Abstract
Background:
Pediatric obstructive sleep apnea (OSA) is associated with cognitive dysfunction, suggesting altered neurotransmitter function. We explored overnight changes in neurotransmitters in the urine of children with and without OSA.
Methods:
Urine samples were collected from children with OSA and from control subjects before and after sleep studies. A neurocognitive battery assessing general cognitive ability (GCA) was administered to a subset of children with OSA. Samples were subjected to multiple enzyme-linked immunosorbent assays for 12 neurotransmitters, and adjusted for creatinine concentrations.
Results:
The study comprised 50 children with OSA and 20 control subjects. Of the children with OSA, 20 had normal GCA score (mean ± SD) (101.2 ± 14.5) and 16 had a reduced GCA score (87.3 ± 13.9; P < .001). Overnight increases in epinephrine, norepinephrine, and γ-aminobutyric acid (GABA) levels emerged in children with OSA; taurine levels decreased. Using combinatorial approaches and cutoff values for overnight changes of these four neurotransmitters enabled prediction of OSA (area under the curve [AUC]: 0.923; P < .0001). Furthermore, GABA and taurine alterations, as well as overnight reductions in phenylethylamine, were more prominent in children with OSA and low GCA than in children with OSA and normal GCA (P < .001), and they reliably discriminated GCA status (AUC: 0.977; P < .0001).
Conclusions:
Pediatric OSA is associated with overnight increases in urinary concentrations of catecholamines indicative of heightened sympathetic outflow. Increases in GABA levels and decreases in taurine levels could underlie mechanisms of neuronal excitotoxicity and dysfunction. Combinatorial approaches using defined cutoffs in overnight changes in concentrations of selected neurotransmitters in urine may not only predict OSA but also the presence of cognitive deficits. Larger cohort studies appear warranted to confirm these findings.
Snoring is a very frequent complaint affecting 10% to 12% of all children1 and is the primary symptom of obstructive sleep apnea (OSA). OSA is the occurrence of repeated events of partial or complete upper airway obstruction during sleep, leading to disruption of normal ventilation, and to hypoxemia and sleep fragmentation. The pathophysiology of pediatric OSA is multifactorial, but adenotonsillar hypertrophy with or without concurrent obesity constitutes the major pathophysiologic mechanism underlying OSA in children.2 Pediatric OSA has been extensively associated with an increased risk for behavioral and mood disturbances, as well as with cognitive deficits. Furthermore, cardiovascular and metabolic morbidities, such as pulmonary hypertension, systemic hypertension, endothelial dysfunction, insulin resistance, and serum lipid alterations, are also frequently observed.3 However, identification of those children who have developed any such OSA-associated morbidities is difficult, and is usually complicated by the need for laborious and onerous test batteries that are not routinely available in most clinical settings. Therefore, such assessments are usually not pursued.
In children with OSA, the heightened risk for increased sympathetic tonic and reactive activity indicative of substantial changes in autonomic nervous system regulation4 can be conveniently assessed, for example, through measurement of catecholamine levels in urine.5‐7 Considering the physiologic importance of neurotransmitters as signaling molecules in the nervous system and the potential alterations that may develop in the context of OSA, assessment of urinary neurotransmitters offers unique opportunities because of their stability, sensitivity, and particularly to the noninvasiveness of this approach.8 The development of multiplexed techniques that enable simultaneous assessment of several neurotransmitters in biologic fluids in general, and in urine in particular, provided us with the opportunity to explore the hypothesis that pediatric OSA would be associated with a unique pattern of alterations in urinary neurotransmitters, which may reflect underlying cognitive deficits.
Materials and Methods
The research protocol was approved by the University of Chicago (protocol 09-115-B) human research ethics committee. Informed consent was obtained from the parents of the children in the study, and age-appropriate assent was obtained from the children. Patients were recruited from the Sleep and Ear Nose and Throat (ENT) clinics of Comer Children’s Hospital, as well as by advertisement. Patients who had genetic or craniofacial syndromes and chronic diseases, such as cardiac disease, diabetes, cerebral palsy, and chronic lung disease of prematurity, or were receiving any psychotropic medications were excluded.
