Abstract
The emergence of extreme-drug-resistant (EDR) bacterial strains in hospital and nonhospital clinical settings is a big and growing public health threat. Understanding the antibiotic resistance mechanisms at the genomic levels can facilitate the development of next-generation agents. Here, comparative genomics has been employed to analyze the rapid evolution of an EDR Acinetobacter baumannii clone from the intensive care unit (ICU) of Rigshospitalet at Copenhagen. Two resistant A. baumannii strains, 48055 and 53264, were sequentially isolated from two individuals who had been admitted to ICU within a 1-month interval. Multilocus sequence typing indicates that these two isolates belonged to ST208. The A. baumannii 53264 strain gained colistin resistance compared with the 48055 strain and became an EDR strain. Genome sequencing indicates that A. baumannii 53264 and 48055 have almost identical genomes—61 single-nucleotide polymorphisms (SNPs) were found between them. The A. baumannii 53264 strain was assembled into 130 contigs, with a total length of 3,976,592 bp with 38.93% GC content. The A. baumannii 48055 strain was assembled into 135 contigs, with a total length of 4,049,562 bp with 39.00% GC content. Genome comparisons showed that this A. baumannii clone is classified as an International clone II strain and has 94% synteny with the A. baumannii ACICU strain. The ResFinder server identified a total of 14 antibiotic resistance genes in the A. baumannii clone. Proteomic analyses revealed that a putative porin protein was down-regulated when A. baumannii 53264 was exposed to antimicrobials, which may reduce the entry of antibiotics into the bacterial cell.
Keywords: Acinetobacter baumannii, antibiotic resistance, comparative genomics, single-nucleotide polymorphism
Introduction
Multidrug-resistant bacterial strains have emerged as the causes of nosocomial infections worldwide (Nikaido 2009). Recently, pandrug-resistant (PDR) bacterial strains, which are resistant to all antimicrobial agents except tigecycline and the polymyxins, and extreme-drug-resistant (EDR) bacterial strains, which are resistant to all antimicrobial agents, were isolated from hospital-acquired infections (Paterson and Doi 2007). There is a huge risk of these “superbugs” extending into the community and threatening public health.
The Gram-negative, nonmotile, aerobic bacterium Acinetobacter baumannii is an example of a fast-evolving organism, which causes healthcare-associated infections (Garnacho-Montero and Amaya-Villar 2010). Acinetobacter baumannii was sensitive to most antibiotics in the 1970s, but now it is resistant to virtually all antibacterial drugs (Howard et al. 2012). Acinetobacter baumannii is responsible for approximately 2–10% of all Gram-negative infections in intensive care units (ICUs) and significantly increased mortality of infected patients (Poirel et al. 2003; Lockhart et al. 2007). Ribotyping and amplified fragment length polymorphism genomic fingerprinting approaches have identified three international groups of epidemic A. baumannii strains: clone I, clone II, and clone III (Dijkshoorn et al. 1996; van Dessel et al. 2004). However, the fingerprinting-based methods can only provide very limited phylogenetic information, and their results cannot identify genetic distinctness within the same clones and among different clones. Thus, whole-genome sequences are required for thorough epidemiological analysis and antibiotic resistance profiling of A. baumannii.
In this study, we have sequenced a colistin-sensitive PDR A. baumannii 48055 strain and an EDR A. baumannii 53264 strain from the ICU of Rigshospitalet in Copenhagen, Denmark. Both these strains have similar antimicrobial resistant profiles and were sequentially isolated from two individuals who had been admitted to ICU, within a 1-month interval. Through comparative genomic analysis, we identified the origin and phylogeny of this A. baumannii strains. We also profiled the drug resistance mechanisms present in the genomes of these A. baumannii strains. We also analyzed the single-nucleotide polymorphisms (SNPs) between the A. baumannii 53264 and 48055 strains to shed light on the mechanisms for the evolution of a PDR strain into an EDR one. Furthermore, we have employed 4-Plex isobaric tag for relative and absolute quantification (iTRAQ)-based quantitative proteomic analysis to investigate the resistance mechanisms of A. baumannii 53264 strain toward three different classes of antibiotics: tobramycin, colistin, and ceftazidime.
Materials and Methods
Bacterial Strains and Patient Background Information
The A. baumannii 48055 strain was isolated from a patient with 50% burn trauma, in the ICU of Rigshospitalet, Copenhagen, Denmark, on September 2010. This patient received antibiotic treatment with meropenem (2 g intravenously [i.v.], three times daily), ciprofloxacin (600 mg i.v., twice daily), and fucidin (500 mg i.v., three times daily) to prevent Gram-negative bacterial infections and treat Staphylococcus aureus bacteremia. After A. baumannii was found in the blood and airway, the treatment was changed to meropenem and colistin (6 million units i.v., once daily) and fucidin (500 mg i.v., three times daily).The treatment was later changed to meropenem (2 g i.v., three times daily), colistin (2 million units, three times daily), and vancomycin (1 g i.v., twice daily). After several operations to remove the necrosed tissues and skin transplantation, the patient recovered and was sent home.
A month later, the A. baumannii 53264 strain was isolated from a patient with 50% burn trauma, in the ICU of Rigshospitalet, Copenhagen, Denmark, on October 2010. This patient received antibiotic treatment with meropenem (1 g i.v., three times daily) and ciprofloxacin (400 mg i.v., twice daily) in the beginning, then with fucidin (500 mg orally, three times daily) due to S. epidermidis bacteremia. The treatment was changed to ceftazidime (1 g i.v., three times daily) with ciprofloxacin (400 mg i.v., twice daily) and vancomycin (1 g i.v., twice daily) to prevent Gram-negative bacterial infection and treat S. haemolyticus bacteremia. After A. baumannii was found in the blood and airway, the treatment was changed to meropenem (1 g i.v., three times daily), ciprofloxacin (400 mg i.v., twice daily), colistin (2 million units i.v., three times daily), and colistin inhalation (2 million units, twice daily). After several operations to remove the necrosed skin tissues and skin transplantation, the patient recovered very well and was sent back to the local hospital to continue treatment until complete recovery.
