Abstract
Interstitial cells of Cajal (ICC) provide a pacemaker signal for coordinated motility patterns in the mammalian gastrointestinal (GI) tract. Kit signaling is required for development and maintenance of ICC, and these cells can be identified by Kit-like immunoreactivity. The zebrafish GI tract has two distinct ICC networks similar to mammals, suggesting a similar role in the generation of GI motility; however, a functional role for Kit-positive cells in zebrafish has not been determined. Analysis of GI motility in intact zebrafish larvae was performed during development and after disruption of Kit signaling. Development of coordinated motility patterns occurred after 5 days post-fertilization (dpf) and correlated with appearance of Kit-positive cells. Disruptions of Kit signaling using the Kit antagonist imatinib mesylate, and in Sparse, a null kita mutant, also disrupted development of coordinated motility patterns. These data suggest that Kit signaling is necessary for development of coordinated motility patterns and that Kit-positive cells in zebrafish are necessary for coordinated motility patterns.
Introduction
The proto-oncogene c-kit is expressed by cells located within the tunica muscularis of the gastrointestinal (GI) tract of all vertebrate species so far examined, including zebrafish.1–4 Early work showed that cells expressing Kit in the GI tract are necessary for initiation and regulation of coordinated muscular contractions. These cells, referred to as interstitial cells of Cajal (ICC), are identified using antibodies to the Kit protein, a specific marker for ICC. Although ICC are necessary for coordinated motility patterns that functionally support mixing and propulsion of luminal contents, complex patterns of muscular contractions result from the integrated activity of several cell types, including smooth muscle cells, enteric neurons, and ICC.5–7 A specific role for ICC in GI motility has been inferred from experiments quantifying GI motility after preventing the development of ICC. For example, experiments examining Kit receptor function showed that peritoneal injection of the neutralizing Kit antibody ACK2 resulted in a severe disruption of GI motility in mice, and a concomitant reduction of Kit immune-positive (Kit+) cells in the small intestine.2,8,9 Similarly, pharmacological inhibition of Kit function using imatinib mesylate on cultured embryonic tissues prevented ICC development and eliminated pacemaker function.10 Those experiments showed that antagonists to the Kit signaling pathway, when applied at the perinatal period, resulted in GI dysmotility and a parallel loss of ICC. Furthermore, mutant mouse models with loss of Kit signaling lack specific classes of ICC and do not have normal motility patterns.2,11–13 Two important examples are the compound heterozygote W/Wv mutant, a Kit mutant with≈90% loss of Kit signaling, and the compound heterozygote Sl/Sld mutant that partially lacks a membrane-bound form of Kit ligand.2,13 Both mutants are viable but have severely dilated intestine, incomplete ICC networks, and disrupted GI motility patterns.
Kit+ cells in the adult zebrafish GI tract form two distinct cellular networks, one deep in the circular muscle layer close to the mucosal border and the other between the inner circular and outer longitudinal muscle layers.14 Zebrafish Kit+ networks are similar in appearance to ICC in the myenteric plexus regions and muscle layers of mice and human muscularis propria. Myenteric ICC form a continuous network around the circumference and along the length of the adult GI tract between the longitudinal and circular muscle layers, and bipolar ICC populate the deep muscular plexus of the small intestine.15 It is well known that ICC generate a spontaneous, rhythmic oscillation in resting membrane potential called the electrical slow wave which paces muscular contractions.5 Therefore, ICC are fundamental for generation and regulation of spontaneous and coordinated muscular contractions in the GI tract. Interaction between the enteric nervous system and ICC are well documented, and bipolar ICC intercalate with enteric motor neurons and function to amplify and distribute neural signals.7,16 Although our previous work demonstrated Kit+ networks that are similar in appearance and in location to ICC networks in human and mouse GI tissues, a functional role for zebrafish Kit+ cells has not been determined.
Kit signaling is also necessary for vertebrate melanocyte development, and null mutants for Kit or its natural ligand Steel Factor (also called stem cell factor and Kit ligand) are lethal in mice, resulting from severe effects on hematopoiesis.17 However, partial loss of function mutants are viable and display coat color deficiency resulting from the failure of melanocyte migration.18 Sparse is a null kita mutant in zebrafish, and homozygous mutants are viable and display a reduced melanocyte embryonic phenotype.19 Hultman and co-workers used morpholino knockdown to establish kita and kitla as functional signaling pairs that promote melanocyte migration and survival during embryogenesis.20 A role for kita in GI motility has been confirmed because Sparse mutants exhibit a reduced contraction frequency and an enlarged GI tract.14 It is clear that kitb and kitlb are not involved in melanocyte survival, but a separate functional role for these orthologs remains unknown.
