January 2009. The study sites are two networks of communities near Iquitos, Peru (the capital of the Amazon province of Loreto). The Zungarococha community is comprised of 4 villages: Zungarococha, Puerto Almendras, Ninarumi, and Llanchama. The Mazan community is located downstream along the Amazon River, and is comprised of 4 villages: Mazan, Santa Cruz, Progresso, and Libertad. The transmission and endemicity is similar in both areas, and as such the data analysis does not separate the two sites.
Sample collection
Patients were followed actively, receiving a visit from the field team at regular intervals during malaria season. During each visit, a blood smear was made. If the patient had a fever detected (≥38.3°C) or reported by patient within 2 days, the blood slide would be immediately observed for parasites by light microscopy. If the slide was positive for parasites, the nurse and physician would draw one 8 ml Vacutainer of blood before treating the patient. Patients who were diagnosed with symptomatic P. falciparum or P. vivax malaria infections (defined as having a body temperature ≥ 38.3°C, a hematocrit of < 30PCV, hemoglobin < 10 gr% or a parasite density of > 5,000 parasites/µl of blood) were treated with artesunate and mefloquine for three days using a directly observed treatment strategy.
Passive cases also entered the study as individuals that went to the community-based health center reporting febrile illness and were diagnosed with malaria. These individuals were processed and followed up in a similar way as an active surveillance febrile patient. Medical histories of all patients, both active and passive, were documented, including recent symptoms of illness and drugs taken.
Healthy non-malaria exposed controls were obtained from donations from healthy individuals living in the city of Iquitos with no prior malaria history.
Statistical analysis
Data were analyzed using Prism (GraphPad). The Mann-Whitney U test was used to identify statistical differences between groups of patients. Student's t-test was used for all other studies.
RESULTS
ICs accumulate in the circulation during malaria in mice
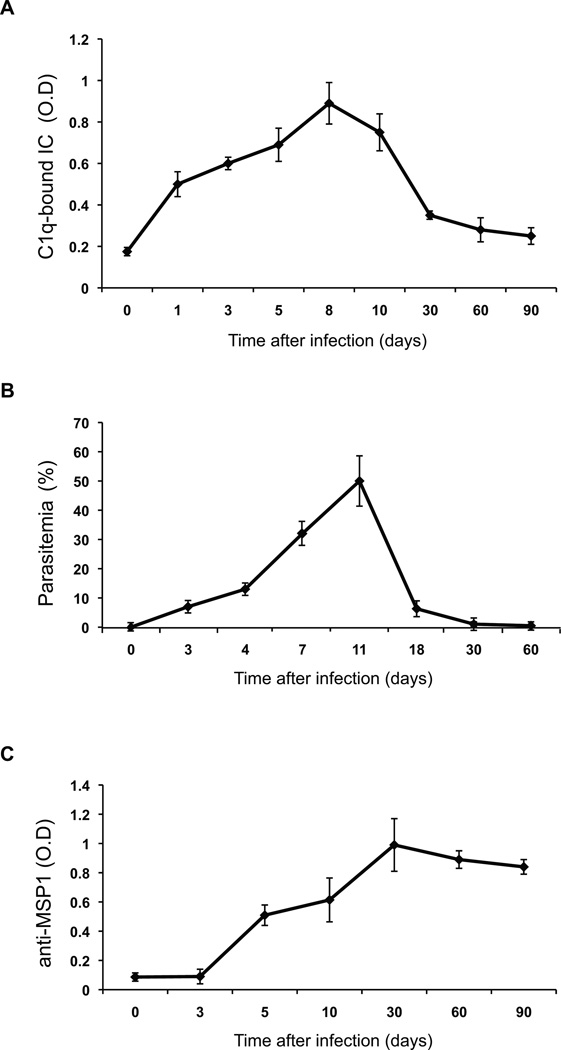
To study IC accumulation during malaria, we first used groups of mice infected with Plasmodium yoelii 17X NL, a rodent malaria parasite frequently used as a model for the human infection. Infections with this parasite are not lethal and mice are able to clear the parasite and recover. The detection of IC was performed in the serum of P. yoelii-infected mice at different times of infection using an indirect ELISA to detect C1q bound to IC. The accumulation of IC in the circulation appears very early, raises with infection and disappears after parasite is cleared (Fig. 1A,B). In contrast to the IC profile, antibodies against a typical parasite antigen, merozoite surface protein 1 (MSP-1) increase during infection, but remain at high levels after infection has been cleared (Fig. 1C). These results indicate that IC are formed and accumulate as a result of infection, but they are removed from the circulation after the parasite is controlled.
FIGURE 1. Circulating IC accumulation in P. yoelii-infected mice.
Serum from mice infected with P. yoelii (n=5) was used to determine: A) ICs (IgM and IgG), detected using an indirect ELISA coated with C1q; B) parasitemia, quantified by Giemsa-stained blood smears; C) anti-MSP-1 antibodies (IgM and IgG), detected using a direct ELISA. Values are expressed as mean ± SD.