Antimicrobial Susceptibility Assay
Susceptibility of the two A. baumannii strains to 18 antimicrobial agents was tested by disc diffusion following the Clinical and Laboratory Standards Institute (CLSI) recommendations using the blood agar plate produced by Statens Serum Institut, Denmark. The A. baumannii 53264 and 48055 strains were resistant to all our tested antibiotics including ampicillin, aztreonam, ceftazidime, ceftriaxone, cefuroxime, chloramphenicol, ciprofloxacin, colistin, gentamicin, imipenem, mecillinam, meropenem, penicillin, piperacillin/tazocin, sulfonamide, tigecycline, tobramycin, and trimethoprim.
To confirm this result, the minimum inhibitory concentration (MIC) was determined by microdilution for the A. baumannii 53264 strain, A. baumannii 48055 strain, and an antibiotic-sensitive A. baumannii strain 52082 to the following six representative antibiotics: tobramycin (aminoglycoside), colistin (antimicrobial peptide), ceftazidime (cephalosporin), tetracycline, ciprofloxacin (fluoroquinolone), and meropenem (carbapenem). Diluted overnight cultures of bacteria were seeded into wells containing serially diluted antibiotic stocks, from concentrations ranging from 0 to 1,024 µg/ml.
Genome Sequencing and Assembly
Whole-genome DNA of the A. baumannii strains were purified using Wizard genomic DNA purification kit (Promega) and sequenced by the Beijing Genomics Institute on an Illumina Hiseq2000 platform generating 90 bp long paired-end reads. Reads were mapped against the genome of A. baumannii ACICU (Genbank accession number CP000863) using Novoalign (Novocraft Technologies) (Krawitz et al. 2010). The best assembly result was then assembled by SOAPdenovo (http://soap.genomics.org.cn/; version 1.05) with filtered data. Average insert sizes were 468 nucleotides, and the average genomic coverage depths were 112–116-fold. Pileups of the read alignments were produced by SAMtools release 0.1.7 (Li et al. 2009).
Genome Comparison
The A. baumannii 53264 and A. baumannii 48055 genomes were first compared with the genomes of 11 A. baumannii strains available from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. A pair-wise genome content distance matrix was computed using Progressive Mauve (Darling et al. 2010), followed by whole-genome alignment. The distance matrix was converted to a heat map using the R heatmap function clustering package (http://www.r-project.org/). Progressive Mauve was then used to compare the genomes of these two strains with the genome sequences of 42 other A. baumannii strains (their genome sequences were downloaded from National Center for Biotechnology Information [NCBI] FTP site). First, the Mauve software computed a genome content distance matrix for all 44 A. baumannii strains, after which a neighbor-joining algorithm was used to produce a phylogenetic guide tree. Phylogenetic tree diagrams were prepared using the software FigTree ver 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Genome Annotation
For genome annotation, the A. baumannii 53264 and A. baumannii 48055 sequence files were submitted to the Rapid Annotations using Subsystem Technology (RAST) Server (Aziz et al. 2008) for bacterial genome annotation. Default settings were used. The RAST-annotated A. baumannii 53264 and A. baumannii 48055 genomes are accessible from the RAST server by logging in with the guest account (userID: guest, password: guest) at the web addresses: http://rast.nmpdr.org/seedviewer.cgi?page=Organism&organism=470.131 (last accessed April 16, 2013) and http://rast.nmpdr.org/seedviewer.cgi?page=Organism&organism=470.130 (last accessed April 16, 2013), respectively.
SNP Comparison and Analysis
The identification of SNPs between the A. baumannii 53264 strain, the A. baumannii 48055 strain, and the annotated A. baumannii ACICU strain (NC_010611) was performed using DNASTAR SeqManNGen and analyzed using DNASTAR SeqMan Pro software version 10.1.1 (DNASTAR, Inc., Madison, WI). Paired-end reads in FASTQ format were mapped against the respective annotated Genbank template file.
Prediction of Antibiotic Resistance Genes
The A. baumannii 53264 and A. baumannii 48055 sequences were also submitted to the Antibiotic Resistance Genes Database (ARDB; Liu and Pop 2009) and the recently described ResFinder database (Zankari et al. 2012), with a 98% threshold for identification of genes involved in antibiotic resistance.
Nucleotide Sequence Accession Numbers
The Whole Genome Shotgun bioproject for A. baumannii 53264 has been deposited at DDBJ/EMBL/GenBank under the accession ALPW00000000. The version described in this article is the first version, ALPW01000000. The Whole Genome Shotgun bioproject for A. baumannii 48055 has been deposited at DDBJ/EMBL/GenBank under the accession AOSP00000000. The version described in this article is the first version, AOSP01000000.
iTRAQ-Based Proteomics Analysis
iTRAQ-based proteomic analysis was used to study the changes in protein expression of the A. baumannii 53264 strain in response to three antibiotics: colistin, tobramycin, and ceftazidime. Proteomics experiments were performed at the Proteomic Core Facility of the Biological Research Center, School of Biological Sciences, Nanyang Technological University, Singapore. A full description of the materials and methods is included as supplementary material, Supplementary Material online.
Results
General Characteristics of A. baumannii 53264 and A. baumannii 48055 Genomes
The clinical isolates A. baumannii 53264 and A. baumannii 48055 were isolated from the ICU of Rigshospitalet at Copenhagen, Denmark. Acinetobacter baumannii 53264 exhibited high resistance to all the 18 tested antibiotics, including tigecycline and the polymyxin, colistin; thus, A. baumannii 53264 is classified as an EDR strain. In comparison, A. baumannii 48055 is a colistin-sensitive PDR strain that was isolated 1 month before A. baumannii 53264. Both A. baumannii strains belong to multilocus sequence type ST208, a molecular type previously reported in European clone II (Runnegar et al. 2010).