The zebrafish GI tract does not contain a stomach, but is otherwise anatomically similar to the human GI tract. Concentric tissue layers surround the lumen beginning with an inner mucosa, circular muscle, a region with relatively high density of enteric neurons and Kit+ cells, and an outer layer of longitudinal muscle.14,21 At 5 days post-fertilization (dpf), the yolk sac is depleted, spontaneous feeding begins, and spontaneous contractions have been detected, but the development of coordinated motor patterns as well as the mechanisms influencing this development have not been characterized in detail. The GI tract is similar to adults at 5 dpf, with circular and longitudinal muscle layers separated by enteric neurons.22,23 Two neurotransmitters, ACh and nitric oxide, modulate motor activity at 5 dpf, verifying that the neurotransmitter receptors and downstream signaling systems are present at this early developmental stage.24 However, tetrodotoxin did not alter motility patterns or the properties of individual contractions at 5 dpf, suggesting that motility is not under tonic control by the enteric nervous system at this age.25 In contrast, neural blockade 2 days later, at 7 dpf, reduced but did not block contraction frequency and contraction propagation distance. Muscular contractions propagate more completely at 7 dpf compared to 4 dpf, consistent with development of regulatory mechanisms during this time period.25 We have shown that Kit+ cells are present at 7 dpf, but not at 5 dpf, and it is possible that Kit+ cells contribute to the development of coordinated motor patterns.14
The objective of this study was to determine the functional role of Kit-positive cells in zebrafish GI motility. We quantified motor patterns in control larvae and compared these with motor patterns before ICC develop, or under conditions when Kit signaling was inhibited.
Materials and Methods
Aquaculture
Wild-type and Sparse mutant zebrafish were obtained from the Zebrafish International Resource Center and were maintained in our facility according to established protocols and in accordance with IACUC guidelines.14 Fish were housed in a rack system with recirculating water (Aquaneering) maintained at 28°C on a 14-h/10-h light/dark cycle. System water was made from deionized water with 240 mg/L Instant Ocean salts and 75 mg/L NaHCO3, resulting in a conductivity≈450 mS and pH≈7.4. Approximately 10% of the water was changed each day. Zebrafish were fed live brine shrimp once each day prepared using decapsulated cysts (Seahorse Source, Fort Peirce, FL). Dry food was fed twice each day (Aquaneering or Decapsulated Artemia Cysts, Jehmco Inc.). Crosses were performed in the morning and embryos were maintained in E3 embryo medium26 in petri dishes (≈25 embryos/dish) in an incubator set to 28°C. Embryos were transferred to clean dishes with fresh media every other day. Beginning at 7 dpf, larvae were fed hatchfry encapsulation, grade 0, by gently blowing powdered food using a Pasteur pipette onto the media to avoid overfeeding (Argent, Redmond, WA).
Imaging of GI motility
Larvae were immobilized for imaging using minor modifications to a previously described technique.27 Zebrafish larvae were anesthetized using 0.16 mg/mL tricaine (MS-222) and immobilized in 0.8% agar prepared with E3 containing tricaine anesthetic. The agar was kept in a warm water bath (≈40°C) to maintain fluidity. Larvae were removed from the incubator and anesthetized, placed in warm agar, gently drawn into a fluorinated ethylene propylene tube (1/32” diameter, Cole Parmer), and mounted in a custom-made slide holder that held a pool of water around the tube to reduce optical distortion and thereby enhance imaging. The tube was manipulated for optimal lateral viewing of the GI tract and single images were captured each second for 10 min using a Spot Insight video camera mounted on a Nikon Diaphot inverted microscope with a 4× or a 10× objective and using Spot software (Diagnostics Instruments Inc.). Image sequences were analyzed using custom written software (Volumetry G6a, Grant Hennig) to create and quantify spatio-temporal maps (STMaps). STMaps were created by calculating the average intensity along columns of pixels in a rectangular region of interest that was drawn over the zebrafish GI tract for each image in the sequence. Contractions are inferred from the change in the average density of the GI tract and contents, and appear as “ripples” or “bands” in STMaps. While precise measurements of external diameter of the GI tract could not be consistently performed due to a lack of contrast, average density STMaps allow the site of initiation of contractions, distance propagated, velocity of propagation, and frequency of events to be calculated. These parameters were calculated for each “ripple” by drawing a line manually over each propagating contraction. The time between sequential contractions was measured and reported as interval. The contraction frequency was calculated and is expressed as the total number of contractions over the 10 min recording period. This technique has been used previously to quantify gut motility.25 In preliminary experiments, we validated this assay by comparing spatial temporal mapping with manual analysis with similar results (data not shown).
Coordinated motility patterns were defined as a series of propagating muscular contractions in the middle and distal segments of the intestine that narrow the lumen and result in mixing and/or propulsion of intestinal contents in the aboral direction. By this definition, muscular contractions primarily support propulsion of luminal contents but also support a mixing function.6 Data presented here quantify GI motility in the middle and distal intestine up to 9 dpf because distance, velocity, and frequency did not appear to change significantly at later developmental stages (11 and 14 dpf). The proximal end of the middle intestine was identified as the point at which the intestinal lumen was obviously narrowed, similar to the defined mid-intestine by Kuhlman and Eisen,22 and Holmberg et al.25 We developed a method to simplify the comparison of motor patterns between multiple larvae to assess the degree of coordination. The motility index (MI) is a binary system to score STMaps as coordinated (1) or uncoordinated (0). Most individual contractions completely propagated from the intestinal bulb to the anus. In contrast, short contractions that do not propagate were not analyzed because they appear to be focal contractions that originate in smooth muscle and propagate passively to neighboring smooth muscle cells. This type of contraction is not regulated by enteric neurons or ICC and would not be scored as coordinated. We defined “coordinated” STMaps as those with at least 75% of contractions fully propagating and with no more than 1 irregular or skipped contraction for each STMap (during the 10 min recording period). We anticipated that approximately 75% of the contractions should fully propagate when regulatory elements are completely developed in the zebrafish GI tract.27
It is possible that fasting until 7 dpf negatively influences zebrafish health and GI motility patterns, because zebrafish normally begin feeding at 5 dpf. Effects of feeding on development were assessed by measuring standard length in fed and in fasted larvae at 7 dpf. Standard length averaged 4.0±0.3 mm (n=5) in fasted larvae, and 3.6±0.8 mm (n=8) in fed larvae, similar to values reported by Parichy et al.28 The effects of fasting at 9 dpf were not evaluated because all larvae in this study were fed beginning at 7 dpf. GI motor patterns were measured in 7 dpf fasted larvae, and in larvae that were fed once per day beginning at 5 dpf. Larvae were not fed on 7 dpf so that intestinal contents would not influence GI motor patterns. Contractions were observed in every fasted larvae (n=7) and in 6 out of 7 fed larvae. Coordinated contractions in the middle intestine were observed in 4 fed larvae and in 3 fasted larvae. No changes were observed in the average number of contractions, frequency, interval, distance, or velocity.