IC induced activation, IC deposition and glomerulonephritis in mice with malaria
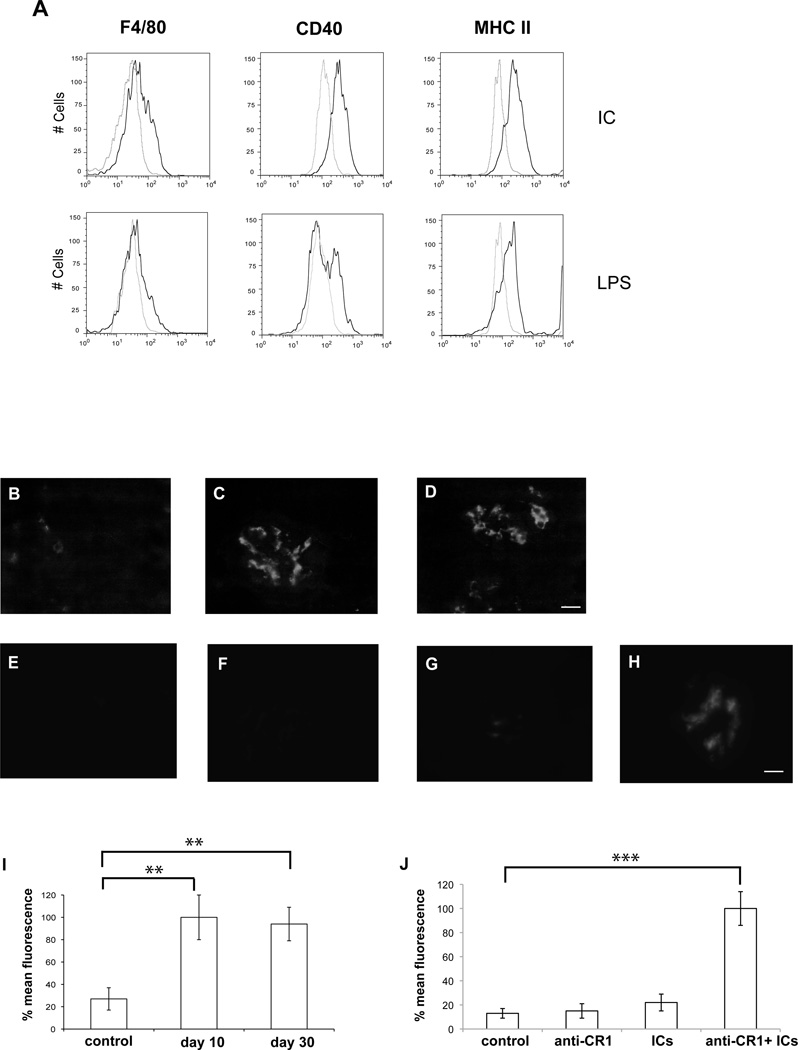
To test whether circulating IC from mice infected with P. yoelii have an activatory effect on macrophages, we purified IC from infected mice and incubated them in vitro with J774 macrophages. We observed a pronounced increase in surface expression of F4/80, CD40 and MHC-II, (Fig. 2A), which is indicative of macrophage activation (19). To determine whether the observed accumulation of IC in circulation results in deposition in the kidneys, as has been observed in other diseases (20, 21), we analyzed histological sections of kidneys from mice infected with P. yoelii at different times after infection. We observed IC deposition after 10 days of infection, which is still detectable after 1 month (Fig. 2B–D, I). Histological observation of kidney slides showed generalized inflammation with glomerular endocapillary proliferation and infiltration of inflammatory interstitial mononuclear cells after 10 days of infection. One month after infection, when parasites have already been cleared, we observed a decrease in inflammatory infiltrates and an increase in interstitial and glomerural capilar permeability with erythrocytic glomerular generalized congestion (Supplementary Fig. 1). These observations coincide with malaria-induced pathology observed in human kidney samples from patients (22–24).
FIGURE 2. Macrophage activation and deposit of IC in the kidneys of P. yoelii-infected mice.
A) FACs plots show surface expression of F4/80, CD40 and MHC-II on J774 macrophages incubated with IC purified from P. yoelii-infected mice (10µg/ml) or LPS (10µg/ml). Light gray line shows control J774 cells with no stimulus. (B–H) Kidney histological sections of groups of five mice stained with anti-IgG antibodies to show IgG deposits in glomeruli. (B–D) Uninfected control (B); 10 days (C) or 30 days (D) after infection with P. yoelii. (E–H) Uninfected mice received different treatments: control (E); injected with anti-CR1 (F), injected with IC (G); injected with anti-CR1 and IC (H). (I) Quantification of IgG deposits by fluorescence in (B–D) P. yoelii infected mice. (J) Quantification of IgG deposits by fluorescence in (E–H). ** p < 0.01 and *** p < 0.001, when each condition is compared to control. Representative images are shown. Bar corresponds to 20 µm.
To confirm that CR1 is needed for IC clearance in our mouse model, we inoculated mice with high concentrations of IC and anti-CR1 blocking antibodies. We found that blocking of CR1 results in IC deposition in the kidneys, which is not observed when IC are administered alone (Fig. 2E–H, J).
