Abstract
For over a decade, spontaneous intestinal neoplasia has been observed in zebrafish (Danio rerio) submitted to the ZIRC (Zebrafish International Resource Center) diagnostic service. In addition, zebrafish displayed preneoplastic intestinal changes including hyperplasia, dysplasia, and enteritis. A total of 195 zebrafish, representing 2% of the total fish submitted to the service, were diagnosed with these lesions. Neoplastic changes were classified either as adenocarcinoma or small cell carcinoma, with a few exceptions (carcinoma not otherwise specified, tubular adenoma, and tubulovillous adenoma). Tumor prevalence appeared similarly distributed between sexes and generally occurred in zebrafish greater than 1 year of age, although neoplastic changes were observed in fish 6 months of age. Eleven lines displayed these preneoplastic and neoplastic changes, including wild-types and mutants. Affected zebrafish originated from 18 facilities, but the majority of fish were from a single zebrafish research facility (hereafter referred to as the primary facility) that has submitted numerous samples to the ZIRC diagnostic service. Zebrafish from the primary facility submitted as normal sentinel fish demonstrate that these lesions are most often subclinical. Fish fed the diet from the primary facility and held at another location did not develop intestinal lesions, indicating that diet is not the etiologic agent.
Introduction
Zebrafish have become an increasingly important model organism in the field of cancer research.1–4 Most cancers in zebrafish models are induced with chemicals or genetically, although spontaneous neoplasms are not uncommon in zebrafish 2 years of age or older.5–7 Specific mutants created as cancer models include tp53M214K (wild-type mutant), which presents with malignant peripheral nerve sheath tumors,8 and the apc/+ (AB mutant), which develops liver and intestinal tumors.9 Increased incidence of intestinal tumors have been observed in zebrafish exposed to DMBA (7,12-dimethylbenz[a]anthracene)10 and incipient intestinal preneoplastic lesions may undergo enhanced promotion when there are co-morbid conditions present, such as the nematode parasite, Pseudocapillaria tomentosa.11 The normal zebrafish intestine has been well-characterized previously12–14 as an agastric simple tubular structure, the mucosa of which is formed by longitudinal folds, that is in many ways similar to the mammalian counterpart, with the exceptions of a submucosa, Peyer's patches, and villi. The anatomic organization of the intestine demonstrates a rostral-to-caudal decreasing of the luminal diameter, lined by columnar epithelium interspersed with mucus (goblet) cells that increase in number caudally. Myenteric neurons and enteroendocrine cells also form components of the zebrafish intestine.
We have observed spontaneous intestinal neoplasia in zebrafish submitted to the Zebrafish International Resource Center (ZIRC) diagnostic service from several facilities since 2000, shortly after the diagnostic center at ZIRC was established. This retrospective study was aimed to provide analysis of the prevalence of these spontaneous intestinal neoplasms identified within the ZIRC diagnostic database over the last 12 years and a descriptive histologic classification of the preneoplastic and neoplastic lesions.
Materials and Methods
Review of historical prevalence and characterization of lesions
The records of 9539 zebrafish that were submitted to the ZIRC diagnostic database between January 4, 2000 and July 3, 2012 were reviewed using the following parameters provided within the ZIRC diagnostic service submitting form: date of submission, submitting facility, age, sex, genetic background, clinical or subclinical submission, and preneoplastic or neoplastic diagnosis within the intestine. Records omitted from the review include those involving species other than zebrafish, and those that were found to be incomplete and therefore unable to be properly evaluated. Fish identified through the database to have preneoplastic or neoplastic changes of the intestine were selected for further review by histopathology at low magnification (200X total magnification) and high magnification (400X total magnification) in order to confirm the original diagnoses and to further characterize the preneoplastic intestinal lesions as hyperplasia and/or dysplasia, as well as to determine whether inflammatory changes were present, and to classify the tumor type. The entire digestive tract, focusing on the intestine, of each fish was examined from oropharynx to excretory vent for three or four serial H&E stained sections.
Diet study
To investigate the possible role of diet, in 2009 we obtained some of the formulated diet mixture and individual components from the facility where we observed a very high prevalence of intestinal lesions. We conducted the following experiment at the Sinnhuber Aquatic Research Center (SARL) at Oregon State University, a laboratory with no history of the lesions described here.15 A total of 200 44-day-old, 5D strain zebrafish were divided into 5 groups, each with two replicate tanks containing 20 fish/tank. Fish were held at 28°C in the SARL recirculating systems. Diet groups were as follows, representing the diet mixture and individual components of the standard diet formulation used at the primary facility: 1) Silver Cup® tropical fish food (Sterling Silver Cup Fish Feeds, Murray, UT, 2) Ziegler® adult zebrafish food (Ziegler Bros, Gardners, PA), 3) TetraMin® tropical fish flakes, 4) Equal mixture of diets 1–3, and 5) the SARL zebrafish diet [comprised of 72% Aquatox Flake (Ziegler Bros), 13% Cyclop-eeze® (Argent Chemical Laboratories, Redmond, WA), and 15% Golden Pearl 300–500 micron diet (Artemia International, Fairview, TX)]. Fish were fed 2–3 times/day to satiation. The study was terminated 6 mo later; all fish were processed for histology and examined for the presence of intestinal lesions. Prevalence of intestinal lesions were compared between fish fed the various diets at SARL to sentinel fish at the primary facility that were fed Diet 4 over the same time periods (2009 and 2010) using an analysis of variance (ANOVA).
Results
The ZIRC diagnostic service, which performs routine postmortem diagnosis of apparently healthy and diseased fish from an average of 26 zebrafish research facilities per year,16 has observed an increasing prevalence of intestinal neoplasia and associated pathology. To better understand this trend, we undertook a systematic survey of all records to characterize these intestinal neoplasms by histopathology and prevalence as described below.