The general characteristics of the A. baumannii 53264 and A. baumannii 48055 strains obtained from the RAST server (Aziz et al. 2008) are presented in table 1. For A. baumannii 53264, we obtained 130 contigs with a total length of 3,976,592 bp and 3,791 predicted coding sequences. For A. baumannii 48055, we obtained 135 contigs with a total length of 4,049,562 bp and 3,858 predicted coding sequences. The average GC% of A. baumannii 53264 and A. baumannii 48055 is 38.93 and 39.00, respectively.
Table 1.
General Characteristics of the Acinetobacter baumannii 53264 and A. baumannii 48055 Genomes as Obtained from the RAST Annotation Server (Aziz et al. 2008)
| Characteristic | Value |
|
|---|---|---|
| A. baumannii 53264 | A. baumannii 48055 | |
| Genome | ||
| Size (bp) | 3,976,592 | 4,049,562 |
| No. of contigs | 130 | 135 |
| G + C content (%) | 38.93 | 39.00 |
| No. of coding sequences | 3,791 | 3,858 |
| No. of subsystems | 440 | 440 |
| No. of RNAs | 62 | 63 |
Acinetobacter Synteny and Phylogeny
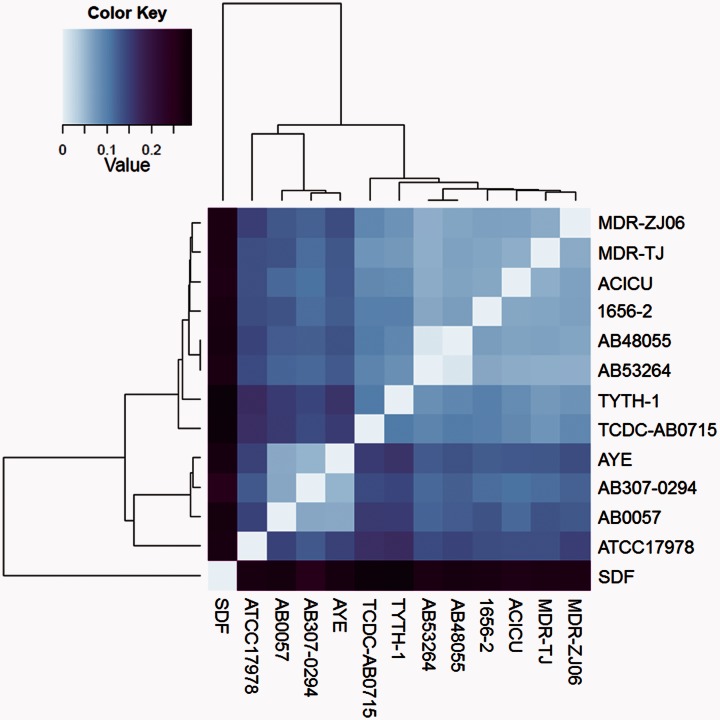
The A. baumannii 53264 and A. baumannii 48055 genomes were first compared with the genomes of 11 A. baumannii strains available from the KEGG database. A pair-wise genome content distance matrix was computed using Progressive Mauve (Darling et al. 2010), followed by whole-genome alignment. The distance matrix was converted to a heat map (fig. 1) using the R statistical package and revealed that the A. baumannii 53264 and A. baumannii 48055 genomes had the greatest amount of similarity to each other. The average nucleotide identity between these two A. baumannii strains and another multidrug resistant strain A. baumannii ACICU (Iacono et al. 2008) from European clone II is 94% as determined by Progressive Mauve (Darling et al. 2010).
Fig. 1.—
Heat map based on a pair-wise distance matrix of whole-genome alignment as computed by Progressive Mauve. Pair-wise genome alignments were performed using the genomes of Acinetobacter baumannii 53264, A. baumannii 48055, and 11 A. baumannii clones whose complete sequences were available in the KEGG database. This heat map was created using the R statistical program (http://www.r-project.org/) with heatmap clustering methods. Dendrograms across the top and left of the diagram indicate the relatedness of the genomes based on genome conservation, while strain names are listed to the right of the heatmap. Distance values range from 0.0 to 0.3 and correspond to a gradient of color steps ranging from light blue (lowest distance value) to dark purple (highest distance value).
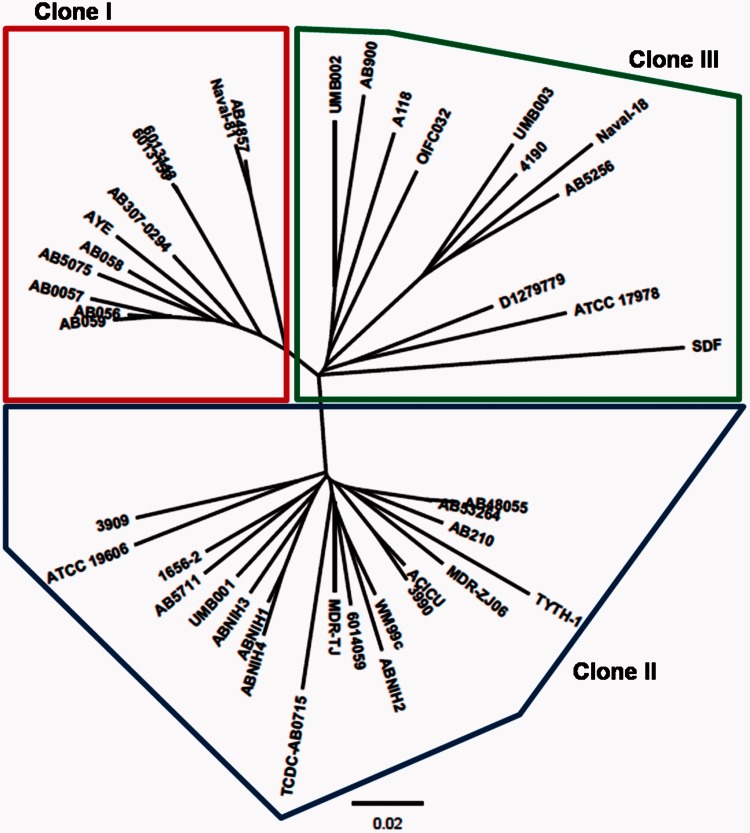
To find out which clonal group the A. baumannii 53264 and A. baumannii 48055 genomes belonged to, Progressive Mauve was then used to compare the genomes of these two strains with the genome sequences of 42 other A. baumannii strains (their genome sequences were downloaded from NCBI FTP site). The phylogenetic tree based on the neighbor-joining algorithm shows that the A. baumannii 53264 and A. baumannii 48055 strains belong to the group of International Clone II A. baumannii strains (fig. 2).
