Abstract
Fatty liver disease in humans can progress from steatosis to hepatocellular injury, fibrosis, cirrhosis, and liver failure. We developed a series of straightforward assays to determine whether zebrafish larvae with either tunicamycin- or ethanol-induced steatosis develop hepatic dysfunction. We found altered expression of genes involved in acute phase response and hepatic function, and impaired hepatocyte secretion and disruption of canaliculi in both models, but glycogen deficiency in hepatocytes and dilation of hepatic vasculature occurred only in ethanol-treated larvae. Hepatic stellate cells (HSCs) become activated during liver injury and HSC numbers increased in both models. Whether the excess lipids in hepatocytes are a direct cause of hepatocyte dysfunction in fatty liver disease has not been defined. We prevented ethanol-induced steatosis by blocking activation of the sterol response element binding proteins (Srebps) using gonzombtps1 mutants and scap morphants and found that hepatocyte dysfunction persisted even in the absence of lipid accumulation. This suggests that lipotoxicity is not the primary cause of hepatic injury in these models of fatty liver disease. This study provides a panel of parameters to assess liver disease that can be easily applied to zebrafish mutants, transgenics, and for drug screening in which liver function is an important consideration.
Introduction
Liver disease affects millions of Americans and a third of adults in the US have fatty liver disease, making this the most common hepatic pathology in the Western world.1,2 Multiple factors contribute to the development and progression of fatty liver disease, including alcohol abuse, obesity, type II diabetes, and viral infection.3 While genetics can contribute to susceptibility to fatty liver disease,4 the rapidly increasing incidence is primarily attributed to the obesity epidemic.5 However, the increasing incidence of fatty liver disease does not correspond to improvements in therapeutic options, as lifestyle changes remain the most effective and often, the only, treatment option. Furthermore, treatments for alcoholic liver disease (ALD), such as prednisolone, have undesirable side effects.6 Thus, a thorough understanding of fatty liver disease pathophysiology and progression is needed to develop better treatment options.
The earliest stage of ALD and nonalcoholic fatty liver disease (NAFLD) is the accumulation of lipid droplets in hepatocytes (steatosis). Multiple factors can cause steatosis, including decreased triglyceride metabolism, increased lipid import or synthesis, and decreased secretion of lipoprotein-triglyceride particles. Lipid-laden hepatocytes are prone to further injury,7,8 but it is not clear whether this is a primary result of lipotoxicity or, instead is secondary to the underlying hepatocellular deficiency or stress that accompanies most cases of fatty liver disease. Indeed, while lipids provide much needed fuel for the continued function of compromised hepatocytes, lipid peroxidation, and excessive lipid accumulation also contribute to disease progression.8 Indeed, approximately one-third of individuals with NAFLD will progress to nonalcoholic steatohepatitis5 and individuals with NAFLD are predisposed to alcoholic liver injury.9–11 However, whether therapies that combat steatosis will be effective at reducing liver injury is not known. The alternative possibility is that lipid accumulation serves as a protective measure taken by hepatocytes to provide an efficient energy resource, and therefore, reducing steatosis may precipitate further hepatocyte dysfunction.
While the hepatocyte carries out most major hepatic functions, essential roles are also provided by the biliary and endothelial cells and by liver macrophages (Kupffer cells). Injury or dysfunction in hepatocytes caused by the toxic byproducts of ethanol metabolism, the consequences of dysregulated insulin signaling, oxidative stress, and/or organelle damage, can affect other hepatic cells. For instance, loss of the fenestrations of the sinusoidal endothelial cells and biliary cell proliferation are commonly found in patients with ALD and NAFLD.12–15 In response, hepatic stellate cells (HSCs) become activated, proliferate, and secrete the extracellular matrix to form a fibrotic scar.16–18 Left unchecked, this scar will replace most of the liver, resulting in cirrhosis.
While rodent models of ALD and NAFLD have unquestionable value, there are limitations. For instance, the degree of steatosis varies greatly depending on the model employed and genetic background. To develop a significant degree of liver disease, rodents commonly require months of exposure to ethanol and coadministration of an additional insult, such as lipopolysaccharide (LPS), or an invasive means of ethanol delivery, such intragastric infusion. Similarly, NAFLD induced through a high fat diet or in genetic models of obesity (i.e., ob/ob and db/db mice)19 does not result in significant injury without additional stressors, such as LPS or tumor necrosis factor20,21 to recapitulate features in patients with fatty liver disease. Therefore, additional models of these diseases are sought.
Zebrafish larvae can complement studies on fatty liver disease traditionally carried out in mammals. The zebrafish liver is mature by 5 days postfertilization (dpf) and studies from several laboratories have demonstrated that hepatobiliary disease in zebrafish larvae is very similar to the disease in mammals.22–26 This likely reflects the high genetic conservation of pathways underlying liver injury24 and common physiological roles played by the liver in both mammals and teleosts. While markers of inflammation have not yet been demonstrated in a larval model of liver disease, the recent identification of HSCs in zebrafish by Yin et al. demonstrated that, like in humans, HSCs are activated in zebrafish with ALD.27 Moreover, the ease of exposing zebrafish to toxins which cause liver disease in humans, such as alcohol27–30 and acetaminophen,23 makes this system increasingly popular for studying liver disease and thus, necessitating a standard set of assays for evaluating hepatic function.
Thus, we developed a series of assays to define the hepatic response of two models of fatty liver disease on the major cells of the hepatic parenchyma: hepatocytes, biliary epithelial cells, endothelial cells, and HSCs. These assays can be easily applied by most zebrafish researchers with a laboratory equipped to apply standard procedures for working with zebrafish larvae and molecular biology techniques. We use these assays to assess the effects of fatty liver disease caused by tunicamycin (Tm) or ethanol (EtOH) on hepatic and biliary function and on HSC activation. We found that some, but not all measures of hepatocyte function were disrupted in both models, whereas some aspects were only affected in response to EtOH. We then used these assays to address a central question in the fatty liver field of whether lipid in hepatocytes contribute to hepatic dysfunction in ALD. These findings demonstrate the utility of zebrafish larvae as a nonmammalian vertebrate system for studying hepatobiliary disease and describe multiple parameters by which liver dysfunction can be assessed.
Materials and Methods
Zebrafish maintenance and larval exposures
Maintenance of adult and larval zebrafish and exposure of larvae to ethanol or tunicamycin were performed as previously described.26–28 For ethanol exposures, 4 dpf larvae were exposed to either 0 or 350 mM (2%) ethanol for 32 h by addition of absolute ethanol (Pharmco-AAPER, Brookfield, CT) to egg water (0.6 g/L Crystal Sea Marinemix; Marine Enterprises International, Baltimore, MD) containing methylene blue (0.002 g/L). For tunicamycin exposures, larvae were exposed from 3–5 dpf to 1 μg/mL tunicamycin (Calbiochem/EMD Chemicals, Gibbstown, NJ) in egg water or dimethyl sulfoxide alone as a control. Dishes containing ethanol were sealed with Parafilm to prevent evaporation. All zebrafish protocols were approved by Mount Sinai School of Medicine and University of California San Francisco's Institutional Animal Care and Use Committees.