Surface expression of CR1 on splenic monocyte/macrophages and B cells is decreased in mice during malaria
Two different receptors play a major role in clearance of IC from circulation: Fc- γ receptor, which binds to naked antibodies, and CR1, which binds to C3b/iC3b already bound to IC. Since we had observed accumulation of IC in infected mice, we tested whether expression levels of these two receptors were altered during malaria.
We found that P. yoelii infection induces a strong decrease of CR1 surface levels in splenic monocyte/macrophages (Fig. 3A,B), which are the major cell type that can internalize IC in circulation. The decrease in CR1 expression is recovered after parasite clearance (Fig. 3A,B) and follows an inverse pattern when compared to circulating IC (Fig. 1), suggesting that the accumulation of IC might be caused by a defect in IC clearance by CR1. Levels of Fc-γIIIA receptor on the surface of monocyte/macrophages are increased during early infection and return to background levels at the peak of infection (day 10) (Fig. 3C,D). A gradual decrease of CR1 surface expression was also observed on B cells during the course of infection (Fig. 3E,F).
FIGURE 3. CR1 surface expression is decreased on mouse splenic monocyte/macrophages and B cells during P. yoelii infection.
FACs plots show surface expression of A) CR1 and C) Fc-γRIII receptor on CD11b+F4/80+ cells and E) CR1 on B200+CD19+ B cells from control (gray line) and P. yoelii infected mice (black line) at different times after infection. The results are representative of three independent experiments with groups of four mice each. B,D,F) percentage variation of average mean fluorescence intensity (MFI) of the surface expression of CR1 (D,F) and Fc-γRIII receptor (D) at the indicated days after infection compared to control uninfected mice processed on each day (n=12 for each day and condition).
We also observed that the decrease in surface CR1 expression is found in monocyte/macrophages from spleen and liver, but not in peritoneal ones (Fig. 4). This coincides with the localization of Plasmodium, which is found in the circulation and accumulates in organs such as liver and spleen, but does not invade the peritoneal cavity.
FIGURE 4. CR1 surface expression is decreased on monocyte/macrophages in spleen and liver.
A) FACs plots show the expression of CR1 on CD11b+F4/80+ cells in spleen, liver and peritoneum in control (grey line) and P.yoelii infected mice (black line) after 10 days of infection. Results are representative of two independent experiments with three infected and three uninfected mice each. B) percentage variation of average mean fluorescence intensity (MFI) of CR1 surface expression compared to cells from control uninfected mice processed in parallel (n=4 for each condition).
The observed decrease in CR1 expression on monocyte/macrophages may be caused by the binding of high concentrations of IC that induce internalization of this receptor, as a response to an inflammatory environment (25, 26) or through another parasite-induced mechanism. We first analyzed whether high concentrations of IC induce a decrease in macrophage CR1 surface expression in vitro. We incubated J774 macrophages with IC in the presence of mouse serum containing complement, and found that surface levels of CR1 do not decrease under these conditions, indicating that binding of IC do not induce a decrease of surface expression of CR1 in vitro (Fig. 5A). To study this question in vivo, mice were injected with either IC, a strong inflammatory stimulus (LPS) or both together. We found that although IC can induce a decrease in CR1 surface expression on macrophages, LPS alone induces also a strong decrease (Fig. 5B–C), suggesting that inflammation is an important mediator of CR1 decrease. Interestingly, both stimuli together do not have an additive effect.
FIGURE 5. Effect of IC and LPS on macrophage surface CR1 expression.
A) J774 macrophages were incubated with commercial OVA-ICs (120 µg/ml) for 30 min before analysis of CR1 expression. Light gray line shows control J774 cells with no stimulus. B) CR1 surface expression in CD11b+F4/80+ cells from mice treated with ICs (4.5 mg/kg), LPS (10 mg/kg) or both, every 3 days during 10 days. C) Average mean fluorescence intensity (MFI) of CR1 surface expression from cells from each group of mice processed in parallel (n=3 for each condition). ** p < 0.01 and *** p < 0.001, when each condition is compared to control.
We wanted to determine whether the observed decrease in the levels of surface CR1 during malaria in the monocyte/macrophage population is caused by decreased expression of CR1 on the membrane of these cells or by selective disappearance of cells expressing this receptor. We transferred labeled splenocytes into mice that were infected with P. yoelii one day after the transfer. Ten days later, analysis of CR1 expression on monocyte/macrophages was performed. We found that the transferred monocyte/macrophages had strongly reduced their expression of surface CR1, compared control mice (Fig. 6). This result confirms that there is a decrease in surface expression levels of CR1 on monocyte/macrophages of P. yoelii-infected mice.
FIGURE 6. CR1 surface expression decrease in monocyte/macrophages transferred into P. yoelii-infected mice.
