Abstract
The uncinate fasciculus is a bidirectional, long-range white matter tract that connects lateral orbitofrontal cortex and Brodmann area 10 with the anterior temporal lobes. Although abnormalities in the uncinate fasciculus have been associated with several psychiatric disorders and previous studies suggest it plays a putative role in episodic memory, language and social emotional processing, its exact function is not well understood. In this review we summarize what is currently known about the anatomy of the uncinate, we review its role in psychiatric and neurological illnesses, and we evaluate evidence related to its putative functions. We propose that an overarching role of the uncinate fasciculus is to allow temporal lobe-based mnemonic associations (e.g. an individual’s name + face + voice) to modify behaviour through interactions with the lateral orbitofrontal cortex, which provides valence-based biasing of decisions. The bidirectionality of the uncinate fasciculus information flow allows orbital frontal cortex-based reward and punishment history to rapidly modulate temporal lobe-based mnemonic representations. According to this view, disruption of the uncinate may cause problems in the expression of memory to guide decisions and in the acquisition of certain types of learning and memory. Moreover, uncinate perturbation should cause problems that extend beyond memory to include social–emotional problems owing to people and objects being stripped of personal value and emotional history and lacking in higher-level motivational value.
Keywords: diffusion tensor imaging, episodic memory, orbitofrontal cortex, schizophrenia, anterior temporal lobe
Introduction
The uncinate fasciculus is one of several long-range white matter association fibre tracts in the human brain. It connects orbitofrontal cortex to the anterior temporal lobes through a direct, bidirectional monosynaptic pathway. Understanding the function and dysfunction of the uncinate fasciculus is of particular interest because of its clinical relevance. The uncinate fasciculus has been implicated in several developmental and psychiatric disorders. Its location makes it particularly susceptible to immediate impact and shearing injuries in head trauma cases and it is commonly implicated in white matter damage associated with traumatic brain injury (Ewing-Cobbs and Prasad, 2011; Johnson et al., 2011; Seo et al., 2012). In addition, the uncinate fasciculus is frequently damaged in surgical treatment of epilepsy (Kucukyuruk et al., 2012) and it is also one of a small group of white matter association tracts to mature, not reaching its developmental peak until the third decade of life (Lebel et al., 2008, 2012; Lebel and Beaulieu, 2011), which perhaps leaves it more susceptible to factors causing psychiatric illness during adolescence and young adulthood (Paus et al., 2008).
It is believed that the information transmission properties of any given white matter tract can be predicted by the function of the regions that it connects. At the same time, functions of cortical regions are determined by their pattern of white matter input and output (Van Essen and Maunsell, 1983; Passingham et al., 2002). By virtue of its geographic placement and connectivity, the uncinate fasciculus is typically associated with the limbic system. As a result, it is assumed that its functions should align with emotion and episodic memory. In actuality, however, the function(s) and information transmission properties of this white matter tract are largely unknown. Advancements in neuroimaging techniques, such as diffusion tensor imaging (DTI), offer the potential to fill in these critical gaps in knowledge. However, to interpret the findings from DTI, we must gain a better understanding of functional tractography. In the absence of a roadmap to guide our understanding of the functional significance of white matter tracts, there is a limit to the new information these endeavours can provide.
The purpose of this review is to summarize what is currently known about the uncinate fasciculus in an effort to move towards a better understanding of its function(s) and dysfunction(s). Inferences have often been made about the function of the uncinate fasciculus based on its anatomical connectivity. Therefore, this review begins with an overview of its physical connections, highlighting current misunderstandings about its connectivity and identifying current gaps in knowledge. Our understanding of the uncinate fasciculus’s anatomy comes from different techniques: autoradiography, gross dissection and DTI. It is widely accepted that radiological tracer studies offer better precision compared with dissection and DTI; however, tracer studies cannot be conducted in humans.
Next, we critically evaluate the literature associating abnormalities in the uncinate fasciculus with a range of neurological and psychiatric disorders. The specific goal is to assess the strength of the evidence linking structural abnormalities of the uncinate fasciculus to specific clinical differences (e.g. differences in symptoms, cognitive function, and treatment outcomes) and to examine how these links contribute to a current understanding of uncinate fasciculus function. The review concludes with an examination of evidence that implicates the uncinate fasciculus in memory, language and socio-emotional processing and a proposed model that more specifically associates the uncinate fasciculus with these cognitive functions.
Anatomy
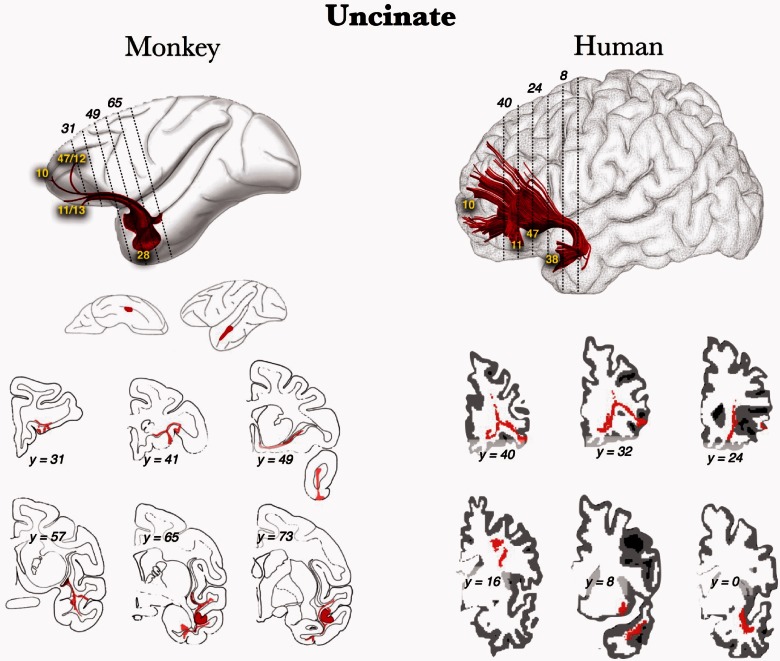
The anatomy of the uncinate fasciculus has been described in detail (Horel and Misantone, 1976; Ebeling and von Cramon, 1992; Ghashghaei and Barbas, 2002; Kier et al., 2004; Kringelbach and Rolls, 2004; Catani and ffytche, 2005; Schmahmann et al., 2008; Choi et al., 2010; Noonan et al., 2010; Peltier et al., 2010; Lehman et al., 2011; Thiebaut de Schotten et al., 2012). Before summarizing these findings, it is important to comment on several common misunderstandings about the uncinate fasciculus. First, in DTI studies, the uncinate fasciculus is often confused with the inferior frontal occipital fasciculus, as they are near each other in the region of the insula (Curran, 1909; Kier et al., 2004). This confusion does not exist in tracer studies because this method relies on axonal transport mechanisms, though it is problematic in the techniques commonly used in humans (dissection and DTI). Thus, some clinically important features of the inferior frontal occipital fasciculus, such as spread of tumour growth in humans, may be incorrectly attributed to the uncinate fasciculus due to its close proximity (Kier et al., 2004). Second, it is often mistakenly stated that the uncinate fasciculus connects the hippocampus to the frontal lobe (Saur et al., 2008), when in fact, most anatomy studies to date do not show any extension into the hippocampus. Last, although the anatomy of the uncinate fasciculus appears to be highly conserved across non-human primates and humans (Fig. 1; Thiebaut de Schotten et al., 2012), there are differences (Croxson et al., 2005). Some caution in extending function from monkeys to humans is in order given the putative role of the human anterior temporal lobe and the uncinate fasciculus in one aspect of language processing, semantic retrieval.
Figure 1.
Thiebaut de Schotten et al.’s (2012) reconstructions of the uncinate fasciculus using post-mortem axonal tracing in monkeys (modified from Schmahmann and Pandya, 2006) and in vivo tractography in humans demonstrates that anatomical connections of the uncinate fasciculus are largely conserved between the human and monkey brain (used with permission from Thiebaut de Schotten et al., 2012).
The uncinate fasciculus is a long-range association fibre connecting the frontal and temporal lobes. In older anatomy texts, the uncinate fasciculus is considered to be part of the ‘temporal stem’, which also contains the anterior commissure, the inferior frontal occipital fasciculus, and Meyer’s loop of the optic tract. The uncinate fasciculus is traditionally considered to be part of the limbic system. The uncinate fasciculus has a distinctive hook shape, arcing around the Sylvian fissure (Schmahmann and Pandya, 2006). For ease of description, the uncinate fasciculus is frequently broken into three parts: a dorsal/temporal segment, a middle/insular segment, and a ventral/frontal extension (Ebeling and von Cramon, 1992). The temporal segment originates from the uncus [Brodmann area (BA) 35], entorhinal and perirhinal cortices (also referred to as cortical nuclei of the amygdala; BA 28, 34 and 36), and the temporal pole/anterior temporal lobe (BA 20 and 38). The uncus is part of the olfactory cortex; entorhinal cortex is closely associated with episodic memory functions of the hippocampus; and perirhinal cortex has a controversial function in high-level object perception and object memory (Murray et al., 2005). The temporal pole and nearby tissue constitute part of the anterior temporal lobe. Portions of the anterior temporal lobe (but not the temporal pole) are believed to play a role in certain types of semantic memory (Patterson et al., 2007) and also in the encoding and storage of social and emotional concepts (Olson et al., 2007, 2013; Zahn et al., 2007; Simmons et al., 2010). Within the greater anterior temporal lobe, there is a sensory-based segregation of function and intraregion connectivity: dorsal = auditory, ventral = visual, medial = olfactory, and polar = multisensory (Grossman et al., 2004; Skipper et al., 2011).