Fig. 2.—
An unrooted phylogenetic tree showing the Acinetobacter baumannii 53264 and A. baumannii 48055 strains in relation to 42 other A. baumannii strains. The clonal groups are as follows: International Clone I (red box), Clone II (blue box), and Clone III (green box). Genome sequences of these 42 sequences were downloaded from NCBI FTP site. This phylogenetic tree was produced by pair-wise genome comparisons by Progressive Mauve. The A. baumannii 53264 and A. baumannii 48055 strains belong to the group of International Clone II A. baumannii strains.
Antibiotic Resistance Profile of the A. baumannii 53264 and 48055 Strains
Using disc diffusion antimicrobial susceptibility testing, the A. baumannii 53264 and 48055 strains were resistant to all 18 tested antibiotics, except for A. baumannii 48055’s sensitivity to colistin. To confirm this result, the minimum inhibitory concentration was determined for A. baumannii 53264, A. baumannii 48055, and an antibiotic-sensitive A. baumannii 52082 strain to the following six representative antibiotics: tobramycin (aminoglycoside), colistin (antimicrobial peptide), ceftazidime (cephalosporin), tetracycline, ciprofloxacin (fluoroquinolone), and meropenem (carbapenem); the antibiogram is presented in table 2. The A. baumannii 52082 strain was sensitive to all six antibiotics (MIC: 1–2 µg/ml). The MIC profiles of the A. baumannii 53264 and 48055 strains were similar, differing only in their sensitivity to colistin (MIC: 128 vs. 2 µg/ml).
Table 2.
Minimum Inhibitory Concentrations (µg/ml) for the EDR Acinetobacter baumannii 53264 and PDR A. baumannii 48055 Strains, and the Antibiotic-Sensitive A. baumannii 52082 Strain toward the Following Six Antibiotics: Tobramycin, Colistin, Ceftazidime, Tetracycline, Ciprofloxacin, and Meropenem
| Antibiotic Tested | Minimum Inhibitory Concentrations (µg/ml) |
||
|---|---|---|---|
| A. baumannii Strain 53264 (EDR) | A. baumannii Strain 48055 (PDR) | A. baumannii Strain 52082 (Sensitive) | |
| Tobramycin | 256 | 256 | 2 |
| Colistin | 128 | 2 | 2 |
| Ceftazidime | 32 | 64 | 2 |
| Tetracycline | >1,024 | >1,024 | 1 |
| Ciprofloxacin | 64 | 64 | 1 |
| Meropenem | 16 | 64 | 1 |
The A. baumannii 53264 and A. baumannii 48055 sequences were submitted to both the recently described ResFinder database (Zankari et al. 2012) and the older ARDB (Liu and Pop 2009) to identify genes involved in antibiotic resistance. Table 3 lists the genes involved in the resistance of these EDR A. baumannii strains to aminoglycosides, beta-lactams, sulphonamides, and tetracyclines.
Table 3.
Antibiotic Resistance Profiles of Acinetobacter baumannii 53264 and A. baumannii 48055 Strains
| Antibiotic Class | Resistance Genea | NCBI DNA Accession | Description of Gene | Description of Gene Productb | Resistance Conferredb | Source |
|---|---|---|---|---|---|---|
| Aminoglycosides | aac(3)-Ia | X15852 | Plasmid R1033 (Tn1696) aacC1 gene for gentamicin acetyltransferase-3-I (AAC(3)-I). | Aminoglycoside N-acetyltransferase, which modifies aminoglycosides by acetylation. | Astromicin Gentamicin Sisomicin | Plasmid R1033, from Pseudomonas aeruginosa |
| aac(6')-Iaf | AB462903 | P. aeruginosa DNA, class 1 integron In123, complete sequence. | Amikacin Dibekacin Isepamicin Netilmicin Sisomicin Tobramyci | P. aeruginosa | ||
| aac(6')-Il | Z54241 | Citrobacter freundii int and aac(6')-I genes. 6'-N-aminoglycoside acetyltransferase. | C. freundii | |||
| aph(3')-Ia | V00359 | Transposon Tn903. | Aminoglycoside O-phosphotransferase, which modifies aminoglycosides by phosphorylation. | Gentamicin Kanamycin Lividomycin Neomycin Paromomycin Ribostamycin | Escherichia coli | |
| aph(3')-Ic | X62115 | Klebsiella pneumoniae plasmid pBWH77 aphA7 gene for neomycin phosphotransferase. | K. pneumoniae | |||
| Aph(3')-VIa | X07753 | Acinetobacter baumanniiaph A-6 gene. | Amikacin Butirosin Gentamicin Isepamicin Kanamycin Neomycin Paromomycin Ribostamycin | A. baumannii | ||
| strA/aph(3'')-Ib | M96392 | Erwinia amylovora plasmid pEa34 transposon Tn5393 streptomycin phosphotransferase (strA) and streptomycin phosphotransferase (strB) genes. | Streptomycin | E. amylovora | ||
| strB/aph(6)-Id | ||||||
| Beta-lactam | blaOXA-23 | HQ700358 | A. baumannii isolate AB210 AbaR4-type multiple antibiotic resistance island, complete sequence. | Class D beta-lactamase. | Carbapenems | A. baumannii |
| blaTEM-1/RblaTEM-1 | AF188200 | E. coli beta-lactamase variant TEM-1D (blaTEM-1D) gene. | Class A beta-lactamase. This enzyme breaks the beta-lactam antibiotic ring open and deactivates the molecule's antibacterial properties. | Cephalosporin Penicillin | E. coli | |
| Fluoroquinolones | No resistance genes found. | |||||
| Fosfomycin | No resistance genes found. | |||||
| Fusidic Acid | No resistance genes found. | |||||
| MLS—Macrolide— Lincosamide— Streptogramin B | No resistance genes found. | |||||
| Phenicol | No resistance genes found. | |||||
| Rifampicin | No resistance genes found. | |||||
| Sulfonamide | sul1 | AY224185 | Escherichia coli isolate Ec1484R sulphonamide resistance protein (sulI) gene. | Sulfonamide-resistant dihydropteroate synthase, which cannot be inhibited by sulfonamide. | Sulfonamide | Escherichia coli |
| sul2 | FM179941 | Pasteurella multocida pCCK1900 plasmid, isolate 1900. | P. multocida | |||
| sul3 | AB281182 | P. aeruginosasul3 gene for dihydropteroatesynthetase. | P. aeruginosa | |||
| Tetracycline | tet(B) | AP000342 | Shigella flexneri 2b plasmid R100 DNA, complete sequence. | Major facilitator superfamily transporter, tetracycline efflux pump. | Tetracycline | Plasmid R100 from S. flexneri |
| Trimethoprim | No resistance genes found. | |||||
| Glycopeptide | No resistance genes found. | |||||
aResistance genes identified with greater than 98.00% sequence identity by ResFinder (Zankari et al. 2012).