Histopathology
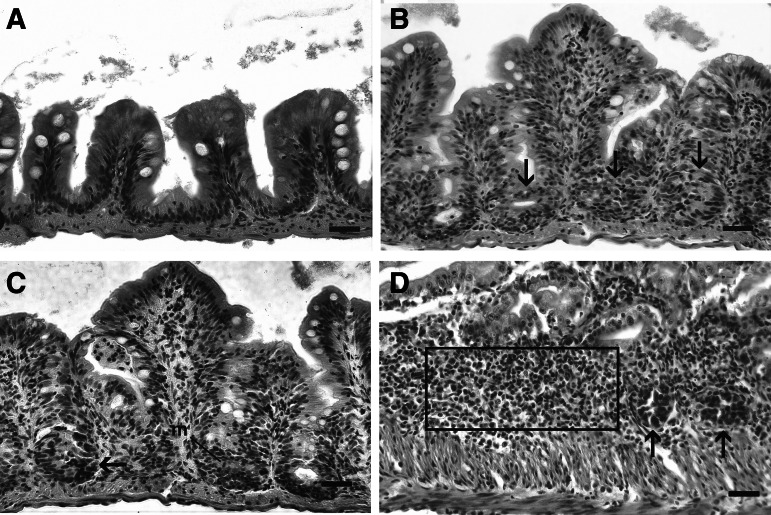
In our re-evaluation of archival samples, we observed the following intestinal pathologic changes that were summarily categorized. Intestinal preneoplastic changes were generally observed in the absence of and concurrent with frank neoplastic disease. Within the course of this study, the most common preneoplastic changes involving the intestinal mucosal epithelium included hyperplasia and dysplasia that often lead to extensive folding and formation of pseudocrypts (Fig. 1). Hyperplastic and dysplastic changes in the mucosal epithelium were characterized as follows: hyperplasia was denoted by an increase in the number of epithelial cells within mucosal folds, which often formed pseudocrypts, while retaining normal microanatomic structure as compared to control fish intestine. Dysplasia was defined by progressive loss of the normal microanatomic structure which may involve disorganization or absence of pre-existing histoanatomic architecture, loss of nuclear polarity, nuclear atypia, cellular pleomorphism, and aberrant mitotic figures (Fig. 1). Hyperplasia and dysplasia occurred independently and occasionally together in fish displaying preneoplastic lesions. Enteritis was typified by intraproprial and intraepithelial (mucosal) infiltrates of intermixed lymphocytes, eosinophilic granule cells, and histiocytes within the affected portion of intestine.
FIG. 1.
Normal and preneoplastic lesions in zebrafish intestines. H&E. Bar=25 μm. (A) Normal zebrafish intestine, lined by a single layer of columnar epithelium. (B) Hyperplasia, with multilayered columnar epithelium and formation of mucosal inter-fold pseudocrypts involving the basal epithelium (arrows). Note pseudostratification of nuclei, but nuclei retain polarity. (C) Dysplasia, with nuclear atypia and cellular pleomorphism. Also, there is loss of normal histological architecture, loss of nuclear polarity, and aberrant mitotic figures (arrow). (D) Enteritis, chronic inflammatory cell infiltrate within the lamina propria (indicated by box). Note two presumptive aberrant pseudocrypt foci (arrows).
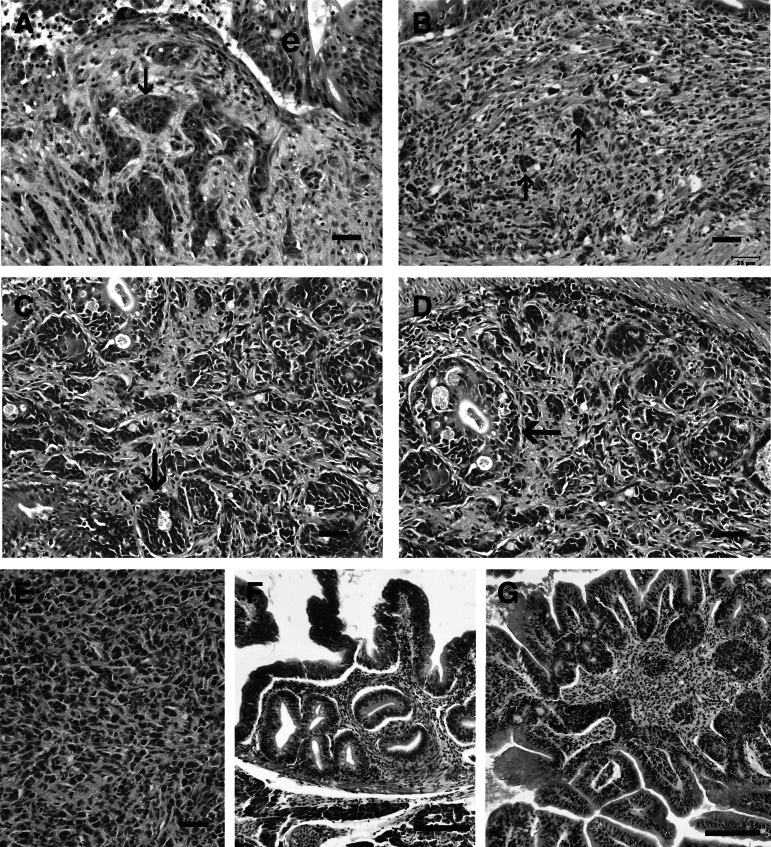
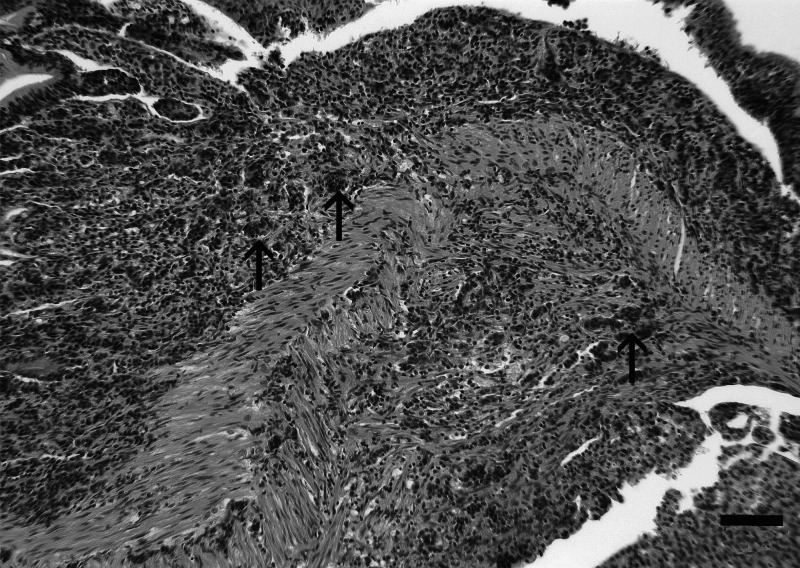
Intestinal tumor types included adenocarcinoma, small cell carcinoma/carcinoid-like tumor, carcinoma not otherwise specified, tubular adenoma, and tubulovillous adenoma. Adenocarcinoma was characterized by randomly oriented and invasive pseudocrypts derived from mucosal epithelium, often resembling pseudoacinar structures replete with intraluminal cellular detritus, as well as nests of polygonal cells within the lamina propria that displayed moderate to extreme cellular and nuclear atypia including aberrant mitotic figures (Fig. 2). Small cell carcinoma/carcinoid-like tumor was comprised of small sheets and nests of round, fusiform, or pleomorphic tumor cells that demonstrated a high degree of nuclear and cytologic atypia, as well as an absence of mitotic figures, that occasionally formed insular or organoid patterns suggestive of a neuroendocrine origin (Fig. 2). Both adenocarcinoma and small cell carcinoma/carcinoid-like tumor frequently elicited intense peri- and intratumoral fibroplasia (scirrhous response) and chronic inflammation. Carcinomatosis, defined as extraintestinal spread of tumor cells throughout the coelomic cavity, was observed occasionally with both adenocarcinoma and small cell carcinoma/carcinoid-like tumor (Fig. 3). Carcinoma not otherwise specified was classified as such because this neoplastic entity was much less differentiated and organized than either adenocarcinoma or small cell carcinoma/carcinoid-like tumor, and indeed in some cases shared characteristics similar to both (Fig. 2). Tubular adenoma and tubulovillous adenoma were rare. Tubular adenomas (Fig. 2) were identified as a focal polypoid mass comprised of tubuloglandular-like structures within the lamina propria formed by hyperplastic epithelium with normal intestinal mucosa immediately adjacent to the mass, while tubulovillous adenomas had a combined pattern. Table 1 summarizes the various histological presentations, emphasizing characteristics that differ.
FIG. 2.