There is disagreement as to whether the temporal segment of the uncinate fasciculus extends into the amygdala proper (Croxson et al., 2005; Petrides and Pandya, 2007; Thiebaut de Schotten et al., 2012) and at least one tracer study provided clear evidence that it did not (Ungerleider et al., 1989). However, several studies have provided clear evidence of monosynaptic connections between the orbital frontal cortex, anterior temporal lobe and amygdala in monkeys. Using radioactive tracers, Ghashghaei and Barbas (2002) and Ghashghaei et al. (2007) detected substantial projections from the caudal orbital frontal cortex and from BA 36 of the anterior temporal lobe to the basolateral and basomedial nuclei of the amygdala; projections from both regions terminated in the basomedial nucleus of the amygdala. Although the aforementioned tracts were unnamed, Schmahmann and Pandya (2006) consider these tracts to be part of the uncinate fasciculus. Direct connections between the orbital frontal cortex and the anterior temporal lobe in monkeys are also well documented (Petrides and Pandya, 1988; Rempel-Clower and Barbas, 2000; Saleem et al., 2008). The caudal orbital frontal cortex projects to entorhinal and perirhinal cortex, the rostral orbital frontal cortex to inferior ventral anterior temporal lobe and to dorsal anterior temporal lobe (Rempel-Clower and Barbas, 2000).
Uncinate fasciculus cell bodies are found in the temporal segment. From there, the uncinate fasciculus passes upward over the lateral nucleus of the amygdala, through the limen insula, and either near or through two smaller white matter tracts, the external capsule and extreme capsule (Catani et al., 2002; Mori et al., 2002). At this point, the uncinate fasciculus is inferior to the inferior frontal occipital fasciculus. From there, it passes into the orbital regions of the frontal lobe (BA 11 and 47), where it has a horizontally-oriented fan shape. This fan splits into two branches, a larger ventro-lateral branch and a smaller medial branch. The ventral branch terminates in the lateral orbitofrontal cortex while the medial branch terminates in the frontal pole (BA 10; Dejerine, 1895; Klingler and Gloor, 1960; Crosby et al., 1962; Thiebaut de Schotten et al., 2012). In adult humans, the uncinate fasciculus has a width of 3–7 mm, a height of 2–5 mm, and a volume of ∼140 mm3 (Ebeling and von Cramon, 1992; Schmahmann and Pandya, 2006).
Findings of hemispheric differences in the uncinate fasciculus volume are discordant. Some authors using DTI methods have reported a leftward bias for uncinate fasciculus volume and fractional anisotropy values (Kubicki et al., 2002; Hervé et al., 2006; Rodrigo et al., 2007; Hasan et al., 2009), whereas other studies using post-mortem dissection methods have reported a rightward bias (Highley et al., 2002; Park et al., 2004). For example, a post-mortem study by Highley et al. (2002) found the right uncinate to be 27% larger in ∼80% of participants studied. The failure to find laterality differences in all subjects could potentially be due to handedness differences, which were not examined by Highley et al. (2002). It is also possible that asymmetry exists in some parts of the uncinate fasciculus but not others (Park et al., 2004; Rodrigo et al., 2007).
Clinical disorders and the uncinate fasciculus
In this section, we discuss evidence for uncinate fasciculus involvement in five disorders: anxiety, schizophrenia, psychopathy, epilepsy and frontotemporal dementia. These disorders were chosen for their prevalence in the uncinate fasciculus literature. Although we do not discuss developmental trajectories or disorders of the uncinate fasciculus in this review, it should be noted that several authors have implicated uncinate fasciculus dysfunction in developmental disorders such as autism and conduct disorder (Sarkar et al., 2012; Travers et al., 2012).
Anxiety and schizophrenia
Several studies have reported altered functional activity in frontal regions, along with increased activations in limbic/paralimbic regions in generalized anxiety disorder and social anxiety disorder (Cooney et al., 2006; Evans et al., 2008; Bishop, 2009). One would expect then, that white matter tracts connecting limbic regions to orbitofrontal cortices, like the uncinate fasciculus, might be structurally impaired in anxiety disorders. However, our review of the DTI literature indicates that the uncinate fasciculus per se plays either a small role, or no role, in anxiety disorders (Table 1). Although several recent studies have provided evidence of a correlation between fractional anisotropy measures in the uncinate fasciculus and various measures of anxiety (Phan et al., 2009; Tröstl et al., 2011; Baur et al., 2012; Hettema et al., 2012), other studies have reported negative correlations (Baur et al., 2012) or no correlation (Han et al., 2008; Liao et al., 2011). It seems more likely that global white matter plays a role in anxiety disorders (Westlye et al., 2011).
Table 1.
Studies providing evidence for or against a particular role of the uncinate fasciculus in various psychiatric disorders
| Evidence type | Summary of findings | |
|---|---|---|
| Anxiety | DTI (Han et al., 2008; Kim and Whalen, 2009; Phan et al., 2009; Liao et al., 2011; Tröstl et al., 2011; Westlye et al., 2011; Baur et al., 2012; Hettema et al., 2012) | Findings are mixed. The uncinate fasciculus appears to play either a small role, or no role, in this disorder. |
| Schizophrenia | DTI (Burns et al., 2003; Kubicki et al., 2005; McIntosh et al., 2008; Price et al., 2008; Kawashima et al., 2009; Voineskos et al., 2010; Kitis et al., 2012; reviewed by Kubicki et al., 2007). | Findings are mixed. The uncinate fasciculus appears to play either a small role, or no role, in this disorder. |
| Psychopathy | DTI (Craig et al., 2009; Motzkin et al., 2011; Sundram et al., 2012). | All DTI studies reported reduced fractional anisotropy values in the right uncinate fasciculus. |
| Volumetric MRI (Raine et al., 2000; Yang et al., 2009,Gregory et al., 2012). | Volumetric studies show bilateral volume losses in regions connected by uncinate fasciculus. |
Similarly, the disconnection hypothesis of schizophrenia (Friston and Frith, 1995) has generated interest in discovering whether abnormalities exist in white matter tracts connecting the prefrontal cortex to other brain regions. Overall, the results of DTI studies measuring changes in uncinate fractional anisotropy values in schizophrenics are contradictory. Several studies have reported reduced fractional anisotropy values in the uncinate fasciculus of schizophrenics (Burns et al., 2003; Kubicki et al., 2005; McIntosh et al., 2008; Price et al., 2008; Kawashima et al., 2009; Voineskos et al., 2010; Kitis et al., 2012). This association tends to be more apparent in older and chronic populations of schizophrenics, or in populations with generally greater symptoms (Mandl et al., 2013). However, other studies have reported increased, or no change in fractional anisotropy values (Highley et al., 2002; Kubicki et al., 2002; Jones et al., 2006; Karlsgodt et al., 2009) relative to healthy control subjects. It is possible that uncinate fasciculus dysfunction correlates with specific symptoms of schizophrenia such as flattened affect and lack of social engagement (Kitis et al., 2012); however, few studies have taken this experimental approach.
Several design flaws, such as small sample sizes and samples that are clinically heterogeneous, are endemic in the DTI literature on schizophrenia, so it is difficult to know whether a true association between the left uncinate fasciculus and schizophrenic symptoms exists. Even if such an association exists, changes in left uncinate fractional anisotropy values do not appear to be particular to schizophrenia, as similar findings have been reported in bipolar disorder (McIntosh et al., 2008; Sussman et al., 2009; Lin et al., 2011) and various anxiety disorders.
Psychopathy and antisocial personality disorder
Craig et al. (2009) measured fractional anisotropy in adult psychopaths and matched control subjects and found significantly reduced mean fractional anisotropy in the right uncinate of psychopaths compared with control subjects (Table 1 and Fig. 2). The volume of the tract, which was measured by the tractography algorithm as the number of trajectories, correlated negatively with antisocial behaviour in both the left and right uncinate. Given the correlation between white matter volume and antisocial behaviour, the authors suggest that their fractional anisotropy findings reflect antisocial behaviour rather than psychopathy. To test this hypothesis, the same group later measured fractional anisotropy in individuals with antisocial personality disorder and in healthy control subjects (Sundram et al., 2012). Participants in the antisocial personality disorder group had significantly reduced fractional anisotropy and significantly increased mean diffusivity compared with control subjects in the right uncinate fasciculus. However, as the antisocial personality disorder group also had significantly reduced fractional anisotropy in several other white matter tracts, this study provides no evidence for an uncinate-specific impairment. An uncinate fasciculus-specific impairment in psychopathic individuals was, however, reported by Motzkin et al. (2011).
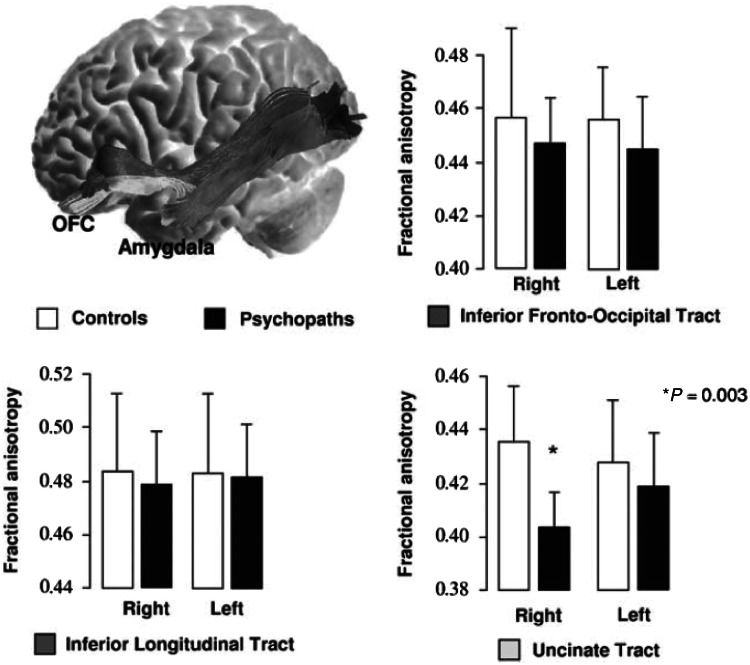
Figure 2.