bAntibiotic resistance conferred is based on ARDB (Liu and Pop 2009).
Antibiotic resistance genes present in the A. baumannii 53264 and 48055 genomes are similar to those found in a wide variety of other bacteria. Acinetobacter baumannii acquires its multiantibiotic resistance phenotype through the acquisition of mobile genetic elements, for example, plasmids and transposons (Fournier, Richet, et al. 2006). From table 3, we note that our A. baumannii strains possess the antibiotic resistance genes, aac(6')-Iaf and sul3, which are similar to genes present in Pseudomonas aeruginosa. Pseudomonas aeruginosa and A. baumannii are the two most prevalent nonfermentative bacteria isolated from hospital patients (Karlowsky et al. 2003) and can be assumed to be in close contact with each other, for example, in an infection site, thus allowing gene transfer. A striking example of this is transfer of an extended-spectrum β-lactamase integron (blaVEB-1) from P. aeruginosa to A. baumannii in a hospital setting (Poirel et al. 2003).
Regarding beta-lactam resistance genes, our EDR A. baumannii strains carry the blaOXA-23 and the blaTEM-1 gene. The blaOXA-23 gene that confers imipenem resistance was first observed in Scotland (Scaife et al. 1995) but was later observed even in China (Zhou et al. 2007), in Bulgaria (Stoeva et al. 2008), in Brazil (Carvalho et al. 2009), and eventually world-wide (Mugnier et al. 2010). The blaOXA-23 gene encodes a Class D beta-lactamase that confers resistance against carbapenems (e.g., imipenem and meropenem) and ceftazidime (Mugnier et al. 2010). The blaTEM-1 gene encodes a Class A beta-lactamase that is found in 90% of ampicillin-resistant Escherichia coli strains (Livermore 1995). Because the blaTEM-1 gene is plasmid borne and utilizes transposon-mediated transfer, this gene spreads easily among bacteria and has been observed in Enterobacteriaceae, P. aeruginosa, and Haemophilus influenza (Bradford 2001).
Comparison of SNPs across A. baumannii Strains
We then studied the nonsynonymous (coding change) SNPs between the A. baumannii ACICU strain (Iacono et al. 2008) and our EDR A. baumannii 53264 and PDR A. baumannii 48055 strains. Table 4 presents the SNPs in three particular genes: ampC, gyrB, and parC.
Table 4.
Nonsynonymous SNPs Observed in the ampC, gyrB, and parC Genes of A. baumannii 53264 and A. baumannii 48055 Strains in Reference to the A. baumannii ACICU Strain
| AB 53264 vs. ACICU |
AB 48055 vs. ACICU |
|||
|---|---|---|---|---|
| DNA Change | Protein Change | DNA Change | Protein Change | |
| SNPs in ampC gene | c.31->T | S11fs | ||
| c.217C>A | R73S | c.217C>A | R73S | |
| c.427C>A | Q143K | c.427C>A | Q143K | |
| c.827T>C | F276S | c.827T>C | F276S | |
| c.828->G | F276fs | c.828->G | F276fs | |
| c.911G>A | S304N | c.911G>A | S304N | |
| c.1001A>C | N334T | c.1001A>C | N334T | |
| c.1114G>A | D372N | c.1114G>A | D372N | |
| SNPs in gyrB gene | c.1738A>G | Y580H | c.1738A>G | Y580H |
| SNPs in parC gene | c.251C>T | S84L | c.251C>T | S84L |
| c.623G>A | G208E | c.623G>A | G208E | |
| c.1982T>C | V661A | c.1982T>C | V661A | |
Note.—fs, frameshift.
Overproduction of the AmpC cephalosporinase by A. baumannii isolates has been shown to be important in conferring high levels of resistance to beta-lactam antibiotics (i.e., ceftazidime) (Corvec et al. 2003). Point mutations within the ampC gene promoter and attenuator regions have been shown to result in the hyperproduction of AmpC in E. coli strains (Nelson and Elisha 1999). In our case, the SNPs detected were within the ampC gene coding sequence. Molecular evolution of beta-lactamases confers extended substrate specificity, thus improving bacterial inactivation of a wider range of antibiotics (Nukaga et al. 1995). In P. aeruginosa, point mutations within the ampC gene resulted in increased beta-lactamase activity of AmpC, which resulted in increased resistance to ceftazidime (Tam et al. 2007). The parC gene encodes subunit A of topoisomerase IV, whereas the gyrB gene encodes DNA gyrase B. These products are the target for inhibition by quinolone-based antibiotics, and mutations within the parC and gyrB genes confer resistance to quinolones (Yoshida et al. 1991; Vila et al. 1997; Eaves et al. 2004).