Intestinal neoplasia in zebrafish. H&E. Bar=25 μm, unless otherwise indicated. (A,B) Small cell carcinoma, with small nests of fusiform to pleomorphic tumor cells (arrows) in the lamina propria that occasionally form organoid patterns. E, epithelium. (C, D) Adenocarcinoma, with tumor cells forming pseudoacinar structures (arrows), complete with a lumen in the most advanced tumors. (E) Carcinoma not otherwise specified in the lamina propria. Less differentiated and organized than the adenocarcinoma and small cell carcinoma. (F) Tubular adenoma, with glandular-like pattern. Bar=100 μm. (G) Tubulovillous adenoma, with the villotubular pattern. Bar=100 μm.
FIG. 3.
Carcinomatosis (adenocarcinoma), characterized by disorganized nests of tumor cells (arrows) infiltrating through the layers of the anterior intestine, with extension into the coelomic cavity. H&E. Bar=50 μm.
Table 1.
Defining Histological Signs of Intestine Presentations as Observed Within Zebrafish Submitted to the Zebrafish International Resource Center Diagnostic Service 2000–2012
| Intestinal presentation | Defining signs |
|---|---|
| Normal intestine | One cell thick layer of columnar epithelial cells lining mucosal folds with basally-oriented oval nuclei; mucosal folds become progressively shorter caudally, causing “villi” (the normal undulating structure of the intestinal wall appears villous, but lacks the true anatomic characteristics of villi) to appear shorter as the intestine approaches the excretory vent (anus); lamina propria, but no submucosa; inner circular and outer longitudinal smooth muscle layers invest the intestine throughout its length. Mucosal mucus (goblet) cells can be observed and increase in number distally. |
| Hyperplastic intestine | Multilayered and increased numbers of epithelial cells, especially within basilar mucosal folds; “piling-up” of mucosal epithelial cells; nuclear pseudostratification; enhanced nuclear basophilia; pseudocrypt formation resulting from increased mucosal folding; anisokaryosis frequently observed and increased mitotic figures. |
| Dysplastic intestine | Features of hyperplastic intestine in addition to increased nuclear and cellular pleomorphism, and occasionally aberrant mitotic figures, the loss of nuclear polarity and disorganization or absence of pre-existing histoanatomic architecture. |
| Intestinal adenocarcinoma | Features of dysplastic intestine plus formation of disorganized pseudocrypts with invasion deep into the lamina propria and frequently through the basement membrane into the underlying muscularis layers; bizarre mitotic figures; neoplastic epithelial cells are pleomorphic and may be columnar, cuboidal or attenuated; hyperchromatic nuclei; annular strictures and fibroplasia frequently accompany tumorigenesis; pseudocrypts formed by the folding of neoplastic mucosal epithelium often resembled pseudoacinar structures that contained intraluminal sloughed rafts of necrotic neoplastic cells. |
| Intestinal small cell carcinoma/carcinoid-like tumor | Sheets and nests of round, polygonal or fusiform cells with minimal cytoplasm; hyperchromatic nuclei with granular chromatin and inconspicuous nucleoli; extensive fibroplasia; tumor cells occasionally formed an insular or organoid pattern characteristic of neuroendocrine tumors. |
| Intestinal tubular/tubulovillous adenoma | Focal adenomatous polypoid structures with clusters of proprial pseudocrypts resembling mammalian glandular colonic crypts. The pseudocrypts often are lined by hyperplastic mucosal epithelium where the cells are crowded and have hyperchromatic nuclei. Increased mitotic figures are observed. Tubulovillous adenoma is essentially similar to tubular adenoma with a combination of both villous and pseudocrypt structures. |
Review of historical prevalence of lesions
The prevalence of preneoplastic changes and neoplastic changes within the intestine among zebrafish submitted to the ZIRC diagnostic database between January 4, 2000 and July 3, 2012 is summarized in Table 2 and involved approximately 2% of the total fish submitted within this period. Of the 2% total fish affected by these intestinal lesions, 1.7% of the fish were submitted as subclinical and 0.3% as clinical. Fish submitted as clinical were those that exhibited clinical signs of any disease when they were collected. Fish submitted as subclinical were healthy-appearing fish; they demonstrated no clinical signs of disease and may have been either from sentinel tanks or collected randomly from main facility tanks as a general health check. Fish classified as neoplastic often displayed preneoplastic changes, but were not counted among the fish with preneoplastic changes for this study, as we considered the tumor formation to be a notable progression following the preneoplastic lesions.