Solutions and drugs
Imatinib mesylate was obtained as a gift from Novartis (Basel, Switzerland) and was dissolved in dH2O water to prepare a 10 mM stock solution. For experiments using imatinib mesylate, embryos were maintained in E3 containing 40 μM embryo medium beginning at 4 dpf. Preliminary experiments showed that imatinib mesylate at 100 μM added shortly after fertilization, or at 4 dpf, was lethal.
Statistics
Results are presented as mean±standard error (SE). The number of different larvae used for each experiment is given as N. Larvae from at least 2 separate clutches were used for every experiment. Motility index data were analyzed using a Fisher's exact test with a confidence interval of 95%. Data involving parameters and age were analyzed using the Chi squared test for trend with alpha <0.05 representing a statistical difference. Data involving comparisons between Imatinib treated and untreated were analyzed using an unpaired Student t test with p<0.05 representing a statistical significance.
Results
GI motor patterns were examined in zebrafish larvae during development beginning at 5 days post-fertilization (dpf) before Kit+ cells are observed, but after feeding begins and spontaneous contractions appear.14 Ring-like contractions that completely occlude the lumen originated close to the transition between the intestinal bulb and the mid-intestine and propagated in both the oral and aboral directions. The region in which propagating contractions occurred is indicated by rectangular bars drawn just beneath the GI tract of a 7 dpf larvae with the middle and distal intestine region marked in black, and the anterior intestinal bulb region marked in gray (Fig. 1A). Three separate regions of coordinated motor patterns have been previously described.22,29 Orally propagating contractions are observed in the intestinal bulb, aborally propagating contractions are observed in the middle intestine, and orally propagating contractions are observed near the anus. Each type of propagating contraction, observed at 5, 7, and 9 dpf, are highlighted in Figure 1B. Contractions in the intestinal bulb were observed in 11 out of 14 5 dpf larvae, and in all 7 and 9 dpf larvae. Contractions near the anus were observed in most larvae that exhibited propagating contractions in the middle intestine. Focal contractions that do not propagate were also observed in each region. This study focused on propagating contractions in the middle intestine. Aborally propagating contractions were easily identified in the STMaps as dark ridges originating distal to the intestinal bulb which propagated in the anal direction, (from left to right; Fig. 1C). Motility patterns became increasingly coordinated with the number of days post-fertilization and this is apparent in the STMaps shown in Figure 1B–D. Contractions in the middle and posterior intestine were observed in 10 of 14 larvae examined at 5 dpf, and 5 STmaps were scored as coordinated (Fig. 1E). Short uncoordinated contractions were observed in 5 larvae; 4 larvae did not have contractions. The total number of propagating contractions per 10 min recording period averaged 6.2±1.2 at 5 dpf (Fig. 1F). The number of propagating contractions nearly tripled to 12.3±1.3 at 7 dpf in the 10 min recording period (p<0.05) and were seen in every larva examined. At 9 dpf, propagating contractions were also observed in 100% of larvae, and coordinated motility was observed in 6 out of 7 larvae. The total number of contractions was 13.6±1.2 per 10 min recording period at 9 dpf (p<0.05).
FIG. 1.