Imaging of live zebrafish
Transgenics
Tg(-3.5fabp10a:gc-EGFP)lri500; Tg(kdrl:HsHRAS-mCherry)s896 double transgenics were obtained from Drs. Torres-Vázquez and Anand-Apte.31 Live larvae were mounted in 2% methylcellulose (Sigma Aldrich, Saint Louis, MO) in left lateral recumbency and imaged using a Nikon SMZ1500 stereomicroscope with a Nikon Digital Sight camera (Melville, NY).
PED6
Larvae were transferred to egg water containing PED6 [N-((6-(2,4-dinitrophenyl)amino)hexanoyl)-1-palmitoyl-2-BODIPY-FL-pentanoyl-sn-glycero-3-phosphoethanolamine] (D23739, Invitrogen, Carlsbad, CA) and rhodamine. After 30 min, larvae were washed with standard egg water, anesthetized, and imaged in right lateral recumbency to visualize the gallbladder.
Oil red O staining
Whole mount oil red O staining
Larvae were fixed in 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) overnight at 4°C. All steps of oil red O staining were performed at room temperature on a slow-moving shaker. After fixation, larvae were transferred to Corning Netwells (Corning, NY) and placed in a 12-well dish, washed in 1× PBS and bleached of pigment using a solution containing: 6 mL sterile H2O, 3.3 mL 30% H2O2 (Fisher Scientific, Pittsburgh, PA) 0.5 mL formamide (Sigma Aldrich) and 0.25 mL 20× SSC buffer (pH=7.0). Larvae were washed in 1× PBS and infiltrated with 85% and 100% propylene glycol (Alfa Aesar, Ward Hill, MA) solutions for 10 min each before submersion in oil red O (Polysciences, Warrington, PA) and stained overnight in the dark. Destaining by washing in 100% and 85% propylene glycol for approximately 30 min each was followed by two washes in 1× PBS and stored in 80% glycerol (Sigma). Larvae were considered positive for hepatic steatosis if 3 or more round lipid droplets were visible within the hepatic parenchyma (see Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/zeb). Occasionally, red staining of the vasculature was noted as branch-like structures within the liver; these were not included in the scoring criteria for steatosis.
Oil red O staining of cryosections
Larvae were fixed in 4% PFA overnight at 4°C, washed with 1× PBS, and infiltrated with 30% sucrose/PBS overnight at 4°C. Larvae were embedded in Tissue-Tek OCT Compound (Sakura Finetek USA, Torrance, CA) and 10 μm sections were mounted on Unifrost slides (Azer Scientific, Morgantown, PA) and stored at −80°C. Before staining with oil red O, slides were warmed to room temperature and immersed in 85% and 100% propylene glycol for 10 min each, and then overnight in oil red O in the dark. Slides were quickly destained the following day in 100% and 85% propylene glycol (∼4 min each), and then washed with 1× PBS. Slides were counterstained with Harris' hematoxylin (Fisher Scientific) for <2 s, washed with running tap water for 1–2 min. Slides were visualized using an Olympus BX41 microscope with a 60× objective (Center Valley, PA) and a Nikon Digital Sight-Ri1 camera.
Histology
Larvae were fixed in 4% PFA overnight at 4°C and embedded in paraffin and 4 μm were obtained as described.32 Hematoxylin and eosin staining was performed by the Mount Sinai Histology Service Shared Resource Facility. Staining for glycogen was performed using the Periodic Acid Schiff's (PAS) Stain kit (Polysciences) as described by the manufacturer's instructions using acidified mercury-free hematoxylin as a counterstain to visualize nuclei. Images were obtained as described above.
TUNEL assay
The TUNEL reaction was carried out on cryosections using the In Situ Cell Death Detection Kit from Roche (Basel, Switzerland) and counterstained stained with Cy3-streptavidin (Sigma) at 1:200 concentration in 1× PBS containing 1% bovine serum albumin (OmniPur, Calbiochem) and 0.1% Tween 20 (OmniPur). Coverslips were mounted using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA). Slides were imaged using a Zeiss Axioplan2 IE epifluorescence microscope using a 40× objective (NA 0.75).
Immunofluorescence
Cryosections
Immunostaining was performed on cryosections. Thawed slides were postfixed for 20 min using ice-cold acetone, dried, and tissue samples were traced with a Super PAP Pen (Daido Sangyo Co., Tokyo, Japan). All steps following postfixation were performed in a humid chamber at room temperature. Slides were blocked with a solution of 1% BSA in PBST (0.1% Tween 20 in 1× PBS) for 30 min before incubation with anti-mouse BSEP (bile salt export pump) to label bile canaliculi (1:1000, Kamiya Biomedical Company, Seattle, WA)33,34 for 2 h at room temperature followed by washing with PBS and incubation with secondary antibody (goat anti-rabbit Alexa Fluor 488; 1:1000, Molecular Probes, Eugene, OR) which contained Cy3-streptavidin (1:200; Sigma) to label hepatocytes. Coverslips were mounted using Vectashield containing DAPI (Vector Laboratories). Slides were visualized on a Leica SP5 DM confocal microscope (Leica Microsystems, Bannockburn, IL) with a 63× NA 1.4 oil objective. The number of BSEP positive structures (canaliculi) and hepatic nuclei were counted for 2–4 Z-stacks per treatment.
Whole larvae
Tg(hand2:EGFP)pd24 larvae were treated as described above, fixed in 2% formaldehyde, and processed for immunohistochemistry as described.35 The antibodies used were, chicken anti-GFP (Aves Labs) at 1:1000, and rabbit anti-laminin (Sigma) at 1:100. Secondary antibodies were obtained from Molecular Probes (goat anti-chicken GFP, 1:200 and donkey anti-rabbit far red, 1:200). Samples were imaged on a Zeiss Pascal confocal microscope. Image processing and cell counting were conducted using Fiji (http://fiji.sc/wiki/index.php/fiji). HSC numbers were determined by counting Tg(hand2:EGFP)-expressing cells inside the liver, excluding cells closely associated with the liver periphery. Statistical analyses were performed using the Student's two-tailed t-test.
Quantitative real-time PCR
cDNA was prepared from total RNA isolated from pools of 15–20 dissected livers using RNeasy Mini kit from Qiagen (Valencia, CA) and reverse-transcribed using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). qPCR was carried out using a Light Cycler 480 (Roche) as previously described30 using PerfeCTa SYBRGreen FastMix (Quanta Biosciences). Values for target gene were normalized to reference gene rpp0, using the comparative threshold method to analyze the data. One sample t-tests were performed to compare each treatment to control for WT larvae. For comparing gonzohi1487 larvae to siblings after alcohol exposure, unpaired, two-tailed t-tests were performed. Primers used are listed in Table S1.
Conventional PCR
Whole larval cDNA was prepared as described above for qPCR. Conventional PCR for EGFP, rpp0, and fabp10 was performed using Phusion High Fidelity Polymerase (New England Biolabs) and visualized by 1% agarose gel electrophoresis. Primers used are listed in Table S1.
Image processing and graphics
Images were cropped and processed using Adobe Photoshop CS4 (Adobe Systems, San Jose, CA). Graphs were plotted using Prism 5.0c (GraphPad Software, Inc., La Jolla, CA).