Table 2.
Prevalence of Preneoplastic and Neoplastic Lesions in Zebrafish Submitted to the Zebrafish International Resource Center Diagnostic Service 2000–2012
| Year | Number of positive cases/total submitted cases | Number of positive clinical fish/total clinical fish | Number of positive subclinical fish/total subclinical fish | Number of affected labs/total submitting labs | Lines of fish affected |
|---|---|---|---|---|---|
| 2000 | 2/41 | 0(0)a/119 | 1(1)/60 | 2/13 | Albino, Brass (AB) |
| 2001 | 3/38 | 3(0)/90 | 0(0)/57 | 2/14 | Albino, 5D |
| 2002 | 3/57 | 1(1)/184 | 4(5)/176 | 2/19 | AB |
| 2003 | 2/47 | 0(0)/157 | 2(0)/432 | 1/16 | AB |
| 2004 | 4/61 | 0(1)/201 | 3(5)/208 | 2/29 | Golden Tupfel Long Fin, AB |
| 2005 | 3/53 | 0(0)/154 | 3(8)/218 | 2/24 | AB |
| 2006 | 4/63 | 0(4)/282 | 4(3)/456 | 2/25 | AB, Ekkwill |
| 2007 | 6/68 | 4(2)/237 | 1(0)/511 | 5/31 | AB, SJA, Wageningen ZF WT Zodiac F5 Line, Coagulation Factor II Mutagenized Transgenic |
| 2008 | 6/71 | 1(0)/143 | 13(6)/1024 | 5/28 | AB, Tupfel Long Fin |
| 2009 | 8/78 | 2(3)/153 | 10(9)/1064 | 6/30 | AB, WIK |
| 2010 | 4/54 | 3(1)/ 57 | 11(5)/1179 | 2/25 | AB |
| 2011 | 12/95 | 0(0)/191 | 39(21)/1277 | 8/44 | AB, Tuebingen, WIK |
| 2012 | 3/53 | 0(0)/49 | 7(8)/860 | 2/25 | AB, Tuebingen |
| Study Totals | 60/779 | 14(12)/2017 | 98(71)/7522 |
Positive clinical and subclinical fish totals are distinguished by fish with neoplastic changes (without parenthesis) and preneoplastic changes (within parenthesis).
It was not uncommon for more than one fish to be affected by preneoplastic or neoplastic changes within a single case submission. By a case basis (several individual fish from one population), 32.2% of the cases included both preneoplastic changes and neoplastic changes amongst the submitted specimens. The mean age of zebrafish with preneoplastic changes was 402 dpf (days post fertilization), with a range of 188–731 dpf. The mean age of zebrafish with neoplastic changes was 477 dpf, with a range of 188–1071 dpf. The affected fish included 107 females and 88 males. Within the affected female population, 58.9% were classified as neoplastic and 41.1% were preneoplastic. The affected male population was classified as 55.7% neoplastic and 44.3% preneoplastic. Eleven genetically distinct lines of zebrafish were connected to the affected populations. The affected fish came from a total of 18 labs, both domestic and international.
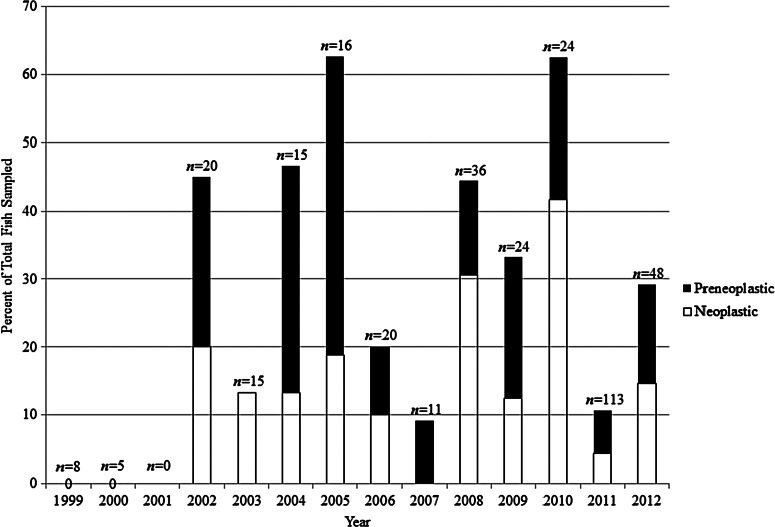
A single zebrafish facility in the USA submits a large volume of diagnostic and normal sentinel zebrafish cases to ZIRC on a regular basis and so it was described as the primary facility for the purposes of this study. Approximately 74% of the fish affected by these intestinal changes, or 144 fish, came from the primary facility. The majority of these fish were part of the facility's sentinel program. The prevalence of intestinal changes amongst this subpopulation of affected fish occurred continuously from 2002 to 2012 (Fig. 4) at an average of approximately 32% of the sentinels affected each year, with a range of 9.1%–62.6%. Preneoplastic and neoplastic intestinal changes occurred at comparable proportion each year.