Splenocytes isolated from a control uninfected mouse were labeled with DDAO and transferred to eight mice (3 × 106 each). DDAO (A) and CR1 (B) levels of CD11b+F4/80+ gated cells before transfer. One day after transfer, four of these mice were infected with P. yoelii. C) CR1 levels of CD11b+F4/80+DDAO+ cells from control (grey line) and infected (black line) mice 10 days after infection. D) Average mean fluorescence intensity (MFI) of CR1 surface expression of cells from infected and control mice processed in parallel (n=4 for each condition), * p < 0.05.
We have also analyzed the relative numbers of monocyte/macrophages in the spleen and peripheral blood of P. yoelii-infected mice and compared it to controls, finding no significant differences (In spleen: control 4.2 ±1.7%; day 10-infected mice 3.8 ±1.0. In blood: control 5.5 ±2.6%; day 10-infected mice 5.7 ±1.9%), indicating that there is no selective disappearance of the monocyte/macrophage population during infection.
Malaria induces decreased internalization of complement-bound IC by monocyte/macrophages
We next wanted to determine whether the decreased levels of CR1 expression on monocyte/macrophages during malaria result in the inhibition of internalization of IC through this receptor.
We first confirmed that monocyte/macrophages from P. yoelii-infected mice efficiently phagocytose control and infected erythrocytes. Similar results were described before for a different strain of Plasmodium (27, 28) and indicate that macrophages from infected mice present similar levels of phagocytosis compared to control mice (Supplementary Fig. 2).
To determine whether the capacity to clear IC in infected mice is specifically decreased during infection, we isolated splenocytes from infected mice at the peak of infection (day 10). Commercially available labeled IC were pre-incubated or not with mouse serum to allow for complement binding before addition to cells. This is important because IC are preferentially internalized through Fc-γ in the absence of complement, but through CR1 in the presence of complement (29–31).
We found that internalization of IC preincubated with serum is strongly inhibited in monocyte/macrophages from mice with malaria compared to these cells isolated from control mice (Fig. 7). This inhibition was not found in the absence of serum, reflecting the intact capacity of monocyte/macrophages to internalize complement-free IC, most likely through Fc receptors.
FIGURE 7. Phagocytosis of complement-bound ICs by monocyte/macrophages is inhibited in P. yoelii infected mice.
Splenocytes from P. yoelii-infected or uninfected control mice were cultured for 30 min in vitro in the presence of FITC-labeled OVA-ICs pre-incubated or not with mouse control serum (complement bound ICs). A) Phagocytosis of IC by CD11b+F4/80+ cells from control mice (gray line) or infected mice (black line). B) Average mean fluorescence intensity (MFI) of FITC-labeled IC in CD11b+F4/80+ cells from control and infected mice (n=3). *** p < 0.001 when compared to control.
Our results indicate that monocyte/macrophages from P. yoelii-infected mice present a specific inhibition of internalization of complement-bound IC, probably contributing to the impaired clearance of IC in infected mice.
To confirm that this inhibition is mediated by the severe decrease in surface expression of CR1 on monocyte/macrophages, we inhibited CR1 in mice by inoculation of anti-CR1 blocking antibodies. We confirmed that these mice had low levels of surface CR1 on immune cells (Fig. 8A), comparable to the levels found during malaria. Monocyte/macrophages from these mice were isolated and tested for their capacity of IC internalization. Similarly to monocyte/macrophages from mice with malaria, we observed deficient IC internalization in the presence of serum and normal internalization in its absence (Fig. 8B–C), confirming that low expression of surface CR1 results in deficient internalization of IC in these cells.
FIGURE 8. Blocking of CR1 in mice inhibits phagocytosis of IC by monocyte/macrophages.
Mice were injected with CR1 blocking antibody (black line) or control antibody (gray line). Five days after injection, splenic CD11b+F4/80+ cells were analyzed for surface expression of CR1 (A) and also were cultured for 30 min in vitro in presence of FITC-labeled OVA-ICs pre-incubated or not with mouse control serum (complement bound ICs) to determine phagocytosis of IC (B). C) Average mean fluorescence intensity (MFI) of FITC-labeled IC in CD11b+F4/80+ cells from control and anti-CR1 injected mice (n=3). ** p < 0.01 when compared to control.
Levels of surface CR1 in circulating monocyte/macrophages from human patients infected with P. falciparum and P. vivax in Peru
To study the relevance of our findings in a mouse malaria model to human disease, we analyzed patients infected with P. falcilparum and P. vivax in Iquitos, Peru. Although the majority of malaria infections occur in Sub-Saharan Africa, studying immune responses to Plasmodium in an African context is complicated by overlapping infections that occur as a result of the high transmission in these hyperendemic areas. In the Peruvian Amazon, P. falciparum transmission is low – 0.49 infections/person/year – and as a result typically each case of falciparum malaria is a single, discrete infection. P. vivax is indeed more prevalent but is still transmitted at relatively low levels, with 1.21 infections/person/year (32, 33). Transmission is restricted to the wet season (January to July) and there is high prevalence of asymptomatic cases in this region (32).