Results
Zebrafish larvae exposed to tunicamycin or ethanol develop fatty liver disease
EtOH induces fatty liver disease in rodents36,37 and fish28–30 and is a major cause of liver disease in humans. Tm blocks N-linked glycosylation of proteins by inhibition of DPAGT1,38,39 causing ER stress and, by an as of yet unknown mechanism, results in fatty liver disease in mammals40–42 and zebrafish.43,44 To determine the effect of EtOH and Tm on liver morphology, we employed a transgenic zebrafish line expressing dsRed specifically in hepatocytes (Tg(fabp10:dsRed)).45 Zebrafish larvae exposed to EtOH from 4–5.5 dpf28,30 or to 1 μg/mL Tm from 3–5 dpf43,44 develop hepatomegaly, indicated as a change from the thin crescent shaped livers in controls to oval shaped livers in EtOH- and Tm-treated larvae (Fig. 1A and previous reports28–30,43). Analysis of liver area by ImageJ displayed a mild increase in liver size in ethanol-treated fish versus controls (38711.5 μm2 in controls vs. 40709.2 μm2 in ethanol-treated larvae, or a 5% total increase in size). However, these calculations were based on two-dimensional images. It is possible that the evident hepatomegaly in our fatty liver disease models would be more pronounced by analysis of liver volume in three-dimensional confocal Z-stacks.
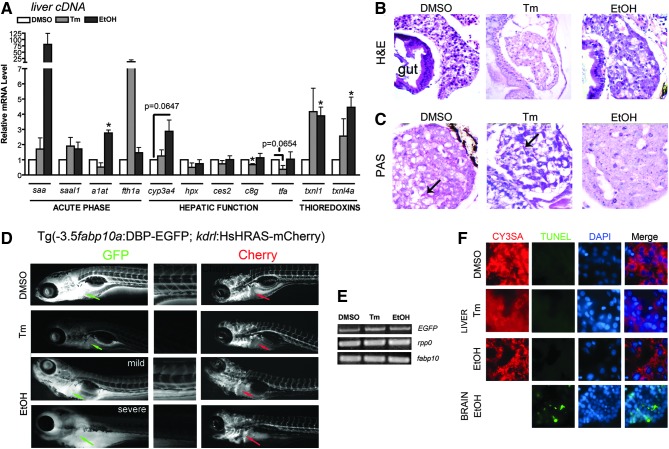
FIG. 1.
Tunicamycin and ethanol cause hepatomegaly and steatosis in zebrafish larvae. (A) Representative brightfield images from control (dimethyl sulfoxide [DMSO]), tunicamycin (Tm), and ethanol-treated larvae expressing dsRed in hepatocytes (Tg:(fabp10:dsRed). (B) Representative whole mount oil red O images showing steatosis development in Tm and ethanol-treated larvae. The incidence of steatosis in each treatment regimen is noted. (C) Representative images from oil red O stained cryosections. Top images show location of the liver (circled) within the larvae. Bar=25 μm. Although hepatomegaly is a highly penetrant phenotype in both Tm and EtOH-treated fish,28,29,43 the section obtained for the Tm image was not through the widest region of the liver and therefore, does not reflect the actual liver size in this sample. Bottom images show accumulation of lipid droplets in hepatocytes of Tm and ethanol-treated larvae. Bar=10 μm.
Staining whole larvae and cryosections through the liver with oil red O, which binds to neutral lipids,46 revealed that livers from both Tm and EtOH-treated larvae have significant lipid content that was readily visible by both methods (Fig. 1B, C). Occasional staining of branch-like structures in the hepatic parenchyma (Supplementary Fig. S1) were not included in the scoring for steatosis. Consistent with our previous findings,28,43 whole mount staining demonstrates that the majority of larvae treated with either Tm or EtOH develop steatosis (Fig. 1B). It is not known whether lipid accumulation in these models is accompanied by hepatic dysfunction, as seen in humans, and the current study was undertaken to address this question.
Hepatocyte function is differentially affected by Tm and EtOH
Hepatocytes are the major parenchymal cell of the liver and are responsible for xenobiotic metabolism, ammonia detoxification, lipid metabolism, glycogen storage, insulin responsiveness, secretion of serum proteins, extracellular matrix synthesis, and synthesis and secretion of bile. To varying degrees, disruption of each of these processes are detected in patients with fatty liver disease.9,47–49 To determine whether similar consequences accompany fatty liver disease in zebrafish, we examined the expression of genes involved in several different hepatocyte functions, assessed hepatocyte integrity, measured the level of hepatocyte glycogen and protein secretion.
We have previously demonstrated that a marker of the acute phase response, serum amyloid A (saa), is strongly upregulated in EtOH-treated larvae.28 Thus, we sought to determine if other acute phase response genes, which signify hepatic injury, or genes involved in a variety of hepatic functions were dysregulated in fatty liver disease (Fig. 2A). Using cDNA prepared from livers dissected from 15–20 treated or control larvae, we found that EtOH treatment caused upregulation of the acute phase genes alpha-1 antitrypsin (a1at or serpina1) and saa, Tm treatment had no significant effect on these genes. Ferritin heavy chain (fth1a), an iron-storage protein that can act as an acute phase responsive protein,50,51 was not affected in the liver of ethanol exposed larvae, but was upregulated in each sample of Tm-treated larvae, although the variability in response prevented this from achieving statistical significance. Neither Tm nor EtOH significantly changed the expression of saal1, a serum amyloid-like protein. These findings suggest that Tm and EtOH have differential efficacy on inducing the acute phase response. We found that Tm and EtOH had different effects on the expression of genes involved in xenobiotic metabolism (cytochrome P450 3A4, cyp3a4, carboxylesterase 2, ces2); complement pathway (complement component 8 gamma polypeptide, c8g); iron homeostasis (hemopexin, hpx, transferring alpha, tfa); and redox signaling (thioredoxin like 1, txnl1, thioredoxin like 4a, txnl4a). In fact, EtOH treatment had little-to-no effect on any of the hepatic function genes, with only a modest upregulation of cyp3a4 (p=0.0647). However, significant upregulation of txnl1 and txnl4a occurred in livers of larvae exposed to EtOH. Modest, but not significant, induction of tnxl1 and tnxl4a was caused by Tm treatment. These data suggest that redox signaling in fatty liver disease is differentially disrupted by EtOH and Tm. Tm treatment modestly decreased expression of c8g and tfa, which encode two proteins secreted by hepatocytes, suggesting that the expression of secretory proteins is suppressed in hepatocytes exposed to Tm.
FIG. 2.
Tunicamycin and ethanol induce hepatic dysfunction. (A) Quantitative, real-time PCR data from liver cDNAs of control (DMSO, white bars), Tm- (gray bars) and EtOH (black bars)-treated larvae. Genes are organized by function. Statistical significance was calculated using the 1-sample t-test. *p<0.05. Bars correspond to mean±SEM. (B) Hematoxylin and eosin staining of livers from DMSO, Tm, and EtOH-treated animals. (C) Periodic acid-Schiff's reagent staining for presence of glycogen. DMSO and Tm-treated larvae contain large glycogen depots (arrow), while EtOH-treated larvae have almost no hepatic glycogen. (D) Fluorescent images from live Tg(fabp10:DBP-EGFP; kdrl:HsHRAS-mCherry) larvae. Expression of DBP-EGFP is drastically reduced in Tm and EtOH-treated larvae but mCherry expression is not affected. Arrow points to the liver. To assist in visualization of DBP-EGFP secretion, a closeup of the tail region is shown. (E) Conventional PCR for EGFP, rpp0, and fabp10 for each treatment. The levels of EGFP mRNA do not correspond to the drastic GFP loss seen in live fish after Tm and EtOH treatment. (F) TUNEL assay for apoptosis. No hepatic apoptosis was observed in any treatment; however, apoptosis was noted in the brain after EtOH treatment.