FIG. 4.
Prevalence of preneoplastic and neoplastic changes amongst the sentinel fish from a single, large zebrafish research facility in the USA (the primary facility cited in the text). There were no positive sentinel fish in 1999 or 2000. No sentinel fish were sampled in 2001.
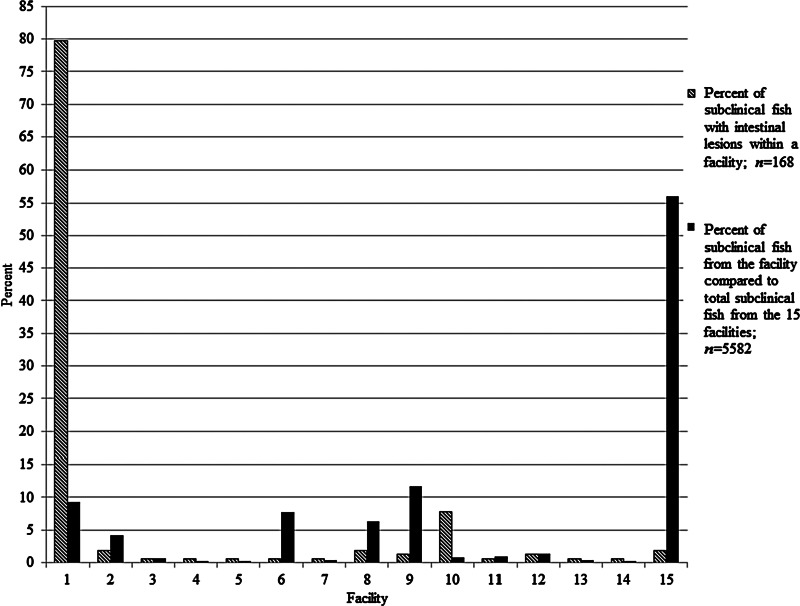
Comparisons of percent subclinical fish with the lesions amongst 15 facilities with a history of the lesions are reported in Figure 5. Close to 80% of the subclinical fish from these facilities that had the intestinal lesions were from the primary facility, while the facility with the next highest prevalence was responsible for less than 10% of the affected fish. The other affected facilities submitted 0.5%–11.7% of the total subclinical fish amongst the affected facilities, with a mean of 3.1% submissions per facility. Another facility submitted over 55% of the samples, but showed about 2% prevalence of the lesions.
FIG. 5.
Prevalence of intestinal preneoplastic and neoplastic changes in subclinical zebrafish relative to total subclinical fish submitted from 15 facilities from 2000–2012. The single, large zebrafish research facility (the primary facility cited in the text) is included.
Table 3 summarizes the prevalence of various intestinal presentations amongst all the fish examined. A total of 82 fish from the entire data set had preneoplastic changes; the majority of which showed only hyperplasia, and some exhibited a combination of hyperplasia and dysplasia. The majority of the 113 tumors were classified as adenocarcinomas or small cell carcinomas/carcinoid-like tumors, whereas the remaining lesions were classified as carcinoma not otherwise specified, tubular adenoma, or tubulovillous adenoma (Table 3). The progression of the neoplastic process to carcinomatosis was observed in 1.5% of fish with neoplastic changes. The majority of the tumors and preneoplastic changes were observed between the anterior and mid-intestine, with rare occurrence in the distal third of the intestine. A total of 14 of 82 (17.1%) fish with preneoplastic lesions exhibited enteritis. Enteritis was observed in five fish with neoplasia, and hence over all prevalence of the former lesion was 9.7% in fish with either preneoplastic or neoplastic lesions. Enteritis was not observed in fish without lesions.
Table 3.
Prevalence of Intestine Presentations as Observed Within Zebrafish Submitted to the Zebrafish International Resource Center Diagnostic Service 2000–2012
| Type | Prevalencea (%) | |
|---|---|---|
| Preneoplastic changes | Hyperplasia only | 67.1 |
| Hyperplasia and dysplasia | 32.9 | |
| Neoplastic changes | Adenocarcinoma | 50.4 |
| Small cell carcinoma/carcinoid-like | 37.2 | |
| Carcinoma not otherwise specified | 9.7 | |
| Tubular adenoma | 1.8 | |
| Tubulovillous adenoma | 0.9 |
Fish classified as neoplastic often displayed preneoplastic changes, but are not counted among the fish with preneoplastic changes for this study, as we considered the tumor formation a notable progression following the preneoplastic lesions. For this reason, prevalence is calculated relative to either the preneoplastic or neoplastic population affected.
Diet study
Most of the fish in all groups survived and appeared healthy after 6 months feeding the various diets. A total of 32 fish (16 fish/tank) were examined from each group, except one tank fed Diet 5 (the SARL zebrafish diet) contained only 13 fish. None of the fish exhibited histological changes consistent with the preneoplastic or neoplastic lesions reported here. The complete lack of lesions in fish fed Diet 4 at SARL was significantly different (p<0.001) compared to the fish fed the same diet prepared at the primary facility, as these fish showed approximately 33% and 63% prevalence of intestinal lesions in 2009 and 2010, respectively (Fig. 3).