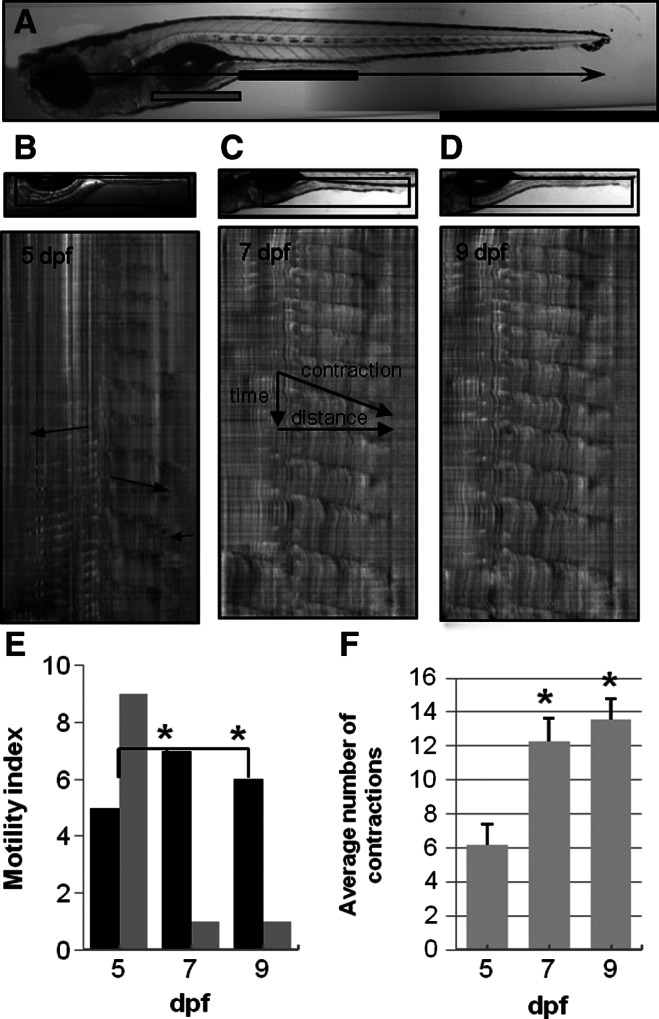
Development of coordinated motility patterns in zebrafish larvae. A 7 dpf larvae is shown in panel A. The anterior bulb and mid-distal intestine are indicated with gray and black bars, respectively. Standard length is shown in panel A and was measured from the snout to the posterior end of the notochord. The region of interest used to construct STMaps is outlined in black in images shown above the STMaps in panels B, C, and D. Patterns of propagating contractions are revealed in STMaps as dark ridges. Short contractions beginning in the mid- and distal intestine and propagating toward the anus are seen at 5 dpf (B). Retrograde contractions are apparent in the anterior region, and short, rapid contractions are apparent near the anal region (arrows, B). Contraction patterns in the mid- and distal intestine were more consistent and individual contractions were longer and more frequent at 7 and 9 dpf (C, D). The motility index, a measure of contraction coordination, increased from 36% (n=14) at 5 dpf to 88% (n=8) at 7 dpf and 86% at 9 dpf (n=7) (E). The number of STMaps showed coordinated motor patterns increased (black bars) and the number of maps scored as uncoordinated decreased (gray bars) between 5 and 7 dpf. The number of propagating contractions per 10 min recording also increased from 5 to 7 dpf and 9 dpf (n=7) (F); *p<0.05.
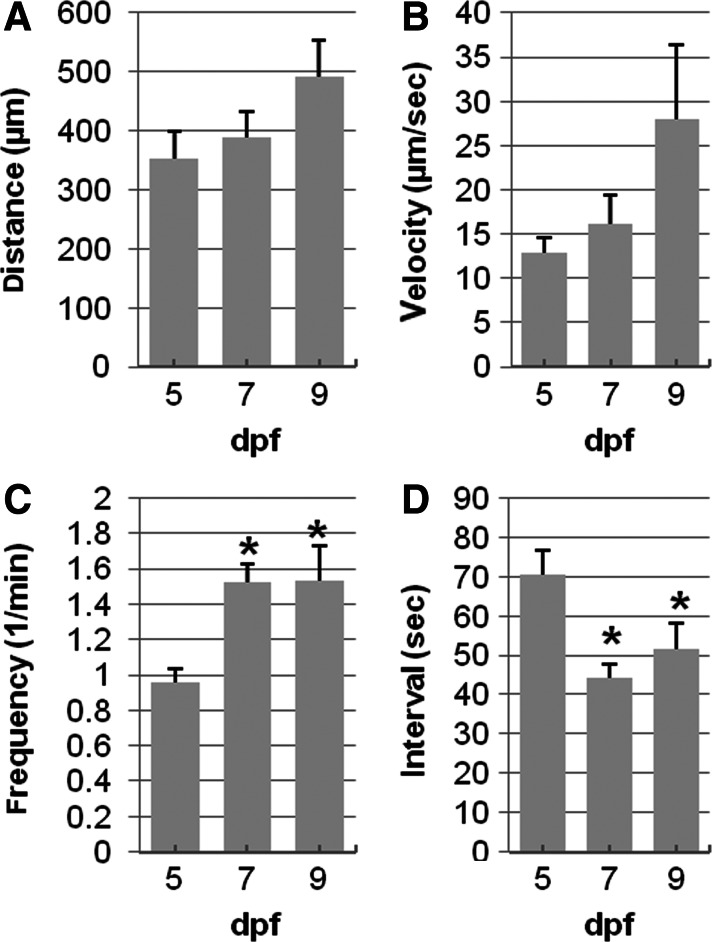
The properties of each propagating contraction were measured, resulting in values for contraction distance, velocity, frequency, and interval (time between contractions) (Fig. 2). The total number of contractions increased between 5 and 7 dpf, but the contraction distance and velocity was unchanged. Contraction frequency increased from 1.08±0.06 contractions/minute at 5 dpf (n=14) to 1.53±0.10 contractions/min at 7 dpf (n=8, *p<0.05). The average interval between successive contractions decreased from 70.3±6.5 sec to 44.3±3.2 sec (P<0.05). Therefore, the propagating motor patterns at 5 dpf occurred less frequently, but the observed contractions were essentially identical in length and in speed compared to contractions at 7 and 9 dpf.
FIG. 2.
Quantification of individual contractions with larvae age. Contraction distance and velocity were not changed significantly between 5 (n=9), 7 (n=8), and 9 dpf (n=7) (A and B). Contraction frequency increased (C) and interval (D), or time between contractions, decreased at 7 dpf compared to 5 dpf; *p<0.05.