Studies of antisocial behaviour and psychopathy consistently report abnormalities of the right uncinate fasciculus. For example, Craig et al. (2009) reported significantly reduced mean fractional anisotropy in the right uncinate fasciculus (P = 0.003) for psychopaths compared with age- and IQ-matched control subjects and no significant differences in fractional anisotropy in the left uncinate fasciculus (P = 0.448) or in two control tracts: the inferior longitudinal tract and the inferior frontal-occipital tract (used with permission from Craig et al., 2009). This pattern of findings is unique and noteworthy because studies of patients with other psychiatric disorders consistently report abnormalities in the left or bilateral uncinate fasciculus rather than right uncinate fasciculus alone.
Interestingly, it has been reported that neural regions linked by the uncinate fasciculus—the orbital frontal cortex and temporal pole—are relatively thinner or have decreased volume in psychopaths and subjects with antisocial personality disorder relative to control subjects (Raine et al., 2000; Yang et al., 2009; Gregory et al., 2012).
In summary, evidence for uncinate fasciculus dysfunction in psychopathy and antisocial personality disorder is limited, as researchers have only recently begun to investigate the matter. Nevertheless, compared with the other clinical disorders reviewed in this manuscript, we find the evidence for the uncinate fasciculus playing a role in these disorders to be more compelling. The cognitive function(s) disrupted by abnormalities of the right uncinate and how this relates to psychopathy is unclear, although it has been proposed that it may relate to particular types of learning and memory.
Epilepsy
Unilateral resection of the anterior temporal lobe has long been used as a treatment for intractable temporal lobe epilepsy (Penfield and Baldwin, 1952; Jensen, 1975, 1976; Jensen and Vaernet, 1977). Although it is rarely noted or commented on, the uncinate fasciculus is frequently severed in these surgeries. Despite the frequency of surgical severance of the uncinate fasciculus, a recent review of the temporal lobe epilepsy surgery literature noted that little could be said about the function of this white matter tract (Kucukyuruk et al., 2012).
Even before resection surgery, temporal lobe epilepsy, especially in the left hemisphere, is strongly associated with decreased white matter integrity in the uncinate fasciculus (Rodrigo et al., 2007; Diehl et al., 2008; McDonald et al., 2008; Riley et al., 2010). It is likely that changes in white matter properties of the uncinate fasciculus are the result of aberrant neuronal morphology or firing and thus epiphenomenal to the disorder because most seizures are localized in the medial and anterior temporal lobe regions. Although it is unlikely that alterations in the uncinate fasciculus cause epilepsy, uncinate fasciculus abnormalities may contribute to certain cognitive deficits commonly associated with temporal lobe epilepsy. Abnormal fractional anisotropy values in the bilateral uncinate fasciculus are correlated with deficits in immediate verbal memory (Diehl et al., 2008; McDonald et al., 2008), delayed memory (Diehl et al., 2008; McDonald et al., 2008; Riley et al., 2010), and confrontational naming/semantic memory as tested with the Boston Naming Test (McDonald et al., 2008) in patients with temporal lobe epilepsy. Similar deficits are reported after unilateral left temporal resection surgery, especially proper naming deficits (Lu et al., 2002; Hamberger and Drake, 2006).
The epilepsy literature also hints that the uncinate fasciculus may have non-mnemonic functions. There is a subgroup of grand mal temporal lobe seizures known as ‘uncinate fits’, so named because the seizure focus is in the uncus, on the medial surface of the anterior temporal lobe at the anterior end of the parahippocampal gyrus. An early case study (Jackson and Colman, 1898) identified an epileptic patient who experienced ‘dreamy states’ and unusual tastes immediately before seizing; post-mortem examination found damage only in the left uncinate fasciculus and the left uncus. Later research found that uncinate fits were also associated with emotional and sexual arousal, olfactory and gustatory hallucinations, and involuntary movement of the face and mouth, including spitting (Penfield and Baldwin, 1952), olfactory hallucination (Efron, 1957; West and Doty, 1995), depressive symptoms (Weil, 1955) and intense anxiety (Parrino, 1971). The olfactory symptoms that accompany uncinate fits do not appear to arise from the uncinate fasciculus, but rather tissue damage to the insula or olfactory cortex (Daly, 1958; Howe and Gibson, 1982). The remaining symptoms are reminiscent of the gustatory, sexual and emotional symptoms of Kluver-Bucy disorder, which can be caused by temporal lobectomy surgery (for a review see Olson et al., 2007).
In general, research on the uncinate fasciculus from the perspective of epilepsy and surgical treatment of epilepsy is surprisingly weak although there is a clinical consensus that it must be important for disease progression and cognitive deficits following temporal resection. Based on this literature, it appears that retrieval of proper names and the retention and/or retrieval of verbal material are disproportionally affected by severance of the left uncinate fasciculus. The proper naming findings are compelling, since several laboratories using different patient groups and different techniques have reported similar findings (Damasio et al., 1996; Tranel et al., 1997; Grabowski et al., 2001; Papagno, 2011). There are also older findings on uncinate fits linking the dominant hemisphere uncinate fasciculus to emotional, sexual and gustatory symptoms. However, our understanding of the uncinate fasciculus from the perspective of epilepsy is at least in part driven by the kinds of tests performed on these patients—typically verbal memory and lexical retrieval—with few studies testing non-verbal memory or emotional processing.
The frontotemporal dementias
Frontotemporal dementia (FTD) is a progressive neurological disease characterized by degeneration of frontal and/or anterior temporal lobe tissue with more medial regions, such as the hippocampus, remaining intact at early stages of the disease. It is a heterogeneous disorder with three prominent subtypes: semantic dementia, behavioural variant FTD, and progressive non-fluent aphasia (Weder et al., 2007). Semantic dementia is a subtype in which general naming deficits are observed, typically in association with deterioration of the temporal lobes, prominently in the left anterior regions (Snowden et al., 1989). Naming deficits are a diagnostic symptom of this disease (Snowden et al., 1989; Hodges et al., 1992; Neary et al., 1998), although patients also usually present with personality changes and are reported by family members to be emotionally distant and lacking in empathy.
Like epilepsy, uncinate fasciculus dysfunction clearly does not cause FTD. However, because the anterior temporal lobe and orbital portions of the frontal lobe are among the earliest regions to experience cell loss in this disorder, the integrity of the uncinate fasciculus is necessarily affected. Several studies have reported an association between decreased fractional anisotropy values/radial diffusivity in the left uncinate fasciculus and semantic dementia (Matsuo et al., 2008; Agosta et al., 2010; Whitwell et al., 2010; Acosta-Cabronero et al., 2011; Galantucci et al., 2011) with a smaller number reporting changes in both the left and right uncinate fasciculus (Matsuo et al., 2008; Galantucci et al., 2011). One study reported that patients with semantic dementia had lower fractional anisotropy values in bilateral uncinate fasciculus compared with both non-fluent aphasics, who exhibit agrammaticism and motor speech errors, and healthy control subjects, suggesting that the uncinate fasciculus is particularly associated with semantic retrieval rather than speech output (Galantucci et al., 2011).
Findings from another subtype of frontotemporal dementia, behavioural variant FTD, complicate this picture. The symptoms of behavioural variant FTD tend to centre around deficits in emotional regulation, social cognition, motivation and decision making, although language deficits typically appear later in the disease progression (Piguet et al., 2011). Patients exhibit apathy, impulsivity, inappropriate sexual behaviours and hoarding (Piguet et al., 2011). Several studies have reported decreased fractional anisotropy values in both the left and right uncinate fasciculus in patients with behavioural variant FTD (Zhang et al., 2009; Piguet et al., 2011; Mahoney et al., 2012). Another study compared patients diagnosed with behavioural variant FTD and Alzheimer’s disease, and found no significant differences between the two groups in fractional anisotropy measures of the uncinate fasciculus, although patients with behavioural variant FTD did, on average, have smaller fractional anisotropy values in both the left and right uncinate fasciculus (Tartaglia et al., 2012). Furthermore, patients with behavioural variant FTD had significant degeneration of the grey matter connected directly to the uncinate fasciculus in the right hemisphere, as compared with the patients with Alzheimer’s disease (Tartaglia et al., 2012). Alternatively, Agosta et al. (2012) found that the damage to the left uncinate fasciculus, as measured by DTI, was the best predictor of behavioural variant FTD diagnosis, as opposed to progressive aphasia, and damage to the left uncinate fasciculus was the best predictor of overall degeneration in these patients. These reports clearly indicate that the uncinate fasciculus is affected in behavioural variant FTD, but the differential effects of the left, right or bilateral uncinate fasciculus degradation remains unclear.
Summary: adult clinical disorders and the uncinate fasciculus
In the preceding paragraphs we reviewed evidence linking uncinate fasciculus dysfunction to three psychiatric and two neurological disorders. Existing evidence fails to support the contention that the uncinate fasciculus has a primary role in anxiety disorders or schizophrenia. Future studies should focus on more distributed networks of white matter as they relate to these disorders, rather than on the uncinate fasciculus alone.