Discussion
Our systematic retrospective survey of the ZIRC diagnostic survey database and histological analysis of archived samples revealed a high incidence of intestinal neoplasia among laboratory-reared zebrafish. Intestinal neoplasia identified in the ZIRC diagnostic database was primarily adenocarcinoma and small cell carcinoma/carcinoid-like tumor. Histomorphologic characteristics of these tumors were used in classification and identification at the tissue and cellular level. These intestinal tumors shared many of the common microscopic characteristics observed in their human counterparts, including small cell carcinoma and adenocarcinoma.17–19
Although the cell of origin for zebrafish small cell carcinoma/carcinoid-like tumor is currently unknown, it is reasonable to postulate that the enteroendocrine cell of the zebrafish intestine may be a likely source, because this intestinal cell type is indicated as a progenitor cell of small cell carcinoma in mice.20 This is further supported by the observation that similar to mice, mitotic figures are not present in these tumors of zebrafish as well, because terminally differentiated enteroendocrine cells do not undergo cell division.21 Although zebrafish and humans share many conserved cancer gene sequences, the molecular studies already conducted in zebrafish tumor models do not conclusively prove that identical molecular mechanisms are responsible for tumor development or more importantly, that zebrafish tumors have the same histogenesis as the human counterpart.22
Further descriptive work at the tissue and cellular levels are prerequisites to molecular based studies. Immunohistochemical identification and confirmation of the cell of origin for these intestinal tumors is imperative and would provide a useful adjunct to histomorphologic classification. For most zebrafish intestinal tumors, there is remarkable conservation of protein antigens that closely parallel human tumors, for which there are current zebrafish models. As an example, both human and zebrafish adenocarcinoma and small cell carcinoma/carcinoid-like tumors retain identical specific protein antigen and cell proliferation markers that are important in identifying and characterizing them, including cytokeratins, chromogranin A,23,24 S-100, synaptophysin, insulin, glucagon, somatostatin, PCNA, and cdx2.25,26 Therefore, it is essential to more fully characterize zebrafish tumors not only at the histomorphologic and cellular levels, but also at the tumor protein (i.e., antigen) level before more fully investigating molecular aspects, such as gene expression, in zebrafish tumors.
Whether spontaneous or induced, zebrafish tumors must be initially approached in a phylogenetic context if they are to be generalized to similar human tumors.22 Generation and development of monoclonal antibodies has advanced since the early experimental procedures,25 which involved using whole tumor cells or protein fractions as immunogens, to molecular approaches using known amino acid sequences that allow creation of immunogens from specific tumor cell peptides. Exploitation of this peptide generated from the amino acid sequence of the antigen of interest would be critical for establishing a zebrafish-specific tumor antigen immunostain panel and potential antigen-directed research modalities applicable to human medicine, such as experimental anti-neoplastic therapies. The intestinal tumors described in this study are currently under immunohistochemical evaluation within our lab. Zebrafish develop common spontaneous neoplasia associated with aging, including spermatocytic seminoma and ultimobranchial gland adenoma that occur in zebrafish at 1.5 to 2 years of age,7,10 and embryonal neuroectodermal tumors of the central nervous system in both juvenile and adult fish.5,27 Although many zebrafish tumors recapitulate their human counterparts in terms of basic histologic appearance, certain molecular characteristics (increased cell proliferation, nuclear atypia, and cellular differentiation) and mechanisms of regulation (cell cycle and apoptosis),3,22,28 there is relatively little understanding of how conserved tumor antigens are between the two species. Some researchers developing zebrafish tumor models consider histologic evaluation as an unnecessary step,29 which would lead to potentially erroneous conclusions because without it as a starting point, obvious tissue and cellular similarities cannot be determined.
Although these intestinal tumors were observed in several facilities, the definitive causative agent is unknown. Possible etiological factors include genetics, water-borne carcinogens, infectious agents, or some combination of these. Knockout mutants in zebrafish are well established as cancer models,1 and these mutants demonstrate an increased propensity towards developing cancerous lesions. For example, apc/+ (AB mutant) zebrafish have been reported to develop intestinal tumors.9 Genetics as a cause of tumorigenesis in this study is unlikely, given that 11 different genetic lines displayed preneoplastic and/or neoplastic changes, including wild-type lines. Additionally, intestinal proliferative lesions and subsequent neoplasia do not appear to be sex-linked, as both males and females are similarly affected.
Whereas the primary facility had the greatest number of affected fish with preneoplastic or neoplastic intestinal lesions, this does not appear to be due to an increased frequency of subclinical submissions. For example, adjusting for this factor, we found that the prevalence was indeed much higher in the primary facility than the others. Moreover, facility 15 submitted the most subclinical fish (approximately 60%) amongst the affected facilities, but showed a low prevalence of the lesions (Fig. 5). We cannot suggest a potential cause of the high prevalence in the primary facility as it appears to be managed and operated no differently than traditional zebrafish facilities with large recirculating systems.
Diet has been implicated in the progression from chronic inflammation to tumorigenesis in the gastrointestinal tract of Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) fed a commercial diet rich in plant products.30 Diet as a cause of the intestinal lesions described here was unlikely as a potential source of carcinogenesis based upon the experiment carried out at SARL. Data from the retrospective study suggest that intestinal lesions may be observed as early as 6 months of age and the sampling at 6 mo yielded no sign of preneoplastic or neoplastic intestinal changes. Although not all fish develop the lesions this young, it would be expected at minima some progression towards these intestinal lesions in some of the fish would have occurred if diet was the cause. The Diet 4 fed at SARL was the exact same diet in regards to formulation and source material that was used at the primary facility, which on average has a prevalence of intestinal changes of 32% per annum. Moreover, the experiment at SARL was conducted with diet prepared at the primary facility in 2009, and the sentinel fish at this location showed approximately 33% prevalence of lesions in 2009 and 63% prevalence in 2010. Nevertheless, our results excluding diet as the cause of these lesions should be considered preliminary at this time because the experiment was terminated after only 6 mo.