The total length of the zebrafish (standard length) and the length of the mid-intestine were measured to exclude the possibility that changes in GI motility result from a simple increase in length of the larvae or the mid-intestine during developmental growth. Our definition for standard length (SL) was identical to Parichy et al.,28 and is represented by the black arrow in Figure 1A. The mid-intestine is indicated by the gray bar just beneath the intestine. SL did not increase during development from 5 to 9 dpf (SL was 4.1±0.04 mm at 5 dpf, 4.1±0.04 mm and 4.1±0.06 mm at 7 and 9 dpf, Fig. 3). Mid-intestine length was also unchanged.
FIG. 3.
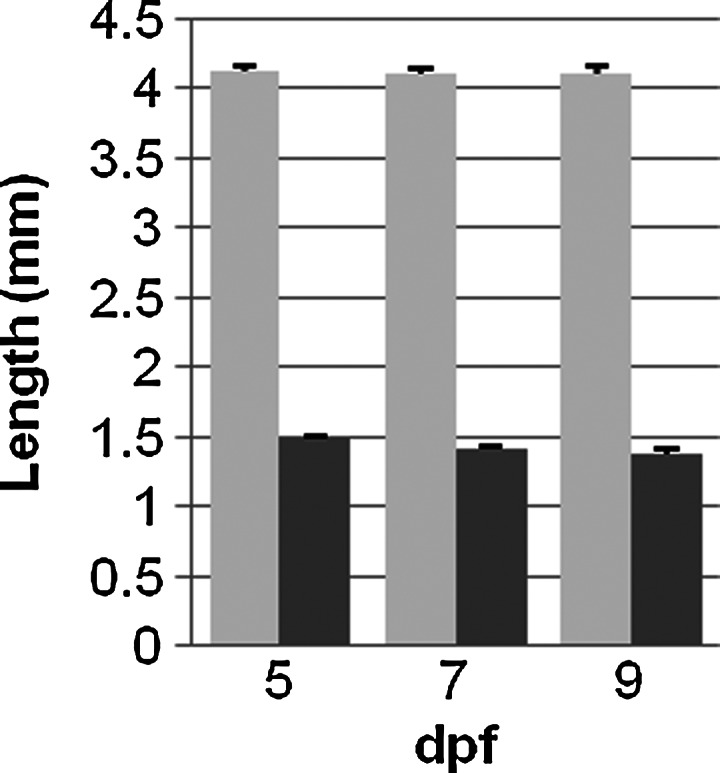
Measurement of larvae and mid intestine length at 5 (n=9), 7 (n=8), and 9 (n=7) dpf. Total larvae length (light bars) and the mid-intestine length (dark bars) did not increase between 5 and 9 dpf; p>0.05.
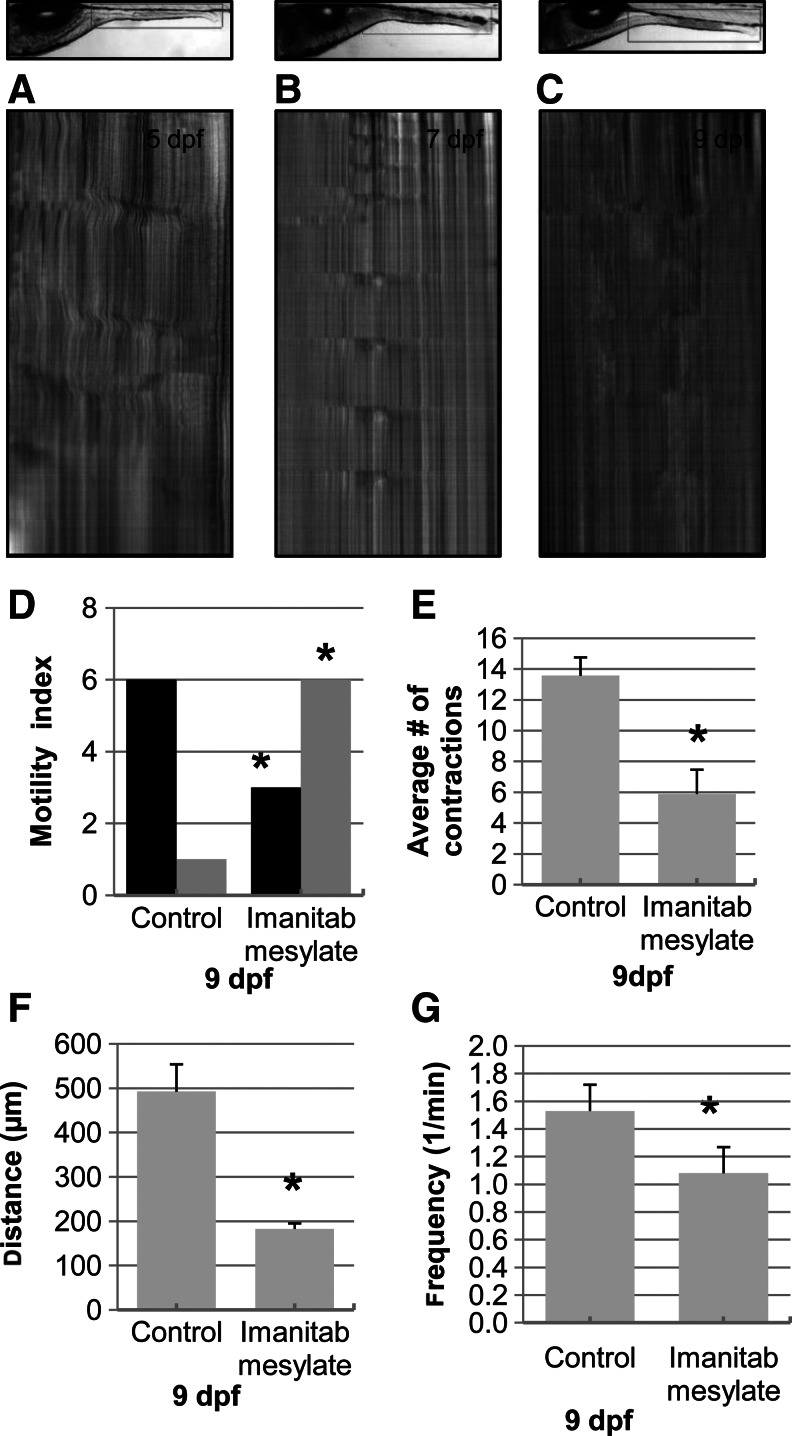
We next explored the role of Kit signaling on GI motility patterns. Imatinib mesylate (40 μM) was added to the embryo water as soon as the GI tract developed at 4 dpf to block Kit activity, and the effects on GI motor patterns were analyzed. STMaps at 5, 7, and 9 dpf from embryos raised in the presence of imatinib mesylate show incomplete and poorly developed motility patterns (Fig. 4A). Imatinib mesylate reduced the number of larvae with coordinated motor patterns, as well as the total number of propagating contractions (Fig. 4). When contractions were present in treated larvae, the average contraction distance was reduced when compared to control larvae. The effects of imatinib mesylate were most pronounced at 9 dpf when just 3 out of 9 larvae displayed a normal motility index, compared to 6 out of 7 control larvae. The total number of contractions for imatinib mesylate-treated larvae was reduced by≈50%, contraction distance was decreased from 492±61 μm to 183±12 μm, and frequency decreased from 1.53±0.5 to 1.08±0.05 contractions/min (Fig. 4D–G).