We found stronger evidence for uncinate fasciculus dysfunction in psychopathy and epilepsy. With regards to epilepsy, further studies of temporal lobe epilepsy in which patients with affected and unaffected uncinate fasciculus can be compared may be critical to elucidating not only the function of the uncinate fasciculus, but also how to preserve emotional processing and verbal memory in treatment of temporal lobe epilepsy. Of note, the epilepsy literature typically includes only a small battery of tests, thus the potential range of deficits has not been fully explored. There is also strong evidence for involvement of uncinate fasciculus degeneration in the clinical diagnosis of FTD, however, it is unclear whether it is more related to semantic retrieval deficits or social–emotional abnormalities.
Recommendations for future researchers interested in investigating the role of the uncinate fasciculus in various psychiatric and neurological disorders include: testing larger and more homogeneous clinical samples; correlating specific symptoms and/or theory-driven test performance, rather than disease presence or absence, with various DTI values; and using more rigorous statistical thresholding procedures. Future studies should also take care to investigate other white matter tracts, as well as measures of global white matter, alongside their investigations of the uncinate fasciculus. In doing so, care must be taken to dissociate the uncinate fasciculus from other nearby tracts. Greater experimental control and rigor will help prevent false positives and also help to elucidate the specific functional involvement of the uncinate fasciculus in psychiatric and neurological disease.
Putative functions of the uncinate fasciculus
The literature refers to three functions of the uncinate fasciculus: associative and episodic memory functions; linguistic functions; and social–emotional functions.
Episodic memory and the uncinate fasciculus
Episodic memory is a record of a person’s experience that holds date information and spatio-temporal relations (Tulving, 1983). Episodic memory formation relies on the function of medial temporal lobe structures, especially the hippocampus and portions of the frontal lobe, such as dorsolateral prefrontal cortex. There is long-standing interest in understanding how fibre pathways that connect these regions function in episodic memory.
Markowitsch (1982) proposed that retrograde amnesia was caused by the disconnection of temporal limbic structures from the frontal lobe ‘The task of the uncinate fascicle will be to guide and channel this information flow to the prefrontal cortex and to transmit preprocessed information back to the temporal cortex for the final act of representation’ (Markowitsch, 1982). His proposal was based on a review of neuropsychological case studies of selective retrograde amnesia, in which the majority of cases involved damage to the ventral frontal lobe and the anterior temporal lobe with presumed involvement of the uncinate fasciculus. Along these lines, Levine et al. (1998) reported the case of Patient ML, who suffered traumatic brain injury after a bicycle accident that resulted in damage to portions of the visual cortex, the hypothalamus, right and left prefrontal cortex, and the uncinate fasciculus. Several years after this incident, the patient continued to suffer from social impairments and severe retrograde memory loss, but only mild anterograde memory loss. Although this case is striking, it is important to note that Patient ML’s brain damage was not limited to the uncinate fasciculus. Indeed, there are no instances of retrograde or anterograde amnesia in which the uncinate alone was destroyed.
Focal unilateral lesions of the human uncinate fasciculus occur from time to time, usually in the context of surgical removal of low-grade gliomas. Papagno et al. (2011) studied 18 right-handed patients before and after left uncinate fasciculus removal. The results showed that effects on memory were modest; short-term memory and visual long-term memory were preserved, while verbal memory as tested by list learning was impaired immediately after surgery but improved to normal levels after 3 months. It is possible that the effects on memory were modest because (i) resection was unilateral; (ii) the wrong type of memory was tested; or (iii) the uncinate fasciculus’s role in memory is redundant with that of other white matter tracts.
The importance of the type of memory tested is highlighted by research on non-human primates. Information flow from the inferior temporal lobe to orbital frontal cortex is dramatically reduced by uncinate fasciculus disconnection (Ungerleider et al., 1989), even though indirect routes between these regions exist (e.g. through the amygdala and medial dorsal nucleus of the thalamus; Eacott and Gaffan, 1992). Uncinate fasciculus disconnection does not impair visual object discrimination learning, suggesting that some associative learning processes are not reliant on the uncinate fasciculus (Parker and Gaffan, 1998; Gaffan et al., 2002). Similarly, concurrent object–reward association learning, reversal learning, configural learning, and delayed matching-to-sample are unimpaired by uncinate fasciculus disconnection in monkeys (Eacott and Gaffan, 1992; Gaffan and Eacott, 1995; Gutnikov et al., 1997). Object-in-place learning is mildly impaired after uncinate fasciculus resection (Browning and Gaffan, 2008). The one type of learning that is consistently impaired after uncinate fasciculus dissection is conditional rule learning. In this task, an instructional cue at the beginning of each trial indicates what types of choices will be rewarded (Gaffan et al., 1988; Gutnikov et al., 1997; Parker and Gaffan, 1998; Bussey et al., 2002).
The DTI literature provides relatively strong support for the role of the uncinate fasciculus in episodic memory. Thomas et al. (2012) followed-up the monkey studies described above; in their study, participants were required to learn face–scene associations through computerized feedback across 1000+ learning trials. Faces were used as stimuli, since portions of the ventral anterior temporal lobe are critical for person memory (Von Der Heide et al., 2013). The results showed that subjects who had faster learning rates also had higher fractional anisotropy values in the left, but not right, uncinate fasciculus. There was no correlation in nearby white matter tracks. One interpretation of these findings is that the uncinate fasciculus was necessary for retrieving information and keeping count as to which face–scene associations were rewarded or punished in the past.
Other relevant DTI studies tend to use a variety of tasks drawn from the neuropsychology literature rather than the relevant monkey literature. Several DTI studies have reported correlations between left uncinate fasciculus white matter fractional anisotropy values and performance on auditory–verbal memory tasks, such as list learning (but not visual memory) by cued or free recall (Diehl et al., 2008; McDonald et al., 2008; Niogi et al., 2008). Such a relationship between the left uncinate fasciculus and verbal memory has been reported not only in adults, but also in children and adolescents (Mabbott et al., 2009). Another study reported correlations between the right uncinate fasciculus and auditory–verbal memory (Fink et al., 2010). Likewise, fractional anisotropy values of the uncinate fasciculus are significantly lower in amnestic mild cognitive impairment (Fujie et al., 2008) and correlate with verbal memory impairment in traumatic brain injury (Niogi et al., 2008) and with age-related reductions on several declarative memory tasks (Engvig et al., 2012).
It is important to note that in some of these studies (Engvig et al., 2012), the uncinate fasciculus was grouped together with nearby structures—the internal capsule—so it is not clear that the uncinate fasciculus per se was associated with memory performance. Although null reports are not commonly reported, Seo et al. (2012) reported finding no correlation between changes in DTI parameters and episodic memory performance in a group of patients with traumatic brain injury.
In summary, the reviewed findings fail to support the contention that the uncinate fasciculus has a general role in episodic memory (Markowitsch, 1982; McCauley et al., 2011). A more likely candidate for this function is the fornix (Metzler-Braddeley et al., 2011). Several studies in monkeys and a related study in humans (Thomas et al., 2012) suggest that the uncinate fasciculus supports some types of associative learning, but perhaps not in the obvious way of forming the actual linkage between two stimuli, such as a face and a place. We draw this conclusion for two reasons. First, cells in the ventral anterior temporal lobe can rapidly form associative pairings between visual stimuli (Sakai and Miyashita, 1991; Eifuku et al., 2010), therefore it is unclear what the uncinate fasciculus or the orbital frontal cortex would add to this process. Second, research in non-human primates has shown that associative learning is not abolished by uncinate fasciculus resection, but rather, that certain types of associative learning are affected. The most compelling finding in this literature is the observed deficit in conditional rule learning after uncinate fasciculus resection. One interpretation of this finding is that the uncinate fasciculus is involved in associating stimuli with various types of rewards, non-rewards, and punishments that when broadly defined, would include error monitoring (Metzler-Braddeley et al., 2011) that may serve to modulate retrieval of correct pairings (Table 2).
Table 2.
A list of psychological functions that do and do not appear to be associated with information transmission through the uncinate fasciculus
| Functions linked to the uncinate fasciculus | Functions not linked to the uncinate fasciculus | |
|---|---|---|
| Episodic memory | Reversal learning; learning from feedback/rewards/punishments; formation of associations that motivate behaviour; value-based updating of stored representations | Encoding and consolidation of common episodic memories including autobiographical memory |
| Language | Retrieval of proper names for people; possibly some aspects of semantic memory retrieval | General linguistic functions, e.g. speech production, speech comprehension, syntax, most aspects of semantic memory |
| Social–emotional processing | Valuation of stimuli; social reward processing; higher-level emotional meaning of concepts | Generation of emotions; personality; motivation; anxiety |
Language and the uncinate fasciculus
The left uncinate fasciculus has frequently been associated with language function because it connects regions of the brain that have putative functions in language: the anterior temporal lobes and portions of the frontal lobes, both of which have been proposed to encode, store and retrieve semantic knowledge (Grossman et al., 2004; Catani and Mesulam, 2008). Based on this evidence, it was hypothesized that the uncinate fasciculus, particularly on the left, was part of the ‘ventral language pathway’ and that it supported semantic naming by relaying sensory information about objects (presumably in ventral temporal cortex) to language supporting regions (in the lateral frontal lobe; Parker et al., 2005). The problem with this logic is that it is based on a rather crude assessment of anatomy. The uncinate does not connect the specific region of the frontal lobe known to be critical for language production i.e. Broca’s area/inferior frontal gyrus, to the anterior temporal lobe; instead, it connects regions in the ventral and medial frontal lobe (which are not commonly associated with linguistic function) to the anterior temporal lobe and surrounding structures.