The SNPs detected in the two A. baumannii strains are identical, except for an additional thymine inserted at position 31 of the ampC gene of A. baumannii strain 53264. The A. baumannii 53264 strain was isolated about a month after the A. baumannii 48055 strain was isolated, which would explain the additional time for mutation. This also lends support to the fact that the EDR A. baumannii 53264 strain has rapidly evolved from the PDR A. baumannii 48055 strain in as short as a 1-month period.
We then analyzed the SNP differences between the EDR A. baumannii 53264 and PDR A. baumannii 48055 strain to find out the reasons behind the evolution of colistin resistance. There were 61 nonsynonymous SNPs detected between the two strains (table 5). From table 5, we noticed two SNPs within the histidine kinase sensor qseC gene; QseC is a highly conserved regulator of virulence that responds to both bacteria signals and host cell factors (Clarke et al. 2006). Inhibition of QseC markedly reduced the virulence of Salmonella enterica serovar Typhimurium (Rasko et al. 2008). Transcriptomic analysis revealed a role for QseC in the antimicrobial peptide (i.e., polymyxins) and oxidative stress resistance responses (Karavolos et al. 2008).
Table 5.
List of SNP Differences between the PDR A. baumannii 48055 and the EDR A. baumannii 53264 Strains
| No. | Feature Name | DNA Change | Protein Change |
|---|---|---|---|
| 1 | LSU ribosomal protein L34p | c.136A>- | 45fs |
| 2 | Biosynthetic aromatic amino acid | c.536G>T | P179H |
| 3 | Transcriptional regulator, LysR family | c.[847G>T]+[847G>G] | Q283K, Q283Q |
| 4 | FIG000988: Predicted permease | c.122T>G | V41G |
| 5 | FIG000906: Predicted permease | c.[866G>T]+[866G>G] | C289C, C289F |
| 6 | FIG000906: Predicted permease | c.[869C>T]+[869C>C] | S290S, S290F |
| 7 | FIG000906: Predicted permease | c.[874A>T]+[874A>A] | I292I, I292F |
| 8 | Acetoacetyl-CoA synthetase (EC | c.[1213C>C]+[1213C>A] | G405G, G405C |
| 9 | FIG00350520: hypothetical protein | c.266T>A | F89Y |
| 10 | FIG022199: FAD-binding protein | c.1206C>A | N402K |
| 11 | Transcriptional regulator, LysR family | c.762C>A | F254L |
| 12 | FIG00350303: hypothetical protein | c.[1502T>T]+[1502T>G] | V501G, V501V |
| 13 | l-carnitinedehydratase/bile acid-inducible | c.[530T>T]+[530T>G] | V177G, V177V |
| 14 | FIG00350277: hypothetical protein | c.2311A>T | F771I |
| 15 | CmaU | c.[27T>T]+[27T>G] | F9L, F9F |
| 16 | CmaU | c.32T>C | V11A |
| 17 | CmaU | c.34C>A | P12T |
| 18 | 4Fe-4S ferredoxin, iron-sulfur binding | c.62C>A | A21D |
| 19 | FIG00350535: hypothetical protein | c.[56T>T]+[56T>G] | V19G, V19V |
| 20 | Cell surface protein | c.[571A>G]+[571A>A] | T191T, T191A |
| 21 | FIG00352920: hypothetical protein | c.[163G>G]+[163G>A] | E55K, E55E |
| 22 | FIG00352920: hypothetical protein | c.[176T>T]+[176T>C] | V59A, V59V |
| 23 | FIG00351830: hypothetical protein | c.[278G>G]+[278G>C] | G93A, G93G |
| 24 | putative hemolysin | c.[50G>T]+[50G>G] | C17C, C17F |
| 25 | Phenylacetic acid degradation protein | c.1004T>G | N335T |
| 26 | Two-component hybrid sensor and regulator | c.1561C>T | H521Y |
| 27 | 5'-nucleotidase (EC 3.1.3.5) | c.[526A>T]+[526A>A] | F176I, F176F |
| 28 | Transcriptional regulator, AraC family | c.322A>C | F108V |
| 29 | Transcriptional regulator, AraC family | c.313A>C | F105V |
| 30 | Xylonatedehydratase (EC 4.2.1.82) | c.419G>C | G140A |
| 31 | Phenylalanine-specific permease | c.[203C>C]+[203C>A] | C68C, C68F |
| 32 | Alcohol dehydrogenase (EC 1.1.1.1) | c.440G>A | G147E |
| 33 | Alcohol dehydrogenase (EC 1.1.1.1) | c.443G>T | G148V |
| 34 | RNA polymerase sigma factor RpoH | c.107G>A | G36E |
| 35 | Sulfatepermease | c.[829A>C]+[829A>A] | C277G, C277C |
| 36 | FIG00351986: hypothetical protein | c.4G>T | P2T |
| 37 | FIG00349989: hypothetical protein | c.[10C>T]+[10C>C] | V4M, V4V |
| 38 | RarD protein | c.689T>G | E230A |
| 39 | RarD protein | c.677A>C | F226C |
| 40 | RarD protein | c.674A>C | V225G |
| 41 | Glycerate kinase (EC 2.7.1.31) | c.571C>G | P191A |
| 42 | Transcriptional regulator, TetR family | c.3C>A | L1F |
| 43 | Long-chain-fatty-acid–CoA ligase (EC 6.2.1.3) | c.1325C>A | G442V |
| 44 | FIG00352445: hypothetical protein | c.[1485T>T]+[1485T>A] | Q495Q, Q495H |
| 45 | FIG00352445: hypothetical protein | c.[1200T>T]+[1200T>A] | Q400Q, Q400H |
| 46 | Cell division protein FtsJ/ribosomal | c.473G>A | A158V |
| 47 | putative hemagglutinin/hemolysin-related protein | c.[592T>T]+[592T>C] | T198T, T198A |
| 48 | Putative hemagglutinin/hemolysin-related protein | c.[589T>T]+[589T>G] | I197I, I197L |
| 49 | Putative hemagglutinin/hemolysin-related protein | c.[436C>T]+[436C>C] | V146I, V146V |
| 50 | Putative hemagglutinin/hemolysin-related protein | c.[205T>T]+[205T>C] | I69I, I69V |
| 51 | Sensory histidine kinase QseC | c.680A>G | V227A |
| 52 | Sensory histidine kinase QseC | c.623G>A | P208L |
| 53 | Putative stomatin/prohibitin-family | c.[172G>T]+[172G>G] | V58V, V58L |
| 54 | Hypothetical protein; putative signal peptide | c.407G>T | A136D |
| 55 | FIG00351726: hypothetical protein | c.[947A>C]+[947A>A] | V316G, V316V |
| 56 | N-carbamoylputrescineamidase (3.5.1.53) | c.388->T | I130fs |
| 57 | FIG00350819: hypothetical protein | c.79C>A | A27S |
| 58 | Sodium-dependent transporter | c.618C>A | M206I |
| 59 | FIG00350872: hypothetical protein | c.713G>T | T238K |
| 60 | Histone acetyltransferase HPA2 | c.[122T>T]+[122T>C] | Q41Q, Q41R |
| 61 | Histone acetyltransferase HPA2 | c.[118T>T]+[118T>C] | T40T, T40A |
Note.—The changes in DNA and protein that occur in A. baumannii 48055 strain, with reference to the A. baumannii 53264 genome sequence.