Histological analyses are a powerful and commonly used clinical approach to diagnose liver disease. Hematoxylin and eosin (H&E) staining (Fig. 2B) of control larvae typically reveals significant vacuolarization of hepatocytes, which also occurred in Tm-treated larvae but was reduced in EtOH-treated samples (Fig. 2B). This vacuolarization likely reflects a postfixation artifact in which glycogen aggregates during processing for paraffin sections (see Fig. 2C). The large extracellular spaces that occur in both Tm and EtOH-treated larvae may reflect dilation of the hepatic vasculature. Imaging of the hepatic vasculature using Tg(fli1a:EGFP)52 confirmed the presence of dilated blood vessels in EtOH-treated larvae (see Supplementary Methods and Supplementary Fig. S2). Measurements of the diameter of the hepatic blood vessels in EtOH-treated Tg(fli1a:EGFP) larvae demonstrated that these were significantly wider than untreated larvae (Supplementary Fig. S2). Finally, the small, round vacuoles within hepatocytes of Tm- and EtOH-treated animals are suggestive of hepatic steatosis, confirming findings from Figure 1 and our previous reports.28,43
Glycogen is a major source of energy and glycogen stores within hepatocytes are depleted in liver disease.48,49,53 The PAS stain labeled glycogen bright pink (Fig. 2C) in hepatocytes of control and Tm-treated larvae, while livers of EtOH-treated larvae contain almost no glycogen (Fig. 2C). Therefore, Tm and EtOH differentially affect hepatic glycogen storage.
We previously demonstrated that zebrafish exposed to Tm or EtOH have significant upregulation of the unfolded protein response (UPR), a homeostatic pathway that attempts to maintain the protein folding capacity of the ER during stressful conditions.28,30,43 Studies in humans and rodent models of fatty liver disease have also detected activation of this pathway.40,54–59 This likely reflects the secretory pathway dysfunction that causes a decrease in protein secretion by hepatocytes in patients with liver disease.47,60 To assess whether hepatocyte protein secretion was impaired by EtOH or Tm exposure, we used a transgenic line in which the vitamin D binding protein (DBP, also called group specific component (gc)),31,61 the albumin equivalent and major serum protein secreted by fish hepatocytes,61 is fused to EGFP and expressed under the hepatocyte specific fabp10 promoter Tg(-3.5fabp10a:gc-EGFP)lri500. This line was crossed to Tg(kdrl:HsHRAS-mCherry)s896 fish in which mCherry is fused to the prenylation sequence in human HRAS, thereby directing it to the membrane of endothelial cells.62 DBP-EGFP secreted by hepatocytes was readily visible by a GFP signal in the vasculature by 3 dpf31 and by 5 dpf can be seen in both the liver and the vasculature of control larvae (Fig. 2D). Fluorescence was dramatically reduced in Tm- and EtOH-treated larvae, without retention of DBP-EGFP in the liver (Fig. 2D). This suggests that either (1) treatment with Tm or EtOH affects the transcription or translation of the transgene, or (2) treatment with Tm or EtOH enhances the degradation of the DBP-EGFP fusion protein, perhaps through ER-associated degradation, as genes encoding key factors in this process are upregulated in larvae treated with Tm or EtOH.30,43 Conventional PCR analysis (Fig. 2E) demonstrated that mRNA expression was equivalent in all samples and immunoblotting demonstrated that the protein produced from the transgene was only moderately reduced in the Tm and EtOH-treated fish (not shown). Thus, we conclude that while both treatments affect the synthesis and possibly secretion of this protein, the major effect is on the acquisition of GFP fluorescence.
Hepatocyte injury in fatty liver disease can culminate in cell death.3,63–65 However, using the TUNEL assay, we did not detect any apoptosis in hepatocytes of Tm or EtOH exposed fish (Fig. 2F), although EtOH treatment did induce TUNEL positive cells in the brain (Fig. 2F). We conclude that Tm and EtOH impair several aspects of hepatocyte function, but that in zebrafish larvae, as in mammalian models of fatty liver disease, this is not sufficient to induce cell death.
Bile canaliculi, but not bile secretion, are disturbed in zebrafish larvae with fatty disease
Cholestasis, the accumulation of bile acids in the liver, has been associated with ER stress both in vitro and in vivo34,66–68 and cholestasis can develop in fatty liver disease.69–72 We assessed the structure of the hepatocyte canaliculi, which is the apical hepatocyte surface through which bile is secreted into preductules, a structure formed by the biliary preductal epithelial cells, which are unique to the livers of fishes and analogous to the mammalian canal of Hering.73,74 The bile salt export pump (BSEP/ABCB11) is a canalicular hepatocyte apical membrane protein that shuttles bile acids out of hepatocytes into the canalicular lumen and a widely used marker of canaliculi.53 Staining with anti-BSEP revealed string-shaped canaliculi in control larvae at 120 and 128 hpf that were evenly distributed throughout the parenchyma (Fig. 3A, first and third panels). However, treatment with Tm or EtOH caused swollen, shortened, and punctate BSEP staining and a reduction in the number of canaliculi per hepatocyte nuclei by 53% and 41%, respectively (Fig. 3A, second and fourth panels). Color images showing the overlay of Bsep staining with DAPI-stained nuclei are shown in Supplementary Figure 3A. These canalicular abnormalities were confirmed by transmission electron microscopic analysis of EtOH-treated larvae (not shown).
FIG. 3.
Biliary alterations by Tm and EtOH. (A) Confocal Z-stack projections of liver labeled with anti-BSEP antibody (top panels) and counterstained with DAPI (bottom panels). Canaliculi from control livers present as long, narrow structures. Treatment with Tm and EtOH results in canalicular attenuation and dilation. Bar=10 μm. Numbers underneath each image correspond to the canaliculi:hepatic nuclei counted for 2–4 Z-stacks per treatment. (B) Live images from larvae treated with rhodamine and PED6 to assess bile secretion. Bile secretion was not affected by Tm or EtOH, as noted by the accumulation of PED6 in the gallbladder (arrow, gb). Bar=1 mm.
PED6 is a BODIPY-tagged phospholipid that becomes fluorescent when cleaved in the gut by phospholipase A2 (PLA2) and can be visualized as it is transferred to hepatocytes via enterohepatic circulation and secreted with bile into the gallbladder.75 We used PED6 to assess bile secretion in live zebrafish treated with Tm and EtOH (Fig. 3B). There was no observable change in PED6 secretion after treatment with Tm or EtOH; there was little retention of fluorescent PED6 in the liver and prominent expression in the gallbladder and intestine regardless of treatment. Therefore, while Tm and EtOH significantly alter canalicular structure, there is no discernable accumulation of bile in the liver or reduced PED6 accumulation in the gallbladder, suggesting that these treatments do not cause cholestasis in zebrafish.