For our study, we used remaining blood from samples taken from participants in a study regarding Malaria Immunology and Genetics in the Amazon, residing in several communities just south of Iquitos, Peru (see Methods). There were 8 symptomatic individuals (fever higher than 38.3°C), from which samples were taken during an uncomplicated malaria infection. However, the majority of the samples were taken from asymptomatic individuals (temperature < 38°C) during a campaign for active detection of infection. Parasitemia in individuals infected with P. vivax ranged between 3–540/µl and in infected with P. falciparum between 2–30,000/µl. As controls, there were 9 samples taken from healthy individuals living in the area who had never reported a previous Plasmodium infection.
We determined the levels of circulating IC in these patients finding that both P. falciparum and P. vivax infected patients show significant increases in the levels of IC (Fig. 9A).
FIGURE 9. Circulating IC levels are increased and CR1 surface expression is decreased on monocyte/macrophages and B cells from Peruvian patients during P. vivax and P. falciparum infection.
A) ICs (IgM and IgG), detected using an indirect ELISA coated with C1q. B–E) CR1 surface expression levels on macrophages CD16+CD10− (B), B cells CD19+CD138− (C), plasmatic cells CD19−CD138+ (D) and neutrophils CD16+CD10+ (E) were analyzed in peripheral blood from healthy controls or patients during P. vivax and P. falciparum infections. Data are expressed as percentage of cells expressing high levels of CR1. * p < 0.05, ** p < 0.01 and *** p < 0.001, when the control group is compared with either P. falciparum or P. vivax infected groups by Mann-Whitney test.
To study CR1 expression on monocyte/macrophages during malaria infection, we first analyzed the percentage of CD16+ CD10− monocyte/macrophages in PBMCs from participants. This population is decreased in peripheral blood of Plasmodium-infected individuals. We found that while healthy control individuals (n=9) have 24.1 ± 7% of CD16+ CD10− monocyte/macrophages in total PBMCs, P. falciparum (n=24) and P. vivax-infected individuals (n=68) have 11.4 ± 7.3 and 10.5 ± 8.4 %, respectively. These results suggests that migration of monocyte/macrophages into other tissues, such as bone marrow and spleen, is taking place in infected individuals (34, 35).
Analysis of CR1 expression on the remaining CD16+ CD10− monocyte/macrophages in peripheral blood showed a significant reduction of CR1 expression on these cells in patients with either P. falciparum or P. vivax infections compared to controls (Fig. 9B). None of these patients showed symptoms of severe or complicated malaria, indicating that the decrease in monocyte/macrophages CR1 can take place in uncomplicated Plasmodium infections. It is possible that patients with severe disease may show even more drastic decreases in CR1 levels. We also analyzed the levels of CR1 surface expression in B cells (identified as CD19+, CD138−), another cell type were CR1 is highly expressed and its levels are regulated during infection and autoimmune diseases (13, 36, 37). Significantly decreased levels of CR1 were observed in B cells of patients with malaria, both in P. falciparum and P. vivax infections (Fig. 9C). Conversely, no differences were observed in CR1 surface levels of neutrophils (identified as CD16+, CD10+) in malaria patients compared to the control group (Fig. 9D).
DISCUSSION
The complement cascade plays a key role in the modulation of inflammatory responses and its activation is crucial to the pathogenesis of various diseases (38). In malaria, decreased levels of CR1 expression on erythrocytes are considered an important factor in the accumulation of IC during disease (39, 40), because of the contribution of erythrocyte CR1 in IC clearance. Erythrocytes transfer IC to macrophages for degradation, probably by transferring the IC from erythrocyte CR1 to macrophage CR1 (41). Macrophages also contribute to the clearance of IC by directly binding IC to CR1 expressed on their membrane. In any case, macrophage CR1 is the final destination of circulatory IC targeted for degradation, however, the role of CR1 expression and function on phagocytic cells in malaria had not been studied before.
Since mice do not express CR1 on the surface of erythrocytes, it constitutes an adequate model to study the role of CR1 on monocytes/macrophages in the IC clearance during malaria. We have demonstrated that monocyte/macrophages from mice with malaria do not internalize complement-bound IC, similarly to macrophages from mice treated with blocking anti-CR1 antibodies. Since monocyte/macrophages play a major role in the clearance of complement-bound IC (5), it is likely that the observed CR1 decrease contributes decisively to the accumulation of circulating IC during malaria.
It is well characterized that accumulation of IC is an essential contributor to inflammatory damage, which is in great part mediated by binding of IC to Fc-γRIII receptors (42). Expression of Fc-γRIII receptor on mice monocyte/macrophages is not decreased, in fact, we found a transient, but significant, increase in its surface expression during the early days of infection. Expression of the equivalent receptor in humans (Fc-γRIIIA) is increased on the monocytes of malaria patients, specially in the ones with malaria-induced anemia (43). We confirmed that inflammatory stimuli decrease CR1 expression, as described before (13, 37), which in turn would reduce IC clearance, contributing to the accumulation of inflammatory IC. Due to increased Fc-γRIIIA expression during malaria, IC would induce a stronger inflammatory response to IC, closing a positive inflammatory feed-back loop in the malaria patient.