Beyond the flawed geography evidence, there is a gaping lack of evidence to support the hypothesis that the uncinate fasciculus plays a general role in language. General language function, excepting verbal memory, is typically unimpaired after unilateral, temporal lobectomy surgery for epilepsy (Parker et al., 2005), and left uncinate fasciculus stimulation during neurosurgery is not associated with general language impairments (Duffau et al., 2009). Also the uncinate fasciculus is not implicated in primary progressive aphasia (Galantucci et al., 2011). Because of this, more recent accounts have discarded the notion that the uncinate fasciculus is part of the ventral language pathway in favour of the extreme capsule, which connects the middle temporal lobe and the ventrolateral prefrontal cortex (Saur et al., 2008).
Although the left uncinate fasciculus does not appear to play a general or exclusive role in language, there is some evidence that it plays a minor supporting role, particularly in lexical retrieval of semantic knowledge. For example, a study of memory in healthy older adults found that performance on a variety of semantic memory tasks, typically involving naming (such as Pyramids and Palm Trees), correlated positively with fractional anisotropy values in the left uncinate fasciculus (de Zubicaray et al., 2011). Temporal lobe resection surgery for epilepsy commonly leads to confrontation naming deficits whereas general language functions remain preserved (Lu et al., 2002; Hamberger and Drake, 2006). The most commonly observed confrontation naming deficit is in proper naming. Papagno et al. (2011) assessed patients with left uncinate fasciculus removal due to surgery for low-grade gliomas and found significant deficits in naming famous faces immediately after surgery and 3 months later. In contrast, other deficits that were present immediately after surgery, such as difficulties in remembering lists of words, and deficits in verbal fluency, improved to normal levels 3 months post-surgery. These findings indicate that the left uncinate fasciculus has redundant functions with other fibre pathways for some aspects of lexical retrieval and verbal memory, with the exception of proper naming.
The association between proper naming and the left uncinate fasciculus was indirectly studied by Damasio et al. (1996, 2004) in their milestone studies of lexical retrieval and the anterior temporal lobes (Tranel et al., 1997). These studies explored the deficits in patients with stable temporal lobe lesions, commonly caused by temporal lobe resection surgery. Patients with lesions restricted to the left anterior temporal lobe, and possibly including damage to the uncinate, had a specific deficit for retrieving the names of persons, and at times, landmarks. However, these patients performed normally when naming animals and objects. It is important to bear in mind that one cannot determine whether the observed deficits were due to uncinate damage per se, or due to other surgical or epilepsy-related damage.
More global semantic retrieval deficits are observed in semantic dementia and there is some evidence, reviewed above, linking semantic dementia to uncinate fasciculus deterioration. However, the literature on semantic dementia is new and typically fails to correlate specific symptoms to the uncinate fasciculus.
These findings strongly indicate that the uncinate fasciculus is not dedicated to general linguistic functions. This should not be taken to imply that the uncinate fasciculus has no role in language; instead, the left uncinate fasciculus appears to have an important, though non-critical, role in semantic retrieval (Table 2). However, it is also likely to be involved in social–emotional processing. The marriage of these two processes is evident when one considers that language is inherently social; you speak to transfer ideas and emotions to another person. The conceptual knowledge that underlies our expression of ideas is coloured by our preferences and emotional history to the degree that emotion is part-and-parcel of our conceptual representations.
Social–emotional processing and the uncinate fasciculus
There is reason to believe that the uncinate fasciculus plays a central role in certain types of social and emotional processing that are instrumental in decision making and shaping behaviour (Table 2). The first line of evidence is anatomical: the uncinate fasciculus connects limbic and paralimbic brain regions. The orbitofrontal cortex has long been considered essential for reward-based decision making (Adolphs et al., 1994; Angrilli et al., 1999; Grabenhorst and Rolls, 2011) and there is evidence that portions of the anterior temporal lobe are involved in the storage and retrieval of social memories including person memory, as well as theory of mind (Olson et al., 2007; Zahn et al., 2007; Simmons et al., 2010).
Second, there is also evidence from some clinical populations suggesting a social–emotional role for the uncinate. For instance, patients with behavioural variant FTD have deficits that are primarily related to social and emotional processing (Piguet et al., 2011), as opposed to other variants of FTD, whose symptoms are more linguistic and mnemonic (Hodges, 2001; Whitwell et al., 2010). Findings of uncinate abnormalities are quite common in this population (Matsuo et al., 2008; Agosta et al., 2010; Whitwell et al., 2010; Acosta-Cabronero et al., 2011; Galantucci et al., 2011). One study reported that patients with semantic dementia and progressive non-fluent aphasia had decreased fractional anisotropy values in the left and right uncinate, respectively, compared with healthy control subjects, but only patients with behavioural variant FTD showed bilateral uncinate fasciculus abnormality and that decreased fractional anisotropy values in the uncinate specifically correlated with behavioural inhibition (Hornberger et al., 2011). Studies have shown grey matter loss in two regions connected by the uncinate fasciculus, the anterior temporal lobe and orbitofrontal cortex, as well as decreased fractional anisotropy values in the right uncinate fasciculus.
Related to this, literature on traumatic brain injury provides some interesting case studies to support our hypothesis. A patient with moderate traumatic brain injury, exhibiting symptoms of depression apathy, and reduced sex drive and self esteem, was described by Zappalà et al. (2012). MRI scans showed damage to this patient’s left uncinate and left cingulate. In another study, Johnson et al. (2011) specifically compared the uncinate fasciculus in 15 children 3 months after traumatic brain injury to 15 healthy control subjects, and discovered that uncinate fasciculus fractional anisotropy measurements were predictive of executive function outcomes, especially emotional control, 1 year later.
Finally, the fifth and most speculative line of evidence suggesting a social role for the uncinate in social–emotional processing comes from case studies of patients suffering from the related disorders of Capgras delusion and reduplicative paramnesia. Capgras delusion is characterized by a patient’s insistence, despite all evidence to the contrary, that his or her loved one has been replaced by an impostor (Hirstein and Ramachandran, 1997; Edelstyn et al., 2001). Patients with reduplicative paramnesia, on the other hand, believe a particular person or place has been duplicated and/or relocated to a familiar place (e.g. a hospital room moved to the patient’s home) (Politis and Loane, 2012).
Hirstein and Ramachandran (1997) put forth a hypothesis that Capgras delusion (and related delusions) results from severed connections between face processing areas and the limbic regions responsible for emotionally ‘colouring’ or ‘tagging’ facial percepts. The logic is that patients with Capgras delusion accurately perceive the face of a familiar loved one but without feeling the expected feelings of warmth, and therefore conclude that it must be someone else’s face.
Regions connected with the uncinate fasciculus have been implicated in Capgras delusion and other delusional misidentification syndromes, although lesions are often diffuse and the evidence for any single region’s involvement is inconclusive (Bouckoms et al., 1986; Drake, 1987). There is, therefore, reason to believe that the uncinate fasciculus might play a limited role in this disorder. Since uncinate lesions have been reported in the absence of symptoms that characterize Capgras delusion, we can be sure that uncinate fasciculus dissection alone is insufficient to produce Capgras delusion. The ablation of a subset of uncinate fasciculus cell bodies, or the cells on which they terminate, may be necessary. Uncinate fasciculus damage may, however, contribute to Capgras-like symptoms.
The uncinate itself has been implicated in at least two studies. Lee et al. (2011) found an uncinate lesion in a patient suffering from reduplicative paramnesia in the absence of other delusions (aside from anasognosia). This patient had an average IQ and normal language and autobiographical memory. Lesions were also observed in several other white matter tracts. Edelstyn et al. (2001) reported a patient with abnormal frontal lobe white matter near or in the uncinate who suffered from episodes of Capgras delusion. Retrieval of stored social semantic information (e.g. pairing a person’s occupation with their face) was also poor in this patient (see also a case of Capgras delusion following temporal lobectomy, Lipson et al., 2003).
Grey matter regions connected by the uncinate are also implicated in the abovementioned disorders, as well as in social and emotional processing in general. Orbitofrontal and anterior temporal regions are involved in behavioural variant FTD and psychopathy (Hodges, 2001; Yang et al., 2009; Hornberger et al., 2011; Gregory et al., 2012), whereas amygdala dysfunction has been implicated in autism spectrum disorder (Dalton et al., 2005; Nacewicz et al., 2006; Kleinhans et al., 2009). Patients with orbitofrontal lesions often have impaired social functioning, as well as impaired theory of mind and empathy (Shamay-Tsoory et al., 2003; Mah et al., 2005).
Although none of the reported findings on their own is convincing, as a whole, the literature indicates that the uncinate fasciculus plays an important, albeit underspecified, role in social–emotional processing.
A proposal and conclusions
After reviewing the literature on uncinate fasciculus function, two credible accounts of uncinate fasciculus function become apparent. The first is a ‘generalist’ hypothesis, which suggests that the uncinate fasciculus is involved in various types of memory, language, social–emotional processing, and perhaps other functions that are yet undiscovered. Although it is easy to critique this hypothesis as a cobbled-together patchwork-quilt hypothesis, it is entirely plausible that certain white matter tracts are best conceived as somewhat general routes of information transfer.