As these mutations occurred within a 1-month period, these 61 SNP changes indicate the fast evolution of the A. baumannii strains. Colistin resistance has been shown to result from a complete loss of lipopolysaccharide (LPS) production by deletion of LpxA, LpxB, and LpxD (Moffatt et al. 2010) or modifications of LPS through mutations in the pmrAB two-component system (Beceiro et al. 2011). EDR strains are defined by their resistance to colistin; however, we were not able to detect any SNP changes that would confer resistance to colistin. Hence, we decided to use iTRAQ-based quantitative proteomics to study the proteome changes in the EDR A. baumannii 53264 strain in response to antibiotics.
Comparative Analysis of Antibiotic-Tolerance-Related Proteins of the EDR A. baumannii 53264 Strain Using iTRAQ
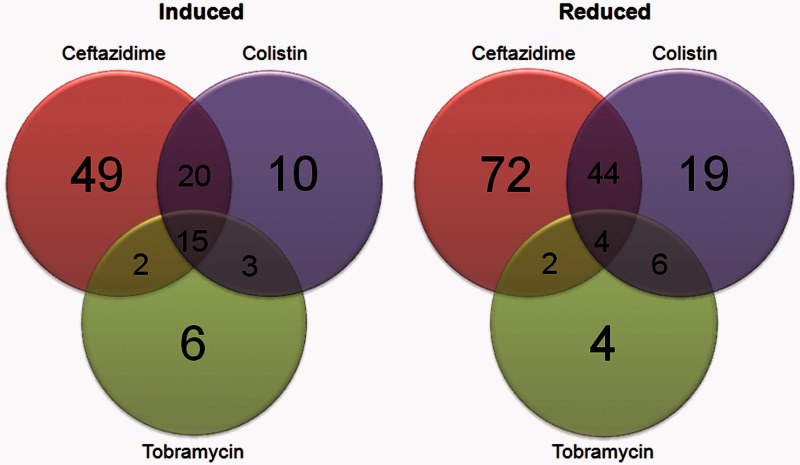
We then studied the stress response of the A. baumannii 53264 strain to treatment by three different classes of antibiotics (colistin, ceftazidime, and tobramycin) to understand the stress response of this EDR A. baumannii clone to various antibiotics. Figure 3 shows the number of proteins whose expression was induced or reduced in the presence of a specific antibiotic (ceftazidime, colistin, or tobramycin).
Fig. 3.—
The Venn diagram on the left shows the number of proteins whose expression was induced in the presence of a specific antibiotic. The Venn diagram on the right shows the number of proteins whose expression was reduced in the presence of a specific antibiotic.
We defined induced proteins as those whose abundance was increased by at least 2-fold versus the control (without antibiotic addition). Conversely, reduced proteins were defined as those whose abundance was decreased by at least 2-fold versus the control. Overlapping regions of the Venn diagrams show the number of proteins whose expression was found to be commonly induced (or reduced) by one or more antibiotics.
There was more similarity in the genes induced (or reduced) in the presence of ceftazidime and colistin when compared with those induced (or reduced) by tobramycin. This indicates that the resistance mechanisms of A. baumannii 53264 strain to ceftazidime and colistin have more in common than its resistance mechanism to tobramycin. A different set of proteins may be required for its resistance to tobramycin. This could be due to the fact that ceftazidime and colistin target the bacteria cell wall (Hayes and Orr 1983) and cell membrane (Falagas et al. 2005), respectively, whereas tobramycin targets the 30S ribosomal subunit (Walter et al. 1999).
Next, we wanted to study the common set of genes that were induced or reduced by all three antimicrobials, as this would indicate a core set of genes essential to the stress response of EDR A. baumannii 53264 to antibiotics. Table 6 lists four proteins that were found to be commonly down-regulated (by at least 2-fold) by A. baumannii 53264 in all three antibiotic treatments. (The list of commonly up-regulated proteins is included as supplementary table S1, Supplementary Material online.)
Table 6.
Proteins Whose Expression Was Down-Regulated by All Three Antibiotics
| Accession No. | Name | Function | No. of Matched Peptides (95%) | %Cov (95%) | Treatment with |
||
|---|---|---|---|---|---|---|---|
| Cef | Col | Tob | |||||
| gi|183211243 | Succinylargininedihydrolase | Arginine and proline metabolism | 31 | 68.9 | 0.45 | 0.43 | 0.43 |
| gi|183209017 | Dihydroorotase | Pyrimidine biosynthesis | 7 | 68.6 | 0.44 | 0.10 | 0.47 |
| gi|183210937 | Long-chain fatty acid transport protein | Membrane transport of long-chain fatty acids | 19 | 60.04 | 0.35 | 0.37 | 0.32 |
| gi|183211425 | Putative porin | Membrane transport | 7 | 62.35 | 0.30 | 0.17 | 0.49 |
Note.—Cef, ceftazidime; Col, colistin; Tob, tobramycin. Percent coverage (%Cov) refers to the percent of the residues in each protein sequence that has been identified at 95% confidence level. Numbers in the Cef, Col and Tob columns refer to the fold change of the protein abundance when compared with the control sample (without antibiotics).