CR1 surface expression is also reduced on B cells in mice and humans infected with Plasmodium. In these cells, CR1 plays an important role in differentiation and activation (44) and its decrease may have important consequences for B cell function during malaria.
It is unlikely that binding of IC to CR1 is a major contributor to the decreased expression found in vivo, since incubation of IC with macrophages in vitro does not have this effect. Additionally, an inflammatory stimulus, such as LPS, has a similar effect as malaria in decreasing CR1 surface expression, suggesting that an inflammation-triggered mediator is responsible for this effect. Injection of IC in addition to LPS has no additional effects in reducing CR1 expression, suggesting again that IC are not inducing a reduction in CR1 expression by receptor binding and internalization. Several studies have also observed decreased CR1 transcript in situations of infection and autoimmunity in the context of an inflammatory environment (25, 26). Since IC are also inflammatory, it is possible that the CR1 decrease observed in vivo is entirely caused by the inflammatory environment and not by internalization of CR1.
We found that the increased concentrations of circulating IC during malaria correlate approximately with parasitemia. The sole presence of IC in blood is not considered immune pathology, but a normal response to infection; however, we have also observed deposition of IC in the kidneys of P. yoelii-infected mice, which is accompanied by glomerulonephritis. Although it is not clear the relative importance of the different factors that may induce glomerulonephritis in malaria, IC deposition is probably one important contributor to kidney pathology (22).
Although CR1-mediated internalization of complement-bound IC is severely impaired, we and others (28) found efficient phagocytosis of control and infected erythrocytes by macrophages of malaria-infected mice. Internalization of IC by macrophages in the absence of complement was also very effective, suggesting that Fc receptor-mediated internalization is not inhibited during malaria. Taken together, these results indicate that the observed impaired CR1-mediated internalization of complement-bound IC is specific, since endocytic/phagocytic processes mediated by other receptors are fully functional in macrophages.
Our study is focused on CR1 expression on monocyte/macrophages, which had not been previously addressed. In contrast, CR1 expression levels on erythrocytes in human malaria patients have been extensively studied. Decreased levels of CR1 expression on erythrocytes during malaria, specially in infections presenting severe anemia, have been characterized (45) and associations between specific CR1 polymorphisms that result in reduced erythrocyte rosetting and severe malaria have been found (16, 17). Interestingly, polymorphisms in the CR1 promoter that result in higher expression have been associated with protection against cerebral malaria (46, 47). These findings cannot be explained by the increase in rosetting, since this would result in increased cerebral malaria, but it is likely that this effect is mediated by the high expression of CR1 in erythrocytes and possibly in macrophages that would contribute to IC clearance and would decrease the inflammatory component of malaria. Indeed, IC are highly inflammatory (4) and are found at high levels in peripheral blood of malaria patients (24, 39, 48).
In our study, no differences were observed between P. vivax and P. falciparum-infected individuals, suggesting that CR1 decreased expression is a common feature of Plasmodium infections.
The decrease in the levels of CR1 expression on peripheral monocyte/macrophages and B cells found in humans is not as pronounced as the decrease found in mice. This might be explained because of intrinsic differences, but also because experimental infections in mice are consistently very acute, compared to the natural variation in the inflammatory response in humans. In particular, our human sample is mostly composed of asymptomatic infected individuals. It is remarkable that such an evident decrease in the expression levels of CR1 in monocyte/macrophages is observed in this group.
Elevated levels of circulating IC are associated with malaria severity (49), further studies will be performed to address whether CR1 expression in monocyte/macrophages is more significantly decreased during severe malaria.
Supplementary Material
Acknowledgements
We thank MR4 for providing us with recombinant MSP-1 antigen contributed by DC Kaslow. We also thank Sandra Gonzalez for her help with mice infections.
Grant Support: C.F.A. was supported by a postdoctoral fellowship from Ministerio de Ciencia e Innovacion, Spain. A.R. was supported by NIH grant 1 R56 AI070907-01A2 and 3 R56 AI070907-01AS1.