The second is a ‘specialist’ hypothesis, which suggests that the uncinate fasciculus is biased towards a particular type of information transfer. Certain findings stood out in our literature review that we can use to infer a more specific explanation of uncinate fasciculus function (Fig. 3). We note that findings from the monkey literature, which offer the strongest causal power, suggest that the uncinate fasciculus is critically involved in some, but not all, mnemonic functions. Converging evidence from DTI and neuropsychology support this contention. The uncinate fasciculus does not appear to play a general role in episodic memory, however, nor is it critical for forming or retrieving many common types of mnemonic associations.
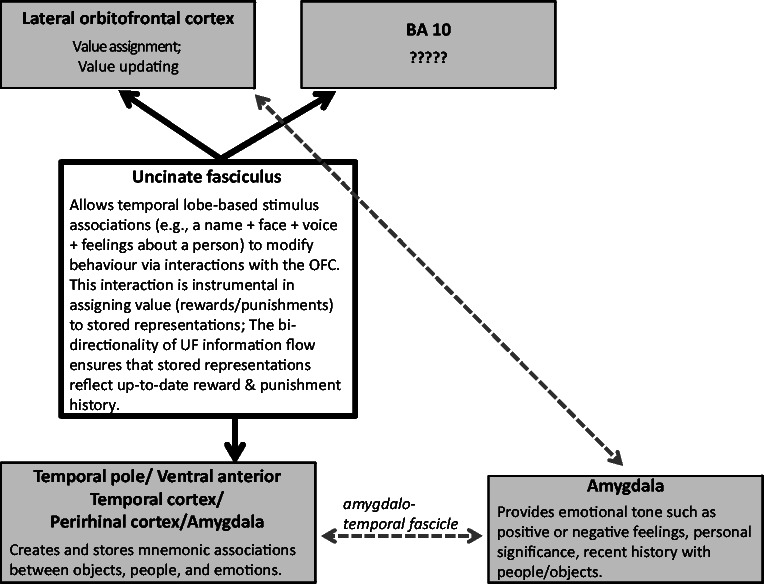
Figure 3.
A proposed model of uncinate fasciculus (UF) function. Non-uncinate fasciculus connectivity is depicted by dashed lines. The information transmission properties of the uncinate fasciculus branch connecting ventral anterior temporal lobe and BA 10 remains unclear. OFC = orbital frontal cortex.
In the following section we lay out the argument that the uncinate fasciculus does not have general mnenomic, linguistic, or social–emotional functions. Instead, its function lies at the intersection of memory and social–emotional processes, in the explicit service of choice. More specifically, we argue that the uncinate fasciculus allows temporal lobe-based mnemonic associations (e.g. a person’s name + face + voice + your feelings about a person) to modify behaviour by interacting with systems in the lateral orbital frontal cortex that are instrumental for making associations between stimuli and rewards, and ultimately, decision making. The bidirectionality of uncinate fasciculus information flow ensures that temporal lobe representations of objects and people reflect up-to-date reward/punishment history.
The mnemonic functions of the uncinate fasciculus are necessarily limited to the types of information, and the type of processing functions, supported by cortical anatomy associated with this tract. Likewise, the social–emotional information that is transmitted by the uncinate should be viewed in light of known functions of the cortical anatomy associated with the uncinate fasciculus. The cortical anatomy of the uncinate fasciculus is described in the following points:
Uncinate fasciculus cell bodies are found in the anterior temporal lobe. Cells in the ventral anterior temporal lobe have rapid association formation capacities. Sakai and Miyashita (1991) reported that single neurons in the ventral anterior temporal lobe of monkeys that initially responded to only one abstract pattern would later respond to a second abstract pattern that had been associated through training with the first. Recordings from these cells show that they can represent an associative pairing between faces and abstract patterns, acquired through training (Eifuku et al., 2010). Tsukiura et al. (2010) showed that successful encoding of person-related semantics with their names was associated with blood oxygen level-dependent activity in the left anterior temporal lobe. Patients with damage to this region have difficulty forming new associations between names and pictures of objects (Sharon et al., 2011). It has been proposed that the rapid association formation capacity of this region underlies some aspects of semantic memory (Eifuku et al., 2010), most likely the association between a visual stimulus such as a face and more abstract forms of semantic knowledge, such as an occupation. The association formation capabilities of the anterior temporal lobe may be reliant on the hippocampus during the initial encoding phase (Alvarez and Squire, 1994; Frankland and Bontempi, 2005; Nieuwenhuis et al., 2011). These structures are strongly interconnected by tracts through the entorhinal cortex and subiculum (Chabardès et al., 2002);
The temporal pole of the anterior temporal lobe has bi-directional interactions with the basomedial nucleus and the border of the basolateral nucleus of the amygdala through a small white matter tract called the ‘amygdalo-temporal fascicle’ (Klingler and Gloor, 1960; Ghashghaei and Barbas, 2002). The amygdala has a known role in emotion generation, social attention, and salience tagging (Adolphs, 2010). The connections between the anterior temporal lobe and the amygdala may serve to give the representations stored in the anterior temporal lobe emotional tone, such as positive or negative feelings, and personal importance and significance (Olson et al., 2013). One plausible role of the uncinate fasciculus is to transmit salience-laden stimulus representations stored in anterior-medial aspects of the temporal lobe to the lateral orbital frontal cortex so that decisions can be made based on the emotional tone or incentive value of the stimuli;
Most uncinate fasciculus fibres terminate in lateral orbital frontal cortex rather than midline orbital frontal cortex. The lateral orbital frontal cortex’s function is variously related to the evaluation of losses related to ongoing behaviour (Kringelbach and Rolls, 2004), or in assigning rewards and punishments to behaviours (Noonan et al., 2010). Of late, more evidence has accrued for the second idea especially in the context of associative learning. For instance, Noonan et al. (2010) showed that when lateral orbital frontal cortex was lesioned, monkeys failed to make the correct associations between behaviours and rewards, and the history of what was rewarded in the past was lost (Noonan et al., 2010). Another group found that lesions to this region in non-human primates disrupted the rapid updating of object value (Rudebeck and Murray, 2011).
Our hypothesis predicts that bilateral damage of the uncinate fasciculus should cause problems with the manner in which memory is used to alter behaviour—approaching or avoiding stimuli based on reward and punishment history. It should also cause problems in the expression of certain memories since the link between associative memory and certain decision processes would be disrupted. It is possible that some types of learning, such as devaluation, would be perturbed by uncinate fasciculus damage, as feedback from lateral orbital frontal cortex to the temporal lobe would be perturbed. These types of learning and memory problems could present clinically as gambling addiction, or poor decision making, for instance. Problems associated with uncinate fasciculus damage should extend beyond memory to include social–emotional problems due to people and objects being stripped of personal value and lacking in higher-level motivational value, potentially explaining aspects of Capgras delusion and some of the symptoms associated with autism spectrum disorder. In autism for instance, failure to form emotional attachments and friendships and failure to learn from praise and other social rewards could potentially be explained by uncinate fasciculus dysfunction. Behavioural variant FTD is characterized by disruptions in affiliative social behaviour and social disinhibition (Mummery et al., 2000; Hodges, 2001), both of which can be conceptualized as disturbances in social reward processing and social learning. Findings reported earlier in psychopaths with reduced fractional anisotropy values in the right uncinate (Craig et al., 2009; Motzkin et al., 2011; Sundram et al., 2012) may reflect difficulties in learning from punishment.
Finally, Papagno (2011) reported that humans with unilateral uncinate fasciculus ablations had a highly specific semantic retrieval deficit in naming famous individuals. At first glance, these findings contradict our proposed model, namely that the unique connectivity of the uncinate fasciculus allows it to play a particular role in affective tagging of memory during storage and retrieval. However, further consideration is required. Salient, emotional information plays a significant role in person identification. Upon seeing a face that you recognize, you automatically activate a host of often salient and emotional, semantic associations beyond the mere name or identity of the person. An image of Marilyn Monroe, for example, evokes not only a name but also semantic and emotional associations, such as her performance in the film Gentlemen Prefer Blondes, her extreme version of female sexuality, her relationships with several famous men such as John F. Kennedy and Joe DiMaggio, and her tragic death by drug overdose. These emotionally laden associations support and strengthen your knowledge of Marilyn Monroe’s identity, even if all you intend to do is access her name. Thus, the findings that person naming is associated with the uncinate fasciculus provides another layer of evidence for the model presented here.
Additional evidence for our proposal is clearly needed and there are many unanswered questions (Box 1). For instance, if our hypothesis is true, the question arises as to why there are no reports of patients with epilepsy who undergo incidental disconnection of the uncinate fasciculus who become psychopathic or exhibited abnormalities in forming emotional associations? As noted above, there is surprisingly little research on the uncinate fasciculus in epilepsy resection patients. Historically, research in these patients has focused on episodic memory and there are few studies of social or emotional processes. Even if such studies did exist, it would be difficult to attribute any social/emotional dysfunction to uncinate fasciculus damage given that the amygdala is destroyed in most instances. In future work, it will be important to examine predictions of our model in resection patients with or without verified uncinate fasciculus disconnection.
Box 1 Unanswered questions in the literature.
There is an extensive literature documenting deficits in encoding and retrieving memories about individuals—their names, feelings of familiarity, or biographical knowledge—after anterior temporal lobe damage from resection or lesions (Grabowski et al., 2001; reviewed in Olson et al., 2013). One question that arises from this review is whether these deficits are best attributed to grey matter loss or white matter disconnection, specifically to the uncinate?
We report a large number of studies that have associated uncinate fasciculus perturbation with various psychiatric disorders. With the exception of psychopathy, most disorders do not appear to be uniquely or even particularly associated with differences in the uncinate fasciculus. Our hypothesis predicts that uncinate perturbation should cause a particular type of social–emotional problem due to the inability to acquire reward or punishment-based associations for people and objects. Within the realm of psychiatric disorders, are there any symptoms or treatment outcomes that can be directly linked to this proposed function?