From table 6, we note the decreased expression of a putative porin (gi|183211425). This outer membrane protein (OMP) was found to be at least 2-fold under expressed in all three antibiotic treatments, with the colistin treatment causing a more than 5-fold decrease in expression of this porin. OMPs are involved in the uptake of antibiotics into the bacterial cell. For example, the OMP OprD of P. aeruginosa has been shown to be important in the uptake of positively charged peptides and carbapenem antibiotics (Nikaido 2003). Also, the expression of the OMP OmpW of A. baumannii was found to be reduced in a colistin-resistant strain (Vila et al. 2007). Hence, down-regulation of porin expression may reduce colistin uptake by the A. baumannii 53264 strain and explain its resistance toward colistin.
Supplementary Material
Supplementary table S1 is available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgments
This work was supported by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Programme and the Start-up Grant (M4330002.C70) from Nanyang Technological University, Singapore. The authors acknowledge Dr Rohan Williams (National University of Singapore) for his valuable discussions.
Literature Cited
- Aziz R, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beceiro A, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;557:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;144:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KR, et al. Dissemination of multidrug-resistant Acinetobacter baumannii genotypes carrying blaOXA-23 collected from hospitals in Rio de Janeiro, Brazil. Int J Antimicrob Agents. 2009;341:25–28. doi: 10.1016/j.ijantimicag.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;10327:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvec S, et al. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J Antimicrob Chemother. 2003;524:629–635. doi: 10.1093/jac/dkg407. [DOI] [PubMed] [Google Scholar]
- Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PloS One. 2010;5(6):e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L, et al. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J Clin Microbiol. 1996;34(6):1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves DJ, et al. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob Agents Chemother. 2004;4810:4012–4015. doi: 10.1128/AAC.48.10.4012-4015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Kasiakou SK, Saravolatz LD. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;409:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Fournier PE, Richet H, Weinstein RA. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42(5):692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23(4):332–339. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- Hayes MV, Orr DC. Mode of action of ceftazidime: affinity for the penicillin-binding proteins of Escherichia coli K12, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1983;122:119–126. doi: 10.1093/jac/12.2.119. [DOI] [PubMed] [Google Scholar]
- Howard A, O'Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3(3):243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono M, et al. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob Agents Chemother. 2008;52(7):2616–2625. doi: 10.1128/AAC.01643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavolos M, et al. Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genomics. 2008;91:458. doi: 10.1186/1471-2164-9-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlowsky JA, et al. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob Agents Chemother. 2003;47(5):1681–1688. doi: 10.1128/AAC.47.5.1681-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz P, et al. Microindel detection in short-read sequence data. Bioinformatics. 2010;26(6):722–729. doi: 10.1093/bioinformatics/btq027. [DOI] [PubMed] [Google Scholar]
- Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;2516:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Pop M. ARDB—Antibiotic Resistance Genes Database. Nucleic Acids Res. 2009;37:D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore DM. beta-Lactamases in laboratory and clinical resistance. Clin Microbiol Rev. 1995;84:557–584. doi: 10.1128/cmr.8.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SR, et al. Antimicrobial resistance among gram-negative bacilli causing infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45(10):3352–3359. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JH, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;5412:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnier PD, Poirel L, Naas T, Nordmann P. Worldwide Dissemination of the blaOXA-23 Carbapenemase Gene of Acinetobacter baumannii1. Emerg Infect Dis. 2010;161:35. doi: 10.3201/eid1601.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E, Elisha BG. Molecular basis of AmpC hyperproduction in clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1999;434:957–959. doi: 10.1128/aac.43.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;674:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukaga M, et al. Molecular evolution of a class C-lactamase extending its substrate specificity. J Biol Chem. 1995;27011:5729–5735. doi: 10.1074/jbc.270.11.5729. [DOI] [PubMed] [Google Scholar]
- Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45(9):1179–1181. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J Clin Microbiol. 2003;41(8):3542–3547. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321:1078–1080. doi: 10.1126/science.1160354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnegar N, et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii in a single institution over a 10-year period. J Clin Microbiol. 2010;48(11):4051–4056. doi: 10.1128/JCM.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife W, Young H-K, Paton RH, Amyes SG. Transferable imipenem-resistance in Acinetobacter species from a clinical source. J Antimicrob Chemother. 1995;363:585–587. doi: 10.1093/jac/36.3.585. [DOI] [PubMed] [Google Scholar]
- Stoeva T, Higgins P, Bojkova K, Seifert H. Clonal spread of carbapenem-resistant OXA-23-positive Acinetobacter baumannii in a Bulgarian university hospital. Clin Microb Infect. 2008;147:723–727. doi: 10.1111/j.1469-0691.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- Tam V, et al. Prevalence of AmpC over-expression in bloodstream isolates of Pseudomonas aeruginosa. Clin Microb Infect. 2007;134:413–418. doi: 10.1111/j.1469-0691.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- van Dessel H, et al. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res Microbiol. 2004;155(2):105–112. doi: 10.1016/j.resmic.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Vila J, Martí S, Sánchez-Céspedes J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2007;596:1210–1215. doi: 10.1093/jac/dkl509. [DOI] [PubMed] [Google Scholar]
- Vila J, Ruiz J, Goñi P, de Anta TJ. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother. 1997;396:757–762. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- Walter F, Vicens Q, Westhof E. Aminoglycoside–RNA interactions. Curr Opin Chem Biol. 1999;36:694–704. doi: 10.1016/s1367-5931(99)00028-9. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Bogaki M, Nakamura M, Yamanaka LM, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob Agents Chemother. 1991;358:1647–1650. doi: 10.1128/aac.35.8.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. Dissemination of imipenem-resistant Acinetobacter baumannii strains carrying the ISAba1–blaOXA-23 genes in a Chinese hospital. J Med Microbiol. 2007;568:1076–1080. doi: 10.1099/jmm.0.47206-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.