A water-borne carcinogen must also be considered as chemical carcinogens such as N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), methylazoxy-methanol acetate (MAMA), and DMBA have all been previously demonstrated to cause neoplasia in zebrafish.31 Proliferative lesions similar in pattern and location to some of the tumors were observed in fry and juvenile zebrafish exposed to DMBA by bath and diet exposure, respectively.10 Although we tended to see the lesions in older fish compared to the findings from the DMBA study, this may be explained by a lower exposure dosage or a less tumorigenic water-borne carcinogen. The typical zebrafish facility also has a very rigorous water filtration system, involving any combination of sand or bead filtration systems, activated carbon filters, reverse osmosis, and UV filtration, resulting in very pure water. Any carcinogen must get past these complexes of filters or be introduced downstream of the filtration process, either as a component of the material used to transport the water or to house the fish.
Several parasites have been implicated as promoters of neoplasia, most notably the nematode Spirocerca lupi, infection with which has been associated with osteosarcoma and esophageal fibrosarcoma in dogs.32 Another agent associated with intestinal neoplasia in zebrafish, while not established as a causative agent, is the nematode Pseudocapillaria tomentosa. Zebrafish that were exposed to both DMBA and P. tomentosa demonstrated a higher prevalence of intestinal tumors than uninfected fish exposed to DMBA.11 Whereas this nematode was implicated in the original diagnosis of several affected fish, it was not prevalent amongst the affected fish in our study.
Other infectious agents are also suspected, whether bacterial or viral. Helicobacter pylori has been previously associated with human gastroesophageal neoplasia, and similar gastric carcinogenesis has been modeled in the Mongolian gerbil.33 Although experimental evidence has not linked bacteria to carcinogenesis in zebrafish to date, the chronic inflammation elicited by certain pathogenic strains of bacteria, and even the natural microbiota of zebrafish could potentially serve as promoters of intestinal carcinogenesis. Viruses have been connected to certain fish cancers,34 such as SLV (salmon leukemia virus) in chinook salmon35 and WDSV (walleye dermal sarcoma virus) in walleye.36 Although endogenous retroviruses have been identified in zebrafish,37 no oncogenic viruses have been currently implicated as the cause of intestinal neoplasia in zebrafish. To date, no naturally occurring pathogenic virus has been isolated from zebrafish.38,39 Transmission studies are currently underway within our lab to evaluate the possibility of an infectious etiology for these intestinal lesions.
Our survey demonstrates that intestinal neoplasia and preneoplastic pathology are common among zebrafish research facilities. The fish surveyed in this study are not a random selection and there is a bias by the volume of cases submitted by the primary facility, but these fish do represent many different research facilities and fish genotypes commonly used in zebrafish research. Based on the continuity of cases through the years and the fact that many of these lesions occur in subclinical fish, we suggest that these lesions could introduce an underlying, unappreciated variable into zebrafish research.
Acknowledgments
The retrospective portion of this study was supported by departmental funds and the Joan Countryman Suite Graduate Fellowship (to CE Paquette), NIH NCRR T32 RR023917 (to TS Peterson), NIH NICHD #P01HD22486, and NIH NCRR P40 RR012546. The diet study was conducted at the Sinnhuber Aquatic Research Lab and supported by NIEHS Center grant P30 ES000210. We would like to thank Dr. Katrina Murray at the Zebrafish International Resource Center, Eugene, Oregon for manuscript review and editing and Dr. Christiane Löhr and the Veterinary Diagnostic Lab at the Oregon State University College of Veterinary Medicine for supplementary diagnostic assistance. Additionally, we would like to thank Benjaporn Somridhivej for statistical support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Faro A. Boj SF. Clevers H. Fishing for intestinal cancer models: Unraveling gastrointestinal homeostasis and tumorigenesis in zebrafish. Zebrafish. 2009;6:361–376. doi: 10.1089/zeb.2009.0617. [DOI] [PubMed] [Google Scholar]
- 2.Liu S. Leach SD. Zebrafish models for cancer. Annu Rev Pathol. 2011;6:71–93. doi: 10.1146/annurev-pathol-011110-130330. [DOI] [PubMed] [Google Scholar]
- 3.Shive H. Zebrafish models for human cancer. Vet Pathol. 2012 doi: 10.1177/0300985812467471. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 4.Aleström P. Holter JL. Nourizadeh-Lillabadi R. Zebrafish in functional genomics and aquatic biomedicine. Trends Biotechnol. 2006;24:15–21. doi: 10.1016/j.tibtech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Peterson TS. Heidel JR. Murray KN. Sanders JL. Anderson WI. Kent ML. Malignant dysembryoplastic neuroepithelial tumor in a zebrafish (Danio rerio) J Comp Pathol. 2012 doi: 10.1016/j.bbr.2011.03.031. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma M. Shrivastav AB. Pandey G. Overviews of the zebrafish model and fish neoplasms. Global J Pharma Res. 2012;1:736–743. [Google Scholar]
- 7.Smolowitz R. Hanley J. Richmond H. A three-year retrospective study of abdominal tumors in zebrafish maintained in an aquatic laboratory animal facility. Biol Bull. 2002;203:265–266. doi: 10.2307/1543433. [DOI] [PubMed] [Google Scholar]