HSCs are activated by Tm and EtOH
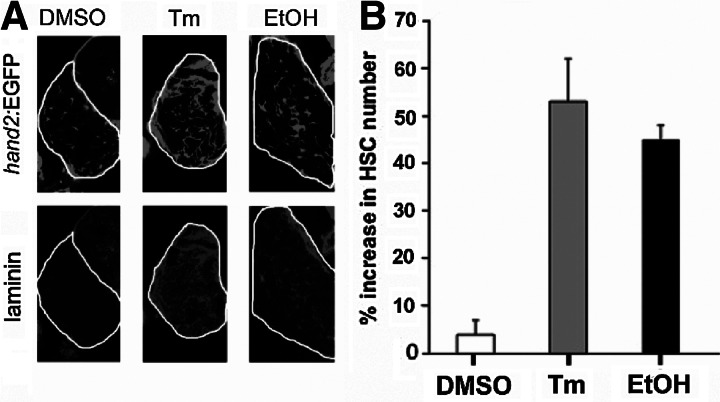
HSCs are quiescent under physiological conditions, but are activated during liver injury to secrete collagen, one of the main components of fibrotic scars.16–18 To determine if Tm and EtOH affect HSCs, we employed a transgenic line that labels both active and inactive HSCs with EGFP (Tg:(hand2:EGFP)).27 We previously described a significant increase in HSC number in response to alcohol, at least partially due to elevated cell proliferation27 (Fig. 4A). Here we demonstrate a similar expansion of HSCs after Tm treatment (Fig. 4A, B). In response to liver injury, HSCs become activated and secrete extracellular matrix proteins, such as laminin and collagen. Whereas there was little laminin deposition in the livers of the control animals, the amount of laminin deposition was greatly increased after, both ethanol and Tm treatment (Fig. 4A, with color overlays shown in Supplementary Fig. 3B), indicative of stellate cell activation. Thus, HSCs activation and increased matrix deposition occurs in fatty liver disease in zebrafish, as in humans with advanced fatty liver disease.
FIG. 4.
Hepatic stellate cells are activated by Tm and EtOH. (A) Single plane confocal images from Tg(hand2:EGFP) larvae with GFP-expressing HSCs (top panels) and labeled with anti-laminin antibody (bottom panels). Little laminin is visible in DMSO-treated larvae, but its deposition is significantly increased after Tm and EtOH treatment. Further, the number of HSCs is significantly increased by both treatments. (B) Quantification of percent increase in HSC number (average±SEM) during treatment with Tm and EtOH. For EtOH treatment, the numbers of HSCs in control and EtOH-treated larvae were counted 1 day after the treatment. Three separate treatments were performed. Total of 30 control and 30 EtOH-treated larvae were analyzed.
Hepatic dysfunction in response to Tm and EtOH does not require lipid accumulation
While several studies indicate that steatosis predisposes to further injury7,8 and that lipotoxicity can cause hepatocyte dysfunction,76 it is not clear whether steatosis per se is required for liver injury in fatty liver disease. To address this, we used the assays described above to ask whether blocking lipid accumulation in EtOH-treated larvae affects hepatic function in ALD.
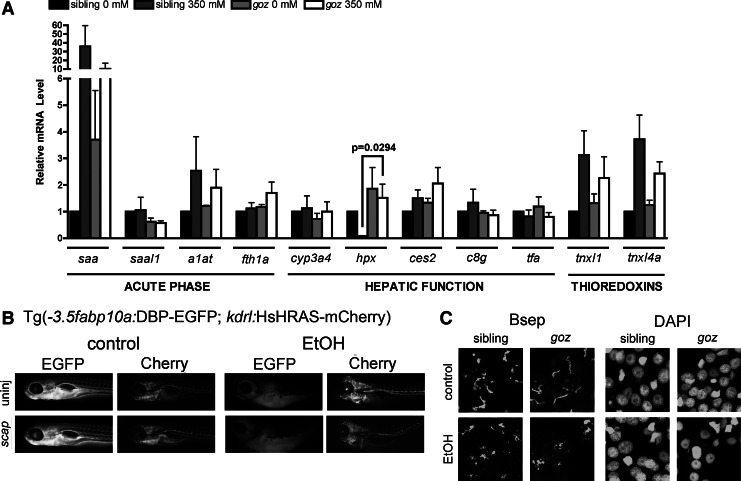
Srebp activation is blocked by mutation of the mbtps1 gene28 and by morpholino mediated knockdown of the Srebp transport protein (scap).28 We treated mbtps1 mutants and scap morphants with alcohol and assessed hepatic gene expression (Fig. 5A), DBP-EGFP secretion (Fig. 5B), and canalicular structure (Fig. 5C). qPCR analysis showed that nearly all acute phase proteins (saal1, a1at, fth1a), hepatic function markers (ces2, c8g, tfa) and genes involved in oxidative stress (tnxl1, tnxl4a) are similarly affected by EtOH in control and mbtps1 mutants (Fig. 5A), with the exception that alcohol exposure reduced hemopexin (hpx) expression in livers of control larvae but did not in mbtps1 mutants. Interestingly, mbtps1 is also required for activation of the Crebh transcription factor which induces expression of several acute phase response genes,77 and mbtps1 mutation did reduce saa upregulation in response to EtOH, but the effect did not achieve statistical significance. Other measures of hepatic function were unchanged in the absence of hepatic lipid, including DBP-EGFP secretion in scap morphants (Fig. 5B) or structural changes to bile canaliculi in goz mutants (Fig. 5C and Supplementary Fig. S3C) or scap morphants (not shown). This data suggests that the hepatic dysfunction caused by EtOH can occur independent of lipid accumulation in hepatocytes.
FIG. 5.
Reducing lipid accumulation in ALD does not improve hepatic function. (A) Quantitative, real-time PCR data from liver cDNAs from gozhi1487 mutants and their nonmutant siblings±EtOH. Statistical significance was calculated using two-way ANOVA with Bonferroni's post-hoc test. Bars correspond to mean±SEM. Significant p-values (p<0.05) correspond to the global effect of either dose or genotype, as noted. No interaction was found between dose and genotype in the ANOVA. (B) Fluorescent images from live Tg(fabp10:DBP-EGFP; kdrl:HsHRAS-mCherry) larvae with scap knockdown±EtOH. (C) Confocal Z-stack projections of liver labeled with anti-BSEP antibody and counterstained with DAPI. No difference in canalicular structure was noted between goz mutants and their nontransgenic siblings in the presence or absence of EtOH.