Abbreviations
- CR1
Complement receptor 1
- IC
Immunecomplexes
- MSP-1
Merozoite surface protein 1
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.June CH, Contreras CE, Perrin LH, Lambert PH, Miescher PA. Circulating and tissue-bound immune complex formation in murine malaria. J Immunol. 1979;122:2154–2161. [PubMed] [Google Scholar]
- 3.Mibei EK, Orago AS, Stoute JA. Immune complex levels in children with severe Plasmodium falciparum malaria. Am J Trop Med Hyg. 2005;72:593–599. [PubMed] [Google Scholar]
- 4.Amigorena S, Bonnerot C. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol Rev. 1999;172:279–284. doi: 10.1111/j.1600-065x.1999.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 5.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haakenstad AO, Mannik M. Saturation of the reticuloendothelial system with soluble immune complexes. J Immunol. 1974;112:1939–1948. [PubMed] [Google Scholar]
- 7.Aguado MT, Mannik M. Clearance kinetics and organ uptake of complement-solubilized immune complexes in mice. Immunology. 1987;60:255–260. [PMC free article] [PubMed] [Google Scholar]
- 8.Nash JT, Taylor PR, Botto M, Norsworthy PJ, Davies KA, Walport MJ. Immune complex processing in C1q-deficient mice. Clin Exp Immunol. 2001;123:196–202. doi: 10.1046/j.1365-2249.2001.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 10.Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita T, Takeda J, Hong K, Kozono H, Sakai H, Inoue K. Monoclonal antibodies to mouse complement receptor type 1 (CR1). Their use in a distribution study showing that mouse erythrocytes and platelets are CR1-negative. J Immunol. 1988;140:3066–3072. [PubMed] [Google Scholar]
- 12.Nardin A, Lindorfer MA, Taylor RP. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol Immunol. 1999;36:827–835. doi: 10.1016/s0161-5890(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 13.Tan SS, O'Toole EM, Kurtz CB, Weis JH. Murine complement receptor gene expression: Cr2 gene transcripts are depressed during a high dose microbial challenge. Immunology. 1993;79:82–88. [PMC free article] [PubMed] [Google Scholar]
- 14.Spadafora C, Awandare GA, Kopydlowski KM, Czege J, Moch JK, Finberg RW, Tsokos GC, Stoute JA. Complement receptor 1 is a sialic acid-independent erythrocyte receptor of Plasmodium falciparum. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000968. e1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe JA, Moulds JM, Newbold CI, Miller LH. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 16.Cockburn IA, Mackinnon MJ, O'Donnell A, Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC, Rowe JA. A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria. Proc Natl Acad Sci U S A. 2004;101:272–277. doi: 10.1073/pnas.0305306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas BN, Donvito B, Cockburn I, Fandeur T, Rowe JA, Cohen JH, Moulds JM. A complement receptor-1 polymorphism with high frequency in malaria endemic regions of Asia but not Africa. Genes Immun. 2005;6:31–36. doi: 10.1038/sj.gene.6364150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingeroth JD, Benedict MA, Levy DN, Strominger JL. Identification of murine complement receptor type 2. Proc Natl Acad Sci U S A. 1989;86:242–246. doi: 10.1073/pnas.86.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Xia XP, Gong SL, Zhao Y. The macrophage heterogeneity: difference between mouse peritoneal exudate and splenic F4/80+ macrophages. J Cell Physiol. 2006;209:341–352. doi: 10.1002/jcp.20732. [DOI] [PubMed] [Google Scholar]
- 20.Arias CF, Ballesteros-Tato A, Garcia MI, Martin-Caballero J, Flores JM, Martinez AC, Balomenos D. p21CIP1/WAF1 controls proliferation of activated/memory T cells and affects homeostasis and memory T cell responses. J Immunol. 2007;178:2296–2306. doi: 10.4049/jimmunol.178.4.2296. [DOI] [PubMed] [Google Scholar]
- 21.Kettritz R. Autoimmunity in kidney diseases. Scand J Clin Lab Invest Suppl. 2008;241:99–103. doi: 10.1080/00365510802150232. [DOI] [PubMed] [Google Scholar]
- 22.Elsheikha HM, Sheashaa HA. Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res. 2007;101:1183–1190. doi: 10.1007/s00436-007-0650-4. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhut M. Auto-antibodies and glomerulonephritis in Plasmodium falciparum malaria. Autoimmunity. 2010;43:640–641. doi: 10.3109/08916931003599088. [DOI] [PubMed] [Google Scholar]
- 24.Pleass RJ. When is a malaria immune complex not an immune complex? Parasite Immunol. 2009;31:61–63. doi: 10.1111/j.1365-3024.2008.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart PH, Jones CA, Finlay-Jones JJ. Peritoneal macrophages during peritonitis. Phenotypic studies. Clin Exp Immunol. 1992;88:484–491. doi: 10.1111/j.1365-2249.1992.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubaniewicz A, Typiak M, Wybieralska M, Szadurska M, Nowakowski S, Staniewicz-Panasik A, Rogoza K, Sternau A, Deeg P, Trzonkowski P. Changed phagocytic activity and pattern of Fcgamma and complement receptors on blood monocytes in sarcoidosis. Hum Immunol. 2012;73:788–794. doi: 10.1016/j.humimm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Shear HL, Nussenzweig RS, Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979;149:1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su Z, Fortin A, Gros P, Stevenson MM. Opsonin-independent phagocytosis: an effector mechanism against acute blood-stage Plasmodium chabaudi AS infection. J Infect Dis. 2002;186:1321–1329. doi: 10.1086/344576. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt RE, Gessner JE. Fc receptors and their interaction with complement in autoimmunity. Immunol Lett. 2005;100:56–67. doi: 10.1016/j.imlet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Whaley K, Ahmed AE. Control of immune complexes by the classical pathway. Behring Inst Mitt. 1989:111–120. [PubMed] [Google Scholar]