Our hypothesis is vague regarding the effects of laterality on uncinate fasciculus function, reflecting the state of the literature. Although psychopathy and antisocial personality disorder are associated with differences in the right uncinate fasciculus, studies of other disorders report differences in the left or bilateral uncinate fasciculus. How do the left and right uncinate fasciculus differ?
Although we propose a transmission route between ventral anterior temporal lobe and the lateral orbital frontal cortex through the uncinate fasciculus, we neglect to discuss information transmission to BA 10 through the uncinate fasciculus. What are the details of information transmission to this region?
The dearth of relevant findings forced us to be vague about several issues, such as laterality effects. We acknowledge that our proposal is speculative, given that supporting evidence is circumstantial (e.g. the uncinate fasciculus’s connections to social and emotional grey matter regions), correlational (e.g. DTI), or based on lesion research, which is often imprecise. Most of the psychiatric and neurological findings were focused on correlating the presence or absence of a psychiatric diagnosis, such as schizophrenia, with differences in white matter tracts with little emphasis on linking white matter changes to specific clinical symptoms. In other cases, the uncinate fasciculus was not clearly delineated from nearby white matter tracts such as the cingulum bundle. Although each individual piece of evidence is weak, the summation of findings indicates that the uncinate fasciculus transmits both social–emotional and mnemonic information, but of a particular type. The particulars of this function are only beginning to be understood. We urge future investigators to use larger and more homogenous clinical populations and to correlate specific symptoms, rather than presence or absence of disease state, with changes in white matter. Also, it would be wise to extend the important findings on memory functions in non-human primates to humans using DTI. To our knowledge, only two groups have achieved this (Metzler-Braddeley et al., 2011; Thomas et al., 2012).
Acknowledgements
We would like to thank Cibu Thomas as well as two anonymous reviewers for intellectual input.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- FTD
frontotemporal dementia
Funding
This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
References
- Acosta-Cabronero J, Patterson K, Fryer TD, Hodges JR, Pengas G, Williams GB, et al. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134:2025–35. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cogntiion? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain. 2010;133:286–99. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex. 2012;22:2705–14. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci USA. 1994;91:7041–5. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Palomba D, Cantagallo A, Maietti A, Stegagno L. Emotional impairments after right orbitofrontal lesion in a patient without cognitive deficits. Neuroreport. 1999;10:1741–6. doi: 10.1097/00001756-199906030-00021. [DOI] [PubMed] [Google Scholar]
- Baur V, Hänggi J, Jäncke L. Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neurosci. 2012;13:4. doi: 10.1186/1471-2202-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bouckoms A, Martuza R, Henderson M. Capgras syndrome with subarachnoid hemorrhage. J Nerv Ment Dis. 1986;174:484–8. doi: 10.1097/00005053-198608000-00008. [DOI] [PubMed] [Google Scholar]
- Browning PGF, Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav Neurosci. 2008;122:477–82. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, et al. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Brit J Psychiat. 2003;182:439–43. [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behav Neurosci. 2002;116:703–15. [PubMed] [Google Scholar]
- Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabardès S, Kahane P, Minotti L, Hoffmann D, Benabid AL. Anatomy of the temporal pole region. Epileptic Disord. 2002;4(Suppl 1):S9–15. [PubMed] [Google Scholar]
- Choi CY, Han SR, Yee GT, Lee CH. A understanding of the temporal stem. J Korean Neurosurg Soc. 2010;47:365–9. doi: 10.3340/jkns.2010.47.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiat Res-Neuroim. 2006;148:55–9. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, et al. Altered connections on the road to psychopathy. Mol Psychiatry. 2009;14:946–53. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T, Lauer EW. Correlative anatomy of the nervous system. New York: MacMillan; 1962. [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TEJ, Robson MD, Pinsk MA, Gross CG, et al. Quantitative investigation of connections of the prefrontal cortex in human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–66. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran EJ. A new association fiber tract in the cerebrum with remarks on the fiber tract dissection method of studying the brain. J Comp Neurol. 1909;19:645–56. [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly D. Uncinate fits. Neurology. 1958;8:250–60. [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Rose SE, McMahon KL. The structure and connectivity of semantic memory in the healthy older adult brain. Neuroimage. 2011;54:1488–94. doi: 10.1016/j.neuroimage.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des centres nerveux. Paris: Rueff et Cie; 1895. [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Drake ME. Postictal Capgras Syndrome. Clin Neurol Neurosurg. 1987;89:271–4. doi: 10.1016/s0303-8467(87)80029-x. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mortiz-Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J Neurol. 2009;256:382–9. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D. Inferotemporal-frontal disconnection: the uncinate fascicle and visual associative learning in monkeys. Eur J Neurosci. 1992;4:1320–32. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–8. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Edelstyn NMJ, Oyebode F, Barrett K. The delusions of Capgras and intermetamorphosis in a patient with right-hemisphere white-matter pathology. Psychopathology. 2001;34:299–304. doi: 10.1159/000049328. [DOI] [PubMed] [Google Scholar]
- Efron R. The conditioned inhibition of uncinate fits. Brain. 1957;80:251–62. doi: 10.1093/brain/80.2.251. [DOI] [PubMed] [Google Scholar]
- Eifuku S, Nakata R, Sugimori M, Ono T, Tamura R. Neural correlates of associative face memory in the anterior inferior temporal cortex of monkeys. J Neurosci. 2010;30:15085–96. doi: 10.1523/JNEUROSCI.0471-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012;33:2390–406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Raunch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR. Outcome after abusive head injury. In: Jenny C, editor. Child abuse and neglect: diagnosis, treatment, and evidence. St. Louis, Missouri: Elsevier Saunders, Inc.; 2011. pp. 451–7. [Google Scholar]
- Fink F, Eling P, Rischkau E, Beyer N, Tomanl B, Klein J, et al. The association between California verbal learning test performance and fibre impairment in multiple sclerosis: Evidence from diffusion tensor imaging. Mult Scler. 2010;16:332–41. doi: 10.1177/1352458509356367. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Fujie S, Namiki C, Nishi H, Yamada M, Miyata J, Sakata D, et al. The role of the uncinate fasciculus in memory and emotional recognition in mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:432–9. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Eacott MJ. Visual learning for an auditory secondary reinforcer by macaques is intact after uncinate fascicle section: indirect evidence for the involvement of the corpus striatum. Eur J Neurosci. 1995;7:1866–71. doi: 10.1111/j.1460-9568.1995.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Easton A, Parker A. Interaction of inferior temporal cortex with frontal cortex and basal forebrain: double dissociation in strategy implementation and associative learning. J Neurosci. 2002;22:7288–96. doi: 10.1523/JNEUROSCI.22-16-07288.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan EA, Gaffan D, Harrison S. Disconnection of the amygdala from visual association cortex impairs visual reward-association learning in monkeys. J Neurosci. 1988;8:3144–50. doi: 10.1523/JNEUROSCI.08-09-03144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–29. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Boles Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S, ffytche D, Simmons A, Kumari V, Howard M, Hodgins S, et al. The antisocial brain: psychopathy matters. Arch Gen Psychiatry. 2012;69:962–72. doi: 10.1001/archgenpsychiatry.2012.222. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Gutnikov SA, Ma YY, Buckley MJ, Gaffan D. Monkeys can associate visual stimuli with reward delayed by 1 s even after perirhinal cortex ablation, uncinate fascicle section or amygdalectomy. Behav Brain Res. 1997;87:85–96. doi: 10.1016/s0166-4328(96)02259-0. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Drake EB. Cognitive functioning following epilepsy surgery. Curr Neurol Neurosci Rep. 2006;6:319–26. doi: 10.1007/s11910-006-0025-8. [DOI] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager SR, Chung A, Hwang J, Daniels MA, et al. Altered cingulate white matter connectivity in panic disorder patients. J Psychiatr Res. 2008;42:399–407. doi: 10.1016/j.jpsychires.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29:1066–79. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Kettenmann B, Ahluwalia V, McCarthy C, Kates WR, Schmitt JE, et al. Pilot multimodal twin imaging study of generalizezd anxiety disorder. Depress Anxiety. 2012;29:202–9. doi: 10.1002/da.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: a post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12:1218–24. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Ramachandran VS. Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc R Soc B. 1997;264:437–44. doi: 10.1098/rspb.1997.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick's disease): clinical features and assessment. Neurology. 2001;56(11 Suppl 4):S6––10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Horel JA, Misantone LJ. Visual discrimination impaired by cutting temporal lobe connections. Science. 1976;193:336–8. doi: 10.1126/science.819992. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Geng J, Hodges JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain. 2011;134:2502–12. doi: 10.1093/brain/awr173. [DOI] [PubMed] [Google Scholar]
- Howe JG, Gibson JD. Uncinate seizures and tumors, a myth reexamined. Ann Neurol. 1982;12:227. doi: 10.1002/ana.410120238. [DOI] [PubMed] [Google Scholar]
- Jackson JH, Colman WS. Case of epilepsy with tasting, movements and “dreamy state” - Very small patch of softening in the left uncinate gyrus. Brain. 1898;21:580–90. [Google Scholar]
- Jensen I. Temporal lobe surgery around the world. Results, complications, and mortality. Acta Neurol Scand. 1975;52:354–73. doi: 10.1111/j.1600-0404.1975.tb05831.x. [DOI] [PubMed] [Google Scholar]
- Jensen I. Temporal lobe epilepsy. Acta Neurol Scand. 1976;53:335–57. doi: 10.1111/j.1600-0404.1976.tb04353.x. [DOI] [PubMed] [Google Scholar]