Discussion
Fatty liver disease affects millions of Americans and can progress to advanced liver disease, liver failure, and death, yet few pharmacologic therapies are available for fatty liver disease patients. Development of new treatments requires animal models for preclinical testing and for identifying the most significant contributions to pathophysiology of this disease. Here we advance the use of zebrafish larvae to study fatty liver disease caused by either Tm or EtOH. We demonstrate the multiple parameters by which liver injury can be assessed in zebrafish and show the differences in hepatic and biliary dysfunction between the two models, though both are able to induce significant hepatic steatosis (Fig. 1). The assays described here are straightforward and adaptable by most zebrafish researchers using standard equipment and procedures. Further, we use these parameters to demonstrate that reducing hepatic steatosis does not improve or exacerbate hepatic injury after EtOH treatment. This suggests that the lipid that accumulates in hepatocytes of ALD patients does not cause the hepatic injury that accompanies this disease. This is similar to findings in a model of NAFLD in which preventing lipid accumulation by using clofibrate does not reduce the secretory pathway stress in hepatocytes that accompanies fatty liver disease.78
We observed dysregulation of thioredoxin expression (txnl1 and txnl4a) by both Tm and EtOH treatment, but differential changes in acute phase genes: while saa and a1at were significantly upregulated by EtOH, only fth1a was affected by Tm. Furthermore, Tm decreased expression of tfa and c8g, while EtOH had no effect (Fig. 2A). These findings suggest that Tm has a more significant effect on hepatic function than EtOH. We did not analyze the expression of these genes extrahepatically and it is possible that they are differentially regulated by Tm and EtOH in other tissues.
Interestingly, the differences in gene expression regulation between Tm and EtOH were not reflected in the histopathology: both treatments showed vascular swelling by H&E staining (Fig. 2B) and congestion of blood vessels was notable with EtOH treatment (Fig. 2B and Supplementary Fig. S1), and glycogen loss was observed only in EtOH-treated livers (Fig. 2C). Furthermore, both treatments caused significant reductions in DBP-EGFP fluorescence and secretion (Fig. 2D), yet neither treatment caused apoptosis (Fig. 2F). Therefore, we conclude that either gene expression data from liver cDNAs may not necessarily reflect the level of liver injury, or that the panel of genes needs to be expanded to other pathways to accurately determine the status of liver function in FLD. These data indicate that both gene expression and histology are important measures of assessing liver injury.
The mechanism of steatosis in ALD and NAFLD is not fully understood. Tm causes ER stress and subsequent steatosis by blocking the activity of DPAGT1, thereby inhibiting N-linked protein glycosylation and causing accumulation of hypoglycosylated and unfoldable proteins in the ER.38,39 The UPR is significantly upregulated after both treatments28,30,43 and ER stress is observed in patients with NAFLD and ALD.58,68 Interestingly, the inability to detect DBP-GFP fluorescence in Tm-treated fish may reflect the propensity of EGFP to aggregate in the oxidizing environment in the ER79 which is further enhanced under conditions that affect ER redox balance. Thus, it is possible that a failure to achieve adequate fluorescence in these transgenics could reflect ER dysfunction both in the failure to properly fold EGFP, the reduction in protein translation that is a part of the UPR and an impairment of the hepatocyte secretory capacity. The mechanism by which ER stress causes steatosis has not yet been clearly defined. Zebrafish present an ideal system to identify the mechanistic links between ER stress and fatty liver disease.28,29,43 Given their rapid development and ease of manipulation, zebrafish larvae can be readily used to assess the requirement of various genes involved in the UPR in the development of fatty liver disease, and to screen for drugs that may alleviate ER stress.
Bile canaliculi are formed between hepatocytes at their apical membranes and the biliary tree. If hepatocytes become injured, bile secretion may become impaired, leading to cholestasis. We determined that bile canaliculi were abnormal and reduced in number after Tm and EtOH treatment (Fig. 3A) but impairment of bile secretion was not detected (Fig. 3B). Therefore, Tm and EtOH do not cause cholestatic liver disease in zebrafish despite their effects on canaliculi. This may be attributed to the unique structure of the biliary tree in fishes: where canaliculi are much shorter than in humans and merge into bile preductules, characterized by the presence of bile preductular epithelial cells (BPDECs).80–82 Electron microscopy revealed that BPDECs appear normal after EtOH treatment (not shown). Therefore, bile flow may be normal since bile preductules, not canaliculi, are the main bile conduit in the fish liver.
Activation of HSCs in response to hepatocyte injury leads to the development of liver fibrosis, which can progress to cirrhosis. We have shown that HSCs are activated in both alcohol27 and Tm induced fatty liver disease in zebrafish (Fig. 4). This is an important distinction from rodents, in which fibrosis only occurs in fatty liver disease models that are administered an additional injury83–85 or lengthy treatment.86,87 Further, these findings highlight the value of the zebrafish model for studying HSC biology as there is a robust response after Tm and EtOH administration.
Liver injury from alcohol abuse is exacerbated when an underlying disease exists, such as obesity-associated NAFLD.6,11 This suggests that the presence of lipids may enhance liver injury from alcohol abuse. We did not observe any improvement in liver function or biliary structure in larvae in which Srebp activation, and therefore, presumably, de novo lipogenesis in hepatocytes, is blocked (Fig. 5). This suggests that liver injury from EtOH treatment is not secondary to hepatic lipid accumulation. Given that redox signaling was disturbed in both wild-type and Srebp deficient mbtps1 mutants, one can postulate that oxidative stress is a mechanism by which liver injury occurs in ALD and indeed this has been widely studied.88 Examining the contribution of oxidative stress to ALD in zebrafish is an important goal and a focus of our ongoing work.
While we demonstrate hepatic and biliary disturbances and dysfunction through a variety of means in this report, we do not know the status of the inflammatory system in zebrafish larvae exposed to Tm or EtOH. While no significant infiltration of leukocytes into the hepatic parenchyma is noted by H&E staining (Fig. 2) the expression of inflammatory cytokines, such as tumor necrosis factor alpha, which is induced in a genetic model of fatty liver disease,25 has yet to be determined in these models. Furthermore, given that there is not 100% penetrance of steatosis in Tm and EtOH-treated zebrafish, it is possible that the alterations in gene expression by qPCR may be masked due to the presence of RNA from nonsteatotic livers. This may also be the case for treated and untreated scap morphants; knockdown of scap does not completely rescue alcoholic steatosis. Therefore, it is possible that reduction of steatosis may have a larger effect on the acute phase response, hepatic function, or oxidative stress responses than we are able to detect. Our current method for detecting steatosis (i.e., oil red O staining) is applied to fixed fish and therefore, this approach is not amenable to selecting larvae with the most hepatic lipid before assessing hepatic function. However, use of the recently developed in vivo lipid imaging approaches89 may allow such selection to allow a more accurate assessment of the impact of steatosis on zebrafish larval liver function.
The incidence of liver disease is expected to rise in the United States due to increases in obesity and metabolic syndrome.2 Elucidating genetic pathways underlying the development of liver disease is crucial to generating pharmacological therapies to combat this growing problem. In this report, we describe multiple parameters that measure hepatic function in zebrafish. These assays can be applied by most zebrafish researchers and can thus, expand the use of this model for studying liver disease pathophysiology and for screening potential therapies to combat liver injury. Further, our findings demonstrate the underlying differences in injury in fatty liver disease models, and can be instructive in understanding the development of liver disease from different sources.