FIG. 4.
Treatment with imatinib mesylate inhibits development of coordinated motility patterns. STMaps from treated larvae (n=16) show fewer contractions that appear disorganized when compared to age-matched control larvae (N=9) (A, B, C, compared to Fig. 1). Imatinib mesylate decreased the number of coordinated motor patterns (black, D), contraction number (E), distance (F), and velocity (G) at 9 dpf; *p<0.05.
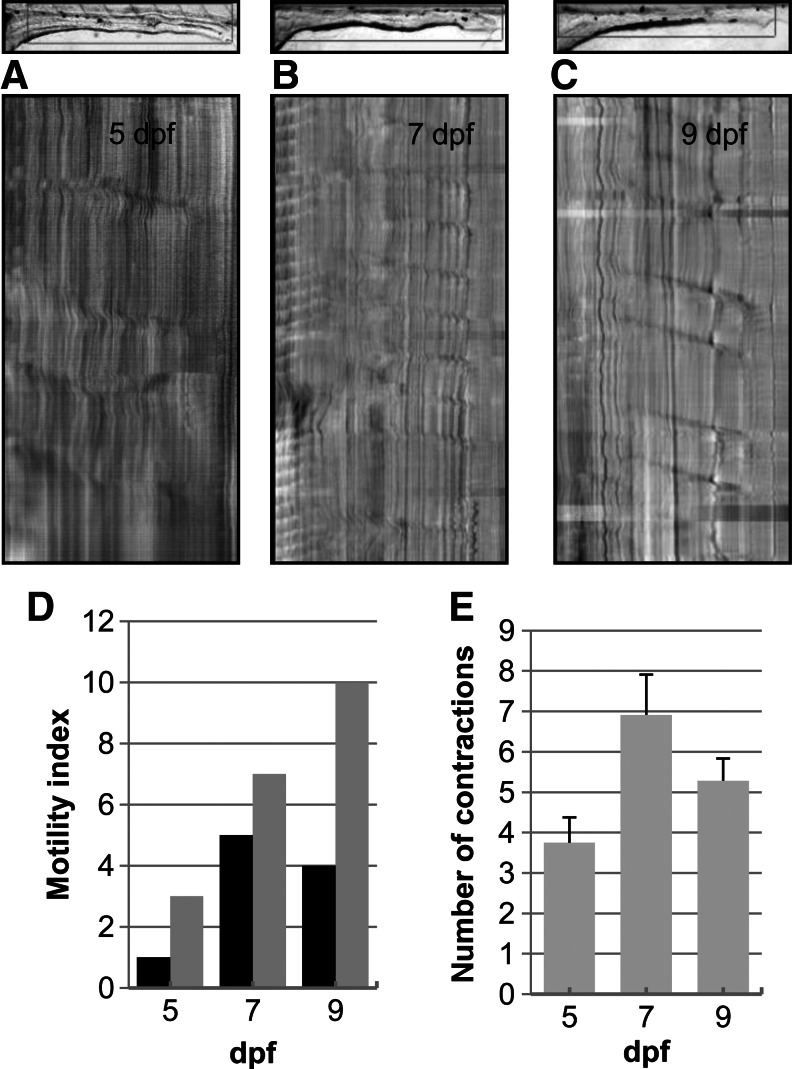
The role of Kit signaling on GI motility patterns was also examined using Sparse, a null kita mutant. Homozygous Sparse mutant larvae display reduced melanocyte numbers and this phenotype was used to identify homozygous mutants.19 STMaps of Sparse homozygous mutant larvae at 5, 7, and 9 dpf are shown in Figure 5A–C. The STMaps show fewer contractions that were poorly coordinated when compared to heterozygous or wild-type larvae (compare to Fig. 1). The motility index and the total number of contraction did not increase in Sparse homozygous mutants during development from 5 to 9 dpf (Fig. 1D and E). No changes were observed in contraction distance, velocity, frequency, or interval during development from 5 to 7 dpf in Sparse. However, comparing motility patterns in Sparse with wild-type larvae at 7 dpf revealed a decrease in the average number of contractions and in the motility index. At 7 dpf the average number of contractions in Sparse was less than wild-type larvae (6.9±1.0 and 12.3±1.3, p<0.05). In 7 dpf Sparse larvae, 5 out of 12 recordings had a motility index of 1 compared to 7 out of 8 recordings in wild-type larvae.