Overnight Polysomnography
Subjects aged from 3 to 12 years were recruited and underwent standard, overnight polysomnography evaluation as previously described,9 with assessment of eight standard EEG channels; bilateral electrooculography; electromyography; two-lead ECG; oronasal airflow measurement using thermistor, nasal pressure transducer, and end-tidal CO2; chest and abdominal movement by respiratory inductance plethysmography; and pulse oximetry including pulse waveform. The polysomnography equipment was Polysmith (Nihon Kohden America Inc). The polysomnography studies were scored per the 2007 American Association of Sleep Medicine guidelines for the scoring of sleep and associated events.10‐12 The proportion of time spent in each stage of sleep was calculated as a percentage of total sleep time. A respiratory event was scored as an obstructive apnea if it was associated with a > 90% fall in signal amplitude for > 90% of the entire event compared with the baseline amplitude, if the event lasted for at least two breaths, and if there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort in the initial part of the event followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, or if the event lasted for at least two missed breaths and was associated with an arousal/ awakening or with a ≥ 3% desaturation. Hypopnea was scored if the event was associated with a ≥ 50% fall in amplitude of the nasal pressure transducer, lasted at least for two breaths, and was associated with an arousal/awakening or ≥ 3% desaturation. The apnea-hypopnea index (AHI) was calculated as the number of instances of apnea or hypopnea per hour of total sleep time (hrTST). The presence of OSA was defined by the presence of an obstructive AHI ≥ 2/hrTST. Control subjects were children who did not snore and whose AHI was < 1/hrTST.
General Cognitive Abilities
A subset of the subjects included in the present study underwent neurocognitive testing by certified psychometricians experienced in working with children and who were blinded to the sleep study and urine results. The neurocognitive assessments were performed the morning after the sleep study, lasted approximately 90 min, and included the core subtests of the Differential Abilities Scales (DAS).13 The DAS is a battery of cognitive tests designed to measure reasoning and conceptual ability in children, or general cognitive abilities. The DAS provides individual subtest scores and the General Conceptual Ability (GCA) composite score that includes the sum of verbal and nonverbal reasoning, memory, and spatial skills. The verbal tests reflect knowledge of verbal concepts and level of vocabulary development, and are also indicative of word retrieval from long-term memory. The core subtests administered included Word Definitions, which measures knowledge of word meanings as demonstrated through spoken language or the ability to formulate definition of words (verbal fluency). The subtest Similarities measures verbal reasoning and knowledge, where inductive reasoning ability or the ability to relate three words to superordinate categories is necessary to earn credit. The nonverbal ability tests measure the child’s inductive and sequential reasoning abilities. The core nonverbal subtest is Matrices, measuring nonverbal reasoning, which involve perception and application of relationships among abstract figures. Sequential and quantitative reasoning tests involve detection of sequential patterns in figures or numbers. The spatial ability tests measure visuospatial construction ability, spatial memory, and spatial reasoning. The core subtest Pattern Construction measures nonverbal reasoning and spatial visualization in reproducing designs with colored blocks, incorporating response time in the individual scoring; recall of designs involves the short-term recall of visual and spatial relationships through reproduction of abstract figures.
The ability score for each subtest is converted to a T score with a mean of 50 and a SD of 10. The sum of the core subtests is then converted to yield a total standard score for the GCA, with a mean of 100 and a SD of 15. Although the raw scores were used in the modeling procedures, we expressed standardized composite scores for descriptive purposes. The DAS was normalized on a large, stratified sample of children across the United States and has good validity and reliability. The GCA scores are indicative of the “psychometric g,” which is the general ability to perform complex mental processing that involves conceptualization and the transformation of information, and has been considered a structural correlate to executive function. For study purposes, we defined abnormal general cognitive ability as ≥ 1 SD below the mean.
Urinary Neurotransmitters
Subjects urinated prior to the sleep study and a first morning urine sample was collected immediately after completing the overnight polysomnographic evaluation (< 60 min after awakening in all cases). Children who urinated during the night were excluded from the study. Urine was collected in midstream after local cleaning of the genital area with a wet sterile cloth, and urine samples were placed in 5-mL tubes containing a filter disc impregnated with 250 μL of 3N hydrogen chloride as preservative. The tubes were stored in a −80°C freezer until assay. A previously validated, multiplexed, enzyme-linked immunosorbent assay method for assessment of several neurotransmitters was used, and the linearity of the response curves within the ranges pertinent to the current study was validated for each of the analytes.14,15 The neurotransmitters included in this study were epinephrine, norepinephrine, dopamine, dihydroxyphenylacetic acid, serotonin, 5-hydroxyindoleacetic acid, glycine, taurine, γ-aminobutyric acid (GABA), glutamate, β-phenylethylamine (PEA), and histamine. All samples were assayed in duplicate and values were retained if they were within 10% of each other. Urine creatinine level was measured for each sample. Individual urine neurotransmitter levels were corrected for corresponding urine creatinine concentration.