- 31.Schifferli JA, Taylor RP. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- 32.Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, Roncal N, Alvarez E, Perez EJ, Gotuzzo E. Clustered local transmission and asymptomatic Plasmodium falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J. 2005;4:27. doi: 10.1186/1475-2875-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres KJ, Clark EH, Hernandez JN, Soto-Cornejo KE, Gamboa D, Branch OH. Antibody response dynamics to the Plasmodium falciparum conserved vaccine candidate antigen, merozoite surface protein-1 C-terminal 19kD (MSP1-19kD), in Peruvians exposed to hypoendemic malaria transmission. Malar J. 2008;7:173. doi: 10.1186/1475-2875-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban BC, Hien TT, Day NP, Phu NH, Roberts R, Pongponratn E, Jones M, Mai NT, Bethell D, Turner GD, Ferguson D, White NJ, Roberts DJ. Fatal Plasmodium falciparum malaria causes specific patterns of splenic architectural disorganization. Infect Immun. 2005;73:1986–1994. doi: 10.1128/IAI.73.4.1986-1994.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickramasinghe SN, Phillips RE, Looareesuwan S, Warrell DA, Hughes M. The bone marrow in human cerebral malaria: parasite sequestration within sinusoids. Br J Haematol. 1987;66:295–306. doi: 10.1111/j.1365-2141.1987.tb06913.x. [DOI] [PubMed] [Google Scholar]
- 36.Arora V, Mondal AM, Grover R, Kumar A, Chattopadhyay P, Das N. Modulation of CR1 transcript in systemic lupus erythematosus (SLE) by IFN-gamma and immune complex. Mol Immunol. 2007;44:1722–1728. doi: 10.1016/j.molimm.2006.07.300. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Kozono Y, Waldschmidt TJ, Berthiaume D, Quigg RJ, Baron A, Holers VM. Mouse complement receptors type 1 (CR1;CD35) and type 2 (CR2;CD21): expression on normal B cell subpopulations and decreased levels during the development of autoimmunity in MRL/lpr mice. J Immunol. 1997;159:1557–1569. [PubMed] [Google Scholar]
- 38.Morgan BP, Walport MJ. Complement deficiency and disease. Immunol Today. 1991;12:301–306. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 39.Stoute JA, Odindo AO, Owuor BO, Mibei EK, Opollo MO, Waitumbi JN. Loss of red blood cell-complement regulatory proteins and increased levels of circulating immune complexes are associated with severe malarial anemia. J Infect Dis. 2003;187:522–525. doi: 10.1086/367712. [DOI] [PubMed] [Google Scholar]
- 40.Owuor BO, Odhiambo CO, Otieno WO, Adhiambo C, Makawiti DW, Stoute JA. Reduced immune complex binding capacity and increased complement susceptibility of red cells from children with severe malaria-associated anemia. Mol Med. 2008;14:89–97. doi: 10.2119/2007-00093.Owuor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emlen W, Carl V, Burdick G. Mechanism of transfer of immune complexes from red blood cell CR1 to monocytes. Clin Exp Immunol. 1992;89:8–17. doi: 10.1111/j.1365-2249.1992.tb06869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willcocks LC, Smith KG, Clatworthy MR. Low-affinity Fcgamma receptors, autoimmunity and infection. Expert Rev Mol Med. 2009;11:e24. doi: 10.1017/S1462399409001161. [DOI] [PubMed] [Google Scholar]
- 43.Ogonda LA, Orago AS, Otieno MF, Adhiambo C, Otieno W, Stoute JA. The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect Immun. 2010;78:2173–2181. doi: 10.1128/IAI.01078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci U S A. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waitumbi JN, Opollo MO, Muga RO, Misore AO, Stoute JA. Red cell surface changes and erythrophagocytosis in children with severe plasmodium falciparum anemia. Blood. 2000;95:1481–1486. [PubMed] [Google Scholar]
- 46.Nagayasu E, Ito M, Akaki M, Nakano Y, Kimura M, Looareesuwan S, Aikawa M. CR1 density polymorphism on erythrocytes of falciparum malaria patients in Thailand. Am J Trop Med Hyg. 2001;64:1–5. doi: 10.4269/ajtmh.2001.64.1.11425154. [DOI] [PubMed] [Google Scholar]
- 47.Teeranaipong P, Ohashi J, Patarapotikul J, Kimura R, Nuchnoi P, Hananantachai H, Naka I, Putaporntip C, Jongwutiwes S, Tokunaga K. A functional single-nucleotide polymorphism in the CR1 promoter region contributes to protection against cerebral malaria. J Infect Dis. 2008;198:1880–1891. doi: 10.1086/593338. [DOI] [PubMed] [Google Scholar]
- 48.Jhaveri KN, Ghosh K, Mohanty D, Parmar BD, Surati RR, Camoens HM, Joshi SH, Iyer YS, Desai A, Badakere SS. Autoantibodies, immunoglobulins, complement and circulating immune complexes in acute malaria. Natl Med J India. 1997;10:5–7. [PubMed] [Google Scholar]
- 49.Thomas BN, Diallo DA, Noumsi GT, Moulds JM. Circulating Immune Complex Levels are Associated with Disease Severity and Seasonality in Children with Malaria from Mali. Biomark Insights. 2012;7:81–86. doi: 10.4137/BMI.S9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.