FIG. 5.
Development of GI motor patterns is incomplete in Sparse mutants. STMaps at 5 dpf (A), 7 dpf (B), and 9 dpf (C) show relatively few contractions when compared to control larvae (see Fig. 1). Motor patterns are poorly coordinated with propagating contractions moving in the oral and the aboral directions (see A). Coordinated motility patterns were observed in 1 of 4 larvae at 5 dpf, 5 of 12 larvae at 7 dpf, and 4 out of 14 larvae at 9 dpf (D). The total number of contractions did not change during development (E); p>0.05.
Discussion
Results presented here show that Kit signaling contributes to development of spontaneous and rhythmic motility patterns in the zebrafish GI tract. Although spontaneous GI contractions begin at 4 dpf, our analysis showed that coordinated motility patterns did not fully develop in the middle intestinal segment until 7 dpf. Previous work did not identify Kit+ cells at 5 dpf, but Kit+ cells were present at 7 dpf.14 Therefore, development of coordinated motility patterns coincides with appearance of Kit+ cells. The data suggest a functional role for zebrafish Kit+ cells in GI motility that is consistent with the role of ICC in the mouse GI tract.14 Binding of Kit ligand to Kit initiates a signaling pathway that is essential for the normal development and the survival of ICC in the mouse GI tract.9,30 We examined the role of Kit signaling in zebrafish using the Kit antagonist imatinib mesylate to inhibit Kit signaling. Imatinib mesylate disrupted development of coordinated motility patterns, suggesting that Kit signaling was necessary for development of coordinated motility patterns in the zebrafish GI tract. The results are consistent with studies in mice where Beckett and co-workers showed that imatinib mesylate disrupted electrical pacemaker activity in jejunal smooth muscle cultures derived from embryonic mice.10 Imatinib mesylate is relatively selective for Kit but also blocks platelet-derived growth factor alpha (PDGFRα).31 Data presented here correlating the appearance of Kit+ cells at 7 dpf and development of coordinated motility patterns at 7 dpf, as well as the dysmotility observed in null Kita Sparse mutants, suggest that imatinib mesylate specifically inhibited Kit in this study. Spontaneous contractions were observed in Sparse but coordinated motility patterns did not develop after 5 dpf. The data show that kita function is required for development of coordinated motility patterns. These results are similar to data from the mouse where severe reductions in ICC density disrupt GI motility patterns, but do not eliminate propulsive contractions that support motility.6,11
Mechanisms controlling GI motility patterns, including smooth muscles, enteric neurons, and ICC, are interconnected and overlapping.6 Distinct and precise roles for each regulatory element are difficult to assign because lesions in two different elements can result in identical disruptions in GI motility, and remaining control elements compensate. For example, myogenic mechanisms compensate in mouse models lacking enteric neurons and in GI tissues after complete neural blockade with tetrodotoxin.7,32,33 In the zebrafish, GI motility patterns are inhibited by tetrodotoxin at 7 dpf but≈50% of the propagating contractions persist and these remaining contractions are essentially normal.25 Therefore, it is possible that myogenic or ICC compensatory regulation are sufficient for development of propagating motor patterns. The role of enteric neurons during development of GI motility in the zebrafish has been examined previously and development of enteric neurons between 3 and 7 dpf was correlated with increasing development of organized GI motility patterns.29 Kuhlman and Eisen screened zebrafish mutants and showed a correlation between the number of enteric neurons and coordination of GI contactions.22 Therefore it is reasonable to conclude that coordinated motor patterns in the zebrafish GI tract are regulated by enteric neurons and by ICC and that diminished influence by a single regulatory element may be compensated. It is also possible that environmental conditions influence development of regulatory mechanisms and that feeding affects ICC development. The results presented in this article correlating inhibition of Kit signaling with disruptions in GI motility patterns, but not complete blockade of GI motility, are consistent with the mouse model system. Future work is necessary to characterize electrical activity, and the presence or absence of an electrical slow wave in the zebrafish GI tract that supports muscle contractions.
In summary, the results in this study show that coordinated GI motility patterns develop between 5 and 7 dpf, and that reduced Kit signaling disrupts development of coordinated GI motility patterns. Pharmacological inhibition of Kit signaling using imatinib mesylate provides evidence for a functional role for the zebrafish Kit orthologues kita. Taken together, these data provide a functional role for Kit-positive cells in coordinated patterns of GI motility.