Supplementary Material
Acknowledgments
We thank Alex Mir and Evan Closser for fish care and maintenance, and Didier Stainier for critical reading of the manuscript. CY thanks Didier Stainier for valuable support during the conduct of this work. Funding was generously provided by the NIH (1F32AA021024-01 and 5T32CA078207 to DLH, K99AA020514 to CY, P20AA017067 to KCS/SL Friedman and 5R01AA018886-02 to KCS), and the UCSF Liver Center Pilot/Feasibility Award (NIH P30DK026743) to CY. Confocal laser scanning microscopy performed at the MSSM Microscopy Shared Resource Facility was supported by the NIH (5R24 CA095823-04, 1 S10 RR0 9145-01) and the NSF (DBI-9724504).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lazo M. Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Lazo M. Hernaez R. Bonekamp S. Kamel IR. Brancati FL. Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levene AP. Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- 4.Romeo S. Kozlitina J. Xing C. Pertsemlidis A. Cox D. Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC. Horton JD. Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Shea RS. Dasarathy S. McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 7.Breitkopf K. Nagy LE. Beier JI. Mueller S. Weng H. Dooley S. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol Clin Exp Res. 2009;33:1647–1655. doi: 10.1111/j.1530-0277.2009.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantena SK. King AL. Andringa KK. Eccleston HB. Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med. 2008;44:1259–1272. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsano LS. Mendez C. Hill D. Barve S. McClain CJ. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27:247–256. [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough AJ. O'Shea RS. Dasarathy S. Diagnosis and management of alcoholic liver disease. J Dig Dis. 2011;12:257–262. doi: 10.1111/j.1751-2980.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- 11.Naveau S. Giraud V. Borotto E. Aubert A. Capron F. Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 12.Chiba M. Sasaki M. Kitamura S. Ikeda H. Sato Y. Nakanuma Y. Participation of bile ductular cells in the pathological progression of non-alcoholic fatty liver disease. J Clin Pathol. 2011;64:564–570. doi: 10.1136/jcp.2011.090175. [DOI] [PubMed] [Google Scholar]
- 13.Farrell GC. Teoh NC. McCuskey RS. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 14.McCuskey RS. Ito Y. Robertson GR. McCuskey MK. Perry M. Farrell GC. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. doi: 10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 15.Ray MB. Mendenhall CL. French SW. Gartside PS. Bile duct changes in alcoholic liver disease. The Veterans Administration Cooperative Study Group. Liver. 1993;13:36–45. doi: 10.1111/j.1600-0676.1993.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 16.Atzori L. Poli G. Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol. 2009;41:1639–1642. doi: 10.1016/j.biocel.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Gea V. Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 19.Schattenberg JM. Galle PR. Animal models of non-alcoholic steatohepatitis: of mice and man. Dig Dis. 2010;28:247–254. doi: 10.1159/000282097. [DOI] [PubMed] [Google Scholar]
- 20.Li Z. Yang S. Lin H. Huang J. Watkins PA. Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 21.Yang SQ. Lin HZ. Lane MD. Clemens M. Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadler KC. Amsterdam A. Soroka C. Boyer J. Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–3572. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 23.North TE. Babu IR. Vedder LM. Lord AM. Wishnok JS. Tannenbaum SR, et al. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci U S A. 2010;107:17315–17320. doi: 10.1073/pnas.1008209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu J. Sadler KC. New school in liver development: lessons from zebrafish. Hepatology. 2009;50:1656–1663. doi: 10.1002/hep.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews RP. Lorent K. Manoral-Mobias R. Huang Y. Gong W. Murray IV, et al. TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development. 2009;136:865–875. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci. 2012;69:3953–3961. doi: 10.1007/s00018-012-1037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin C. Evason KJ. Maher JJ. Stainier DY. The bHLH transcription factor Hand2 marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology. 2012 doi: 10.1002/hep.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Passeri MJ. Cinaroglu A. Gao C. Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howarth DL. Passeri M. Sadler KC. Drinks like a fish: using zebrafish to understand alcoholic liver disease. Alcohol Clin Exp Res. 2011;35:826–829. doi: 10.1111/j.1530-0277.2010.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howarth DL. Vacaru AM. Tsedensodnom O. Mormone E. Nieto N. Costantini LM, et al. Alcohol disrupts endoplasmic reticulum function and protein secretion in hepatocytes. Alcohol Clin Exp Res. 2012;36:14–23. doi: 10.1111/j.1530-0277.2011.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J. Farage E. Sugimoto M. Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol. 2010;10:76. doi: 10.1186/1471-213X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabaliauskas NA. Foutz CA. Mest JR. Budgeon LR. Sidor AT. Gershenson JA, et al. High-throughput zebrafish histology. Methods. 2006;39:246–254. doi: 10.1016/j.ymeth.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi TF. Sadler KC. Crosnier C. Stainier DY. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol. 2008;18:1565–1571. doi: 10.1016/j.cub.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng PP. Severijnen LA. van der Weiden M. Willemsen R. Kros JM. A crucial role of caldesmon in vascular development in vivo. Cardiovasc Res. 2009;81:362–369. doi: 10.1093/cvr/cvn294. [DOI] [PubMed] [Google Scholar]
- 35.Field HA. Ober EA. Roeser T. Stainier DY. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol. 2003;253:279–290. doi: 10.1016/s0012-1606(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 36.You M. Crabb DW. Molecular mechanisms of alcoholic fatty liver: role of sterol regulatory element-binding proteins. Alcohol. 2004;34:39–43. doi: 10.1016/j.alcohol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 37.You M. Fischer M. Deeg MA. Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–29347. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 38.Bassik MC. Kampmann M. Knocking out the door to tunicamycin entry. Proc Natl Acad Sci U S A. 2011;108:11731–11732. doi: 10.1073/pnas.1109035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heifetz A. Keenan RW. Elbein AD. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979;18:2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- 40.Wu J. Rutkowski DT. Dubois M. Swathirajan J. Saunders T. Wang J, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto K. Takahara K. Oyadomari S. Okada T. Sato T. Harada A, et al. Induction of liver steatosis and lipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JS. Zheng Z. Mendez R. Ha SW. Xie Y. Zhang K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett. 2012;211:29–38. doi: 10.1016/j.toxlet.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cinaroglu A. Gao C. Imrie D. Sadler KC. Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology. 2011;54:495–508. doi: 10.1002/hep.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakur PC. Stuckenholz C. Rivera MR. Davison JM. Yao JK. Amsterdam A, et al. Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish. Hepatology. 2011;54:452–462. doi: 10.1002/hep.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong PD. Munson CA. Norton W. Crosnier C. Pan X. Gong Z, et al. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- 46.Fowler SD. Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33:833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 47.Baraona E. Lieber CS. Effects of alcohol on hepatic transport of proteins. Annu Rev Med. 1982;33:281–292. doi: 10.1146/annurev.me.33.020182.001433. [DOI] [PubMed] [Google Scholar]