- Jensen I, Vaernet K. Temporal lobe epilepsy. Follow-up investigation of 74 temporal lobe resected patients. Acta Neurochir (Wien) 1977;37:173–200. doi: 10.1007/BF01402126. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing-Cobbs L. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J Int Neuropsychol Soc. 2011;17:663–73. doi: 10.1017/S1355617711000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJC, Shergill SS, O'Sullivan M, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–8. doi: 10.1002/hbm.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–9. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Nakamura M, Bouix S, Kubicki M, Salisbury DF, Westin CF, et al. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: a diffusion tensor imaging study. Schizophr Res. 2009;110:119–26. doi: 10.1016/j.schres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. Am J Neuroradiol. 2004;25:677–91. [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitis O, Ozalay O, Zengin B, Haznedaroglu D, Eker MC, Yalvac D, et al. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin Neurosci. 2012;66:34–43. doi: 10.1111/j.1440-1819.2011.02293.x. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiatry. 2009;166:467–75. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol. 1960;115:333–69. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–20. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukyuruk B, Richardson RM, Wen HT, Fernandez-Miranda JC, Rhoton AL., Jr Microsurgical anatomy of the temporal lobe and its implications on temporal lobe epilepsy surgery. Epilepsy Res Treat. 2012;2012:769825. doi: 10.1155/2012/769825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–47. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–55. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee K, Shinbo M, Kanai H, Nagumo Y. Reduplicative paramnesia after a right frontal lesion. Cogn Behav Neurol. 2011;24:35–9. doi: 10.1097/WNN.0b013e31821129b7. [DOI] [PubMed] [Google Scholar]
- Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–402. doi: 10.1523/JNEUROSCI.0595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, Mcintosh AR, Toth JP, et al. Episodic memory and the self in a case of isolated retrograde amnesia. Brain. 1998;121:1951–73. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Liao W, Xu Q, Mantini D, Ding J, Machado-de-Sousa JP, Hallak JEC, et al. Altered gray matter morphometry and resting-state functional and structural connectivity in social anxiety disorder. Brain Res. 2011;1388:167–77. doi: 10.1016/j.brainres.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. J Affect Disord. 2011;131:299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Lipson SE, Sacks O, Devinsky O. Selective emotional detachment from family after right temporal lobectomy. Epilepsy Behav. 2003;4:340–2. doi: 10.1016/s1525-5050(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40:1608–21. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, Smith ML, Rockel C. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Mah LWY, Arnold MC, Grafman J. Deficits in social knowledge following damage to ventromedial prefrontal cortex. J Neuropsych Clin N. 2005;17:66–74. doi: 10.1176/jnp.17.1.66. [DOI] [PubMed] [Google Scholar]
- Mahoney C, Rosser MN, Fox NC, Warren JD. 019 Profiles of white matter degeneration in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2012;83:e1. [Google Scholar]
- Mandl RCW, Rais M, van Baal GCM, van Haren NEM, Cahn W, Kahn RS, et al. Altered white matter connectivity in never-medicated patients with schizophrenia. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22075. Advance Access published on March 28, 2012, doi: 10.1002/hbm.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ. Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animals and man. Neurosci Biobehav Rev. 1982;6:351–80. doi: 10.1016/0149-7634(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Mizuno T, Yamada K, Akazawa K, Kasai T, Kondo M, et al. Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology. 2008;50:605–11. doi: 10.1007/s00234-008-0379-5. [DOI] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Bigler ED, Chu Z, Yallampalli R, Oni MG, et al. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. J Neurotrauma. 2011;28:503–16. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Maniega SM, Lymer KS, McKirdy J, Hall J, Sussman JED, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–92. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Metzler-Braddeley C, Jones DK, Belaroussi B, Aggleton JP, O'Sullivan MJ. Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. J Neurosci. 2011;31:13236–45. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magent Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. J Neurosci. 2011;31:17348–57. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Murray EA, Graham KS, Gaffan D. Perirhinal cortex and its neighbours in the medial temporal lobe: contributions to memory and perception. Q J Exp Psychol. 2005;58B:378–96. doi: 10.1080/02724990544000077. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–28. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis ILC, Takashima A, Oostenveld R, McNaughton BL, Fernández G, Jensen O. The neocortical network representing associative memory reorganizes with time in a process engaging the anterior temporal lobe. Cereb Cortex. 2011;22:2622–33. doi: 10.1093/cercor/bhr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–21. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TEJ, Sallet J, Buckley MJ, Rushworth MFS. Separate value compairson and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107:20547–52. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8:123–33. doi: 10.1093/scan/nss119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep. 2011;11:553–9. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134:405–14. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: a diffusion tensor MRI study. Neuroimage. 2004;23:213–23. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia. 1998;36:259–71. doi: 10.1016/s0028-3932(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Parrino JJ. Reduction of seizures by desensitization. J Behav Ther Exp Psychiatry. 1971;2:215–8. [Google Scholar]
- Passingham RE, Stephan KE, Kötter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3:606–16. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, Pruvo JP, Godefroy O, Le Gars D. Microsurgical anatomy of the temporal stem: clincal relevance and correlations with diffusion tensor imaging fiber tracking. J Neurosurg. 2010;112:1033–8. doi: 10.3171/2009.6.JNS08132. [DOI] [PubMed] [Google Scholar]
- Penfield W, Baldwin M. Temporal lobe seizures and the technic of subtotal temporal lobectomy. Ann Surg. 1952;136:625–34. [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–72. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Politis M, Loane C. Reduplicative paramnesia: a review. Psychopathology. 2012;45:337–43. doi: 10.1159/000337748. [DOI] [PubMed] [Google Scholar]
- Price G, Cercigani M, Parker GJM, Altmann DR, Barnes TRE, Barker GJ, et al. White matter tracts in first-episode psychosis: a DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39:949–55. doi: 10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–27. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–65. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer SC, et al. Altered white matter integrity in temporal lobe epilepsy: Association with cognitive and clinical profiles. Epilepsia. 2010;51:536–45. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, et al. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol. 2007;17:1663–8. doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–78. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–5. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506:659–93. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell'Acqua F, Fahy T, Deeley Q, et al. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol Med. 2012;43:401–11. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schmell S, Kümmerer D, Kellmeyer P, Magnus-Sebastian V, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J, Pandya D. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JP, Kim OL, Kim SH, Chang MC, Kim MS, Son SM, et al. Neural injury of uncinate fasciculus in patients with diffuse axonal injury. NeuroRehabilitation. 2012;30:323–8. doi: 10.3233/NRE-2012-0762. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Sharon T, Moscovitch M, Gilboa A. Rapid neocortical acquisition of long-term arbitrary associations independent of the hippocampus. Proc Natl Acad Sci USA. 2011;108:1146–51. doi: 10.1073/pnas.1005238108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20:813–25. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper LM, Ross LA, Olson IR. The sensory and categorical topography of the anterior temporal lobe. Neuropsychologia. 2011;49:3419–29. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–82. [Google Scholar]
- Sundram F, Deeley Q, Sarkar S, Daly E, Latham R, Craig M, et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48:216–29. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Sussman JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–8. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Tartaglia MC, Zhang Y, Racine C, Laluz V, Neuhaus J, Chao L, et al. Executive dysfunction in frontotemporal dementia is related to abnormalities in frontal white matter tracts. J Neurol. 2012;259:1071–80. doi: 10.1007/s00415-011-6300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Thomas C, Walker L, Pierpaoli C, Baker C. The role of the uncinate fasciculus in human visual-associative learning. J Vis. 2012;12:1189. [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–27. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Travers BG, Adluru M, Ennis C, Tromp DPM, Destiche D, Doran S, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröstl J, Sladky R, Hummer A, Kraus C, Moser E, Kasper S, et al. P01-182 - Reduced connectivity in the uncinate fiber tract between the frontal cortex and limbic subcortical areas in social phobia (abstract) Eur Psychiatry. 2011;26(Suppl 1):182. [Google Scholar]
- Tsukiura T, Mano Y, Sekiguchi A, Yomogida Y, Hoshi K, Kambara T, et al. Dissociable roles of the anterior temporal regions in successful encoding of memory for person identity information. J Cogn Neurosci. 2010;22:2226–37. doi: 10.1162/jocn.2009.21349. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. New York: Oxford University Press; 1983. [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76:473–84. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JHR. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci. 1983;6:370–5. [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, et al. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–504. doi: 10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: a meta-analysis and empirical study. Front Hum Neurosci. 2013;7:17. doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder ND, Aziz R, Wilkins K, Tampi RR. Frontotemporal dementias: a review. Ann Gen Psychiatry. 2007;6:15. doi: 10.1186/1744-859X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil AA. Depressive reactions associated with temporal lobe-uncinate seizures. J Nerv Ment Dis. 1955;121:505–10. doi: 10.1097/00005053-195506000-00002. [DOI] [PubMed] [Google Scholar]
- West SE, Doty RL. Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia. 1995;36:531–42. doi: 10.1111/j.1528-1157.1995.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Bjørnebekk A, Grydeland H, Fjell AM, Walhovd KB. Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiatry. 2011;68:369–77. doi: 10.1001/archgenpsychiatry.2011.24. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–87. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry. 2009;66:986–94. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:6430–5. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappalà G, Thiebaut de Schotten M, Eslinger PJ. Traumatic brain injury and the frontal lobes: What can we gain with diffusion tensor imaging? Cortex. 2012;48:156–65. doi: 10.1016/j.cortex.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain. 2009;132:2579–92. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]