Acknowledgments
This work was supported by NIH grants DK071588 and DK57061. We thank Amanda Diamond and Brittany Heatherington for standard length measurements for fed and fasted 7dpf larvae.
Disclosure Statement
No competing financial interests exist.
References
- 1.Burns AJ. Herbert TM. Ward SM. Sanders KM. Interstitial cells of Cajal in the guinea pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- 2.Maeda H. Yamagata A. Nishikawa S. Yoshinaga K. Kobayashi S. Nishi K, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 3.Ward SM. Burns AJ. Torihashi S. Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu LW. Thuneberg L. Huizinga JD. Development of pacemaker activity and interstitial cells of Cajal in the neonatal mouse small intestine. Dev Dyn. 1998;213:271–282. doi: 10.1002/(SICI)1097-0177(199811)213:3<271::AID-AJA4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Sanders KM. Koh SD. Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Ann Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 6.Huizinga JD. Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastroint Liver Physiol. 2009;296:G1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 7.Huizinga JD. Martz S. Gil V. Wang XY. Jimenez M. Parsons S. Two independent networks of interstitial cells of Cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Frontiers Neurosci. 2011;5:93. doi: 10.3389/fnins.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huizinga JD. Thuneberg L. Kluppel M. Malysz J. Mikkelsen HB. Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 9.Torihashi S. Ward SM. Nishikawa S. Nishi K. Kobayashi S. Sanders KM. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- 10.Beckett EA. Ro S. Bayguinov Y. Sanders KM. Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 11.Hennig GW. Spencer NJ. Jokela-Willis S. Bayguinov PO. Lee HT. Ritchie LA, et al. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol Motil. 2010;22:e138–151. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders KM. Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen HB. Malysz J. Huizinga JD. Thuneberg L. Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol Motility. 1998;10:11–26. doi: 10.1046/j.1365-2982.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 14.Rich A. Leddon SA. Hess SL. Gibbons SJ. Miller S. Xu X, et al. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev Dyn. 2007;236:903–911. doi: 10.1002/dvdy.21086. [DOI] [PubMed] [Google Scholar]
- 15.Huizinga JD. Zarate N. Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: Basic and clinical science. Gastroenterology. 2009;137:1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders KM. Hwang SJ. Ward SM. Neuroeffector apparatus in gastrointestinal smooth muscle organs. J Physiol. 2010;588:4621–4639. doi: 10.1113/jphysiol.2010.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besmer P. Manova K. Duttlinger R. Huang EJ. Packer A. Gyssler C, et al. The kit-ligand (steel factor) and its receptor c-kit/W: Pleiotropic roles in gametogenesis and melanogenesis. Development. 1993:125–137. [PubMed] [Google Scholar]
- 18.Baxter LL. Hou L. Loftus SK. Pavan WJ. Spotlight on spotted mice: A review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res. 2004;17:215–224. doi: 10.1111/j.1600-0749.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 19.Parichy DM. Rawls JF. Pratt SJ. Whitfield TT. Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- 20.Hultman KA. Bahary N. Zon LI. Johnson SL. Gene duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genetics. 2007;3:e17. doi: 10.1371/journal.pgen.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace KN. Akhter S. Smith EM. Lorent K. Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Kuhlman J. Eisen JS. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev Dyn. 2007;236:118–127. doi: 10.1002/dvdy.21033. [DOI] [PubMed] [Google Scholar]
- 23.Wallace AS. Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–382. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg A. Olsson C. Holmgren S. The effects of endogenous and exogenous nitric oxide on gut motility in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2006;209:2472–2479. doi: 10.1242/jeb.02272. [DOI] [PubMed] [Google Scholar]
- 25.Holmberg A. Olsson C. Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol. 2007;210:1084–1091. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- 26.Westerfield M. Danio rerio. 4th. Univ. of Oregon Press; Eugene, OR: 2000. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. [Google Scholar]
- 27.Petzold AM. Bedell VM. Boczek NJ. Essner JJ. Balciunas D. Clark KJ, et al. SCORE imaging: Specimen in a corrected optical rotational enclosure. Zebrafish. 2010;7:149–154. doi: 10.1089/zeb.2010.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parichy DM. Elizondo MR. Mills MG. Gordon TN. Engeszer RE. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238:2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holmberg A. Schwerte T. Pelster B. Holmgren S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2004;207:4085–4094. doi: 10.1242/jeb.01260. [DOI] [PubMed] [Google Scholar]
- 30.Rich A. Miller SM. Gibbons SJ. Malysz J. Szurszewski JH. Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastroint Liver Physiol. 2003;284:G313–320. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 31.Bardsley MR. Horvath VJ. Asuzu DT. Lorincz A. Redelman D. Hayashi Y, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennig GW. Gregory S. Brookes SJ. Costa M. Non-peristaltic patterns of motor activity in the guinea-pig proximal colon. Neurogastroenterol Motility. 2010;22:e207–217. doi: 10.1111/j.1365-2982.2009.01453.x. [DOI] [PubMed] [Google Scholar]
- 33.Burns AJ. Roberts RR. Bornstein JC. Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009;18:196–205. doi: 10.1053/j.sempedsurg.2009.07.001. [DOI] [PubMed] [Google Scholar]