- 48.Krahenbuhl L. Lang C. Ludes S. Seiler C. Schafer M. Zimmermann A, et al. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 2003;23:101–109. doi: 10.1034/j.1600-0676.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 49.Van Horn CG. Ivester P. Cunningham CC. Chronic ethanol consumption and liver glycogen synthesis. Arch Biochem Biophys. 2001;392:145–152. doi: 10.1006/abbi.2001.2433. [DOI] [PubMed] [Google Scholar]
- 50.Koorts AM. Viljoen M. Ferritin and ferritin isoforms II: protection against uncontrolled cellular proliferation, oxidative damage and inflammatory processes. Arch Physiol Biochem. 2007;113:55–64. doi: 10.1080/13813450701422575. [DOI] [PubMed] [Google Scholar]
- 51.Koorts AM. Viljoen M. Ferritin and ferritin isoforms I: Structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem. 2007;113:30–54. doi: 10.1080/13813450701318583. [DOI] [PubMed] [Google Scholar]
- 52.Lawson ND. Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 53.Lam P. Soroka CJ. Boyer JL. The bile salt export pump: clinical and experimental aspects of genetic and acquired cholestatic liver disease. Semin Liver Dis. 2010;30:125–133. doi: 10.1055/s-0030-1253222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji C. Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 55.Ji C. Mehrian-Shai R. Chan C. Hsu YH. Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kammoun HL. Chabanon H. Hainault I. Luquet S. Magnan C. Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutkowski DT. Wu J. Back SH. Callaghan MU. Ferris SP. Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroder M. Sutcliffe L. Consequences of stress in the secretory pathway: the ER stress response and its role in the metabolic syndrome. Methods Mol Biol. 2010;648:43–62. doi: 10.1007/978-1-60761-756-3_3. [DOI] [PubMed] [Google Scholar]
- 59.Ye R. Jung DY. Jun JY. Li J. Luo S. Ko HJ, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hannuksela ML. Liisanantti MK. Nissinen AE. Savolainen MJ. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953–961. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- 61.Noel ES. Reis MD. Arain Z. Ober EA. Analysis of the Albumin/alpha-Fetoprotein/Afamin/Group specific component gene family in the context of zebrafish liver differentiation. Gene Expr Patterns. 2010;10:237–243. doi: 10.1016/j.gep.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Chi NC. Shaw RM. De Val S. Kang G. Jan LY. Black BL, et al. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes Dev. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baroni GS. Marucci L. Benedetti A. Mancini R. Jezequel AM. Orlandi F. Chronic ethanol feeding increases apoptosis and cell proliferation in rat liver. J Hepatol. 1994;20:508–513. doi: 10.1016/s0168-8278(05)80498-2. [DOI] [PubMed] [Google Scholar]
- 64.Benedetti A. Brunelli E. Risicato R. Cilluffo T. Jezequel AM. Orlandi F. Subcellular changes and apoptosis induced by ethanol in rat liver. J Hepatol. 1988;6:137–143. doi: 10.1016/s0168-8278(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 65.Feldstein AE. Canbay A. Angulo P. Taniai M. Burgart LJ. Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 66.Bernstein H. Payne CM. Bernstein C. Schneider J. Beard SE. Crowley CL. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol Lett. 1999;108:37–46. doi: 10.1016/s0378-4274(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 67.Bochkis IM. Rubins NE. White P. Furth EE. Friedman JR. Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malhi H. Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tung BY. Carithers RL., Jr. Cholestasis and alcoholic liver disease. Clin Liver Dis. 1999;3:585–601. doi: 10.1016/s1089-3261(05)70086-6. [DOI] [PubMed] [Google Scholar]
- 70.McGill DB. Steatosis, cholestasis, and alkaline phosphatase in alcoholic liver disease. Am J Dig Dis. 1978;23:1057–1060. doi: 10.1007/BF01072878. [DOI] [PubMed] [Google Scholar]
- 71.Afshani P. Littenberg GD. Wollman J. Kaplowitz N. Significance of microscopic cholangitis in alcoholic liver disease. Gastroenterology. 1978;75:1045–1050. [PubMed] [Google Scholar]
- 72.Sorrentino P. Tarantino G. Perrella A. Micheli P. Perrella O. Conca P. A clinical-morphological study on cholestatic presentation of nonalcoholic fatty liver disease. Dig Dis Sci. 2005;50:1130–1135. doi: 10.1007/s10620-005-2719-1. [DOI] [PubMed] [Google Scholar]
- 73.Hampton JA. Lantz RC. Goldblatt PJ. Lauren DJ. Hinton DE. Functional units in rainbow trout (Salmo gairdneri, Richardson) liver: II. The biliary system. Anat Rec. 1988;221:619–634. doi: 10.1002/ar.1092210208. [DOI] [PubMed] [Google Scholar]
- 74.Hinton DE. Pool CR. Ultrastructure of the liver in channel catfish Ictalurus punctatus (Rafinesque) J Fish Biol. 1976;8:209–219. [Google Scholar]
- 75.Hama K. Provost E. Baranowski TC. Rubinstein AL. Anderson JL. Leach SD, et al. In vivo imaging of zebrafish digestive organ function using multiple quenched fluorescent reporters. Am J Physiol Gastrointest Liver Physiol. 2009;296:G445–G453. doi: 10.1152/ajpgi.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. doi: 10.1053/j.gastro.2012.02.003. e6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang K. Shen X. Wu J. Sakaki K. Saunders T. Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 78.Soon RK., Jr. Yan JS. Grenert JP. Maher JJ. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology. 2010;139:1730–1739. doi: 10.1053/j.gastro.2010.07.046. 9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aronson DE. Costantini LM. Snapp EL. Superfolder GFP is fluorescent in oxidizing environments when targeted via the Sec translocon. Traffic. 2011;12:543–548. doi: 10.1111/j.1600-0854.2011.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blair JB. Ostrander GK. Miller MR. Hinton DE. Isolation and characterization of biliary epithelial cells from rainbow trout liver. In Vitro Cell Dev Biol Anim. 1995;31:780–789. doi: 10.1007/BF02634120. [DOI] [PubMed] [Google Scholar]
- 81.Hinton DE. Couch JA. Architectural pattern, tissue and cellular morphology in livers of fishes: relationship to experimentally-induced neoplastic responses. EXS. 1998;86:141–164. doi: 10.1007/978-3-0348-8853-0_4. [DOI] [PubMed] [Google Scholar]
- 82.Okihiro MS. Hinton DE. Partial hepatectomy and bile duct ligation in rainbow trout (Oncorhynchus mykiss): histologic, immunohistochemical and enzyme histochemical characterization of hepatic regeneration and biliary hyperplasia. Toxicol Pathol. 2000;28:342–356. doi: 10.1177/019262330002800215. [DOI] [PubMed] [Google Scholar]
- 83.Koteish A. Yang S. Lin H. Huang X. Diehl AM. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 2002;277:13037–13044. doi: 10.1074/jbc.M101632200. [DOI] [PubMed] [Google Scholar]
- 84.Schaffert CS. Duryee MJ. Hunter CD. Hamilton BC., 3rd DeVeney AL. Huerter MM, et al. Alcohol metabolites and lipopolysaccharide: roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. 2009;15:1209–1218. doi: 10.3748/wjg.15.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von Montfort C. Beier JI. Guo L. Kaiser JP. Arteel GE. Contribution of the sympathetic hormone epinephrine to the sensitizing effect of ethanol on LPS-induced liver damage in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1227–G1234. doi: 10.1152/ajpgi.00050.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rinella ME. Elias MS. Smolak RR. Fu T. Borensztajn J. Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–1076. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rinella ME. Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Cederbaum AI. Lu Y. Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 89.Carten JD. Bradford MK. Farber SA. Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish. Dev Biol. 2011;360:276–285. doi: 10.1016/j.ydbio.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.