- 8.Berghmans S. Murphey RD. Wienholds E, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haramis AG. Hurlstone A. van der Velden Y, et al. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitsbergen JM. Tsai HW. Reddy A, et al. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol Pathol. 2000;28:705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- 11.Kent ML. Bishop-Stewart JK. Matthews JL. Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52:654–658. [PubMed] [Google Scholar]
- 12.Wallace KN. Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 13.Wallace KN. Akhter S. Smith EM. Lorent K. Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Menke AL. Spitzbergen JM. Wolterbeek APM. Woutersen RA. Normal anatomy and histology of the adult zebrafish. Toxicol Pathol. 2011;39:759–775. doi: 10.1177/0192623311409597. [DOI] [PubMed] [Google Scholar]
- 15.Kent ML. Buchner C. Watral VG, et al. Development and maintenance of a specific pathogen free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ. 2011;95:73–79. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent ML. Spitsbergen JM. Matthews JM. Fournie JW. Westerfield M. Eugene (OR): Zebrafish International Resource Center; c2006–2012. Diseases of zebrafish in research facilities [Internet] [cited 2012 Sep 25] [Google Scholar]
- 17.Brenner B. Tang LH. Klimstra DS. Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: A review. J Clin Oncol. 2004;22:2730–2739. doi: 10.1200/JCO.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu GS. The endodermal origin of digestive and respiratory tract APUD cells. Histopathologic evidence and a review of the literature. Am J Pathol. 1979;96:5–17. [PMC free article] [PubMed] [Google Scholar]
- 19.Lingeman CH. Garner FM. Comparative study of intestinal adenocarcinomas of animals and man. J Natl Cancer Inst. 1972;48:325–346. [PubMed] [Google Scholar]
- 20.Cheng H. Leblond CP. Origin, differentiation, and renewal of the four main epithelial cell types in the mouse small intestine III. Entero-endocrine cells. Am J Anat. 1974;141:503–520. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- 21.Lauren P. The cell structure and secretion in intestinal cancer. With reference to benign epithelial tumors of the bowel. Acta Pathol Microbiol Scand Suppl. 1961;152:1–151. [PubMed] [Google Scholar]
- 22.Amatruda JF. Patton EE. Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol. 2008;271:1–34. doi: 10.1016/S1937-6448(08)01201-X. [DOI] [PubMed] [Google Scholar]
- 23.Lai M. Lu B. Xing X. Xu E. Ren G. Huang Q. Secretagogin, a novel neuroendocrine marker, has a distinct expression pattern from chromogranin A. Virchows Arch. 2006;449:402–409. doi: 10.1007/s00428-006-0263-9. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence B. Gustafsson BI. Kidd M. Pavel M. Svejda B. Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrin Metab Clin North Am. 2011;40:111–134. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Fink LM. Clarke SM. Monoclonal antibodies as diagnostic reagents for the identification and characterization of human tumor antigens. Prog Clin Pathol. 1984;9:121–133. [PubMed] [Google Scholar]
- 26.Moskaluk CA. Zhang H. Powell SM. Cerilli LA. Hampton GM. Frierson HF. Cdx2 protein expression in normal and malignant human tissues: An immunohistochemical survey using tissue microarrays. Mod Pathol. 2003;16:913–919. doi: 10.1097/01.MP.0000086073.92773.55. [DOI] [PubMed] [Google Scholar]
- 27.Kagan RA. Pinkerton ME. Kinsel MJ. Neuronal embryonal tumors in fish. Vet Pathol. 2010;47:553–559. doi: 10.1177/0300985809359600. [DOI] [PubMed] [Google Scholar]
- 28.Kloppel G. Tumor biology and histopathology of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2007;21:15–31. doi: 10.1016/j.beem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Stoletov K. Klemke R. Catch of the day: Zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 30.Dale OB. Tørud B. Kvellestad A. Koppang HS. Koppang EO. From chronic feed-induced intestinal inflammation to adenocarcinoma with metastases in salmonid fish. Am J Cancer Res. 2009;69:4355–4362. doi: 10.1158/0008-5472.CAN-08-4877. [DOI] [PubMed] [Google Scholar]
- 31.Spitsbergen JM. Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—Advantages and current limitations. Toxicol Pathol. 2003;31:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvir E. Clift SJ. Williams MC. Proposed histological progression of the Spirocerca lupi induced oesophaeal lesions in dogs. Vet Parasitol. 2010;168:71–77. doi: 10.1016/j.vetpar.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Honda S. Fujioka T. Tokieda M. Satoh R. Nishizono A. Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Am J Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 34.Quakenbush SL. Rovnak J. Casey RN, et al. Genetic relationship of tumor-associated piscine retroviruses. Mar Biotechnol. 2001;3:S88–S99. doi: 10.1007/s10126-01-0030-5. [DOI] [PubMed] [Google Scholar]
- 35.Eaton WD. Kent ML. A retrovirus in chinook salmon (Oncorhynchus tshawytscha) with plasmacytoid leukemia and evidence for the etiology of disease. Am J Cancer Res. 1992;52:6496–6500. [PubMed] [Google Scholar]
- 36.Martineau D. Bowser PR. Renshaw RR. Casey JW. Molecular characterization of a unique retrovirus associated with a fish tumor. J Virol. 1992;66:596–599. doi: 10.1128/jvi.66.1.596-599.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C. Steiner LA. Genome structure and thymic expression of an endogenous retrovirus in zebrafish. J Virol. 2004;78:899–911. doi: 10.1128/JVI.78.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crim MJ. Riley LK. Viral diseases in zebrafish: What is known and unknown. ILAR J. 2012;53:135–143. doi: 10.1093/ilar.53.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitsbergen JM. Buhler DR. Peterson TS. Neoplasia and neoplasm associated lesions in laboratory colonies of zebrafish emphasizing key influences of diet and aquaculture system design. ILAR J. 2012;53:114–125. doi: 10.1093/ilar.53.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]