Statistical Analysis
Data are expressed as mean ± SEM unless otherwise indicated. Significant differences within groups were analyzed using unpaired t tests or analysis of variance for continuous variables and χ2 tests for categorical variables. Bonferroni corrections were applied for multiple comparisons. In addition, receiver operating characteristic curves (ROC) were constructed for individual overnight changes in urinary neurotransmitter levels using previously published approaches.16 Simply, a cutoff criterion was initially identified using ROC analyses for each of the urinary neurotransmitter analytes, and then each individual subject was scrutinized as to whether they fulfilled the cutoff values for the number of analytes. Then a ROC analysis for those fulfilling one, two, or more than two cutoffs of the previously identified, significantly predictive analytes was performed to determine the predictive performance of the combinatorial approach. Statistical analyses were performed using SPSS software, version 17.0 (IBM). All P values reported are two-tailed with statistical significance set at < .05.
Results
A total of 50 children with OSA were included in the study. Their demographic characteristics and polysomnographic findings are shown in Table 1 along with those of 20 age-, sex-, ethnicity-, and BMI-matched control subjects. Cognitive testing could only be performed in 36 of the children with OSA; 20 of the 36 children with OSA who were tested had normal general cognitive ability (OSAn) (GCA score: 101.2 ± 14.5) and 16 had reduced general cognitive ability (OSAab) (GCA score: 87.3 ± 13.9; P < .001).
Table 1.
—Demographic, Polysomnographic, and Urinary Neurotransmitter Overnight Changes in 50 Children With OSA and 20 Matched Control Subjects
| Characteristics | OSA Group (n = 50) | Control Group (n = 20) | P Value |
| Age, y | 6.2 ± 1.6 | 6.4 ± 1.4 | NS |
| Sex, male, % | 54 | 55 | NS |
| White ethnicity, % | 70 | 70 | NS |
| BMI Z score | 1.22 ± 0.78 | 1.05 ± 0.84 | NS |
| Apnea-hypopnea index, events/h | 9.24 ± 0.91 | 0.37 ± 0.19 | <.001 |
| Sao2 nadir, % | 84.5 ± 3.9 | 93.1 ± 2.7 | <.001 |
| TAI/hrTST | 16.7 ± 6.2 | 11.1 ± 4.9 | <.04 |
| GCAab | 16/36 | … | |
| Overnight percent change in | |||
| Norepinephrine | 114.3 ± 23.8 | −9.3 ± 3.3 | <.0001 |
| Epinephrine | 132.7 ± 10.5 | 0.8 ± 2.1 | <.0001 |
| Dopamine | 13.2 ± 2.9 | 1.4 ± 0.2 | NS |
| DOPAC | −30.5 ± 4.8 | −23.2 ± 3.9 | NS |
| Serotonin | 51.6 ± 6.9 | 38.7 ± 5.6 | NS |
| 5-HIAA | −13.8 ± 2.7 | −7.8 ± 2.6 | NS |
| Glycine | −23.7 ± 3.8 | −16.6 ± 3.2 | NS |
| Taurine | −105.2 ± 11.0 | 9.8 ± 1.7 | <.0001 |
| GABA | 45.2 ± 8.0 | −7.8 ± 1.8 | <.0001 |
| Glutamate | 9.2 ± 3.6 | −17.8 ± 4.7 | NS |
| PEA | −51.4 ± 10.6 | −29.5 ± 9.8 | NS |
| Histamine | −40.4 ± 9.3 | −44.2 ± 9.6 | NS |
Data for changes in urine neurotransmitter level overnight are shown as mean ± SEM; all other values are shown as mean ± SD. 5-HIAA = 5-hydroxyindoleacetic acid; DOPAC = dihydroxyphenylacetic acid; GABA = gamma-aminobutyric acid; GCAab = low general cognitive ability; hrTST = hours of total sleep time; NS = not significant; OSA = obstructive sleep apnea; PEA = β-phenylethylamine; Sao2 = hemoglobin oxygen saturation; TAI = total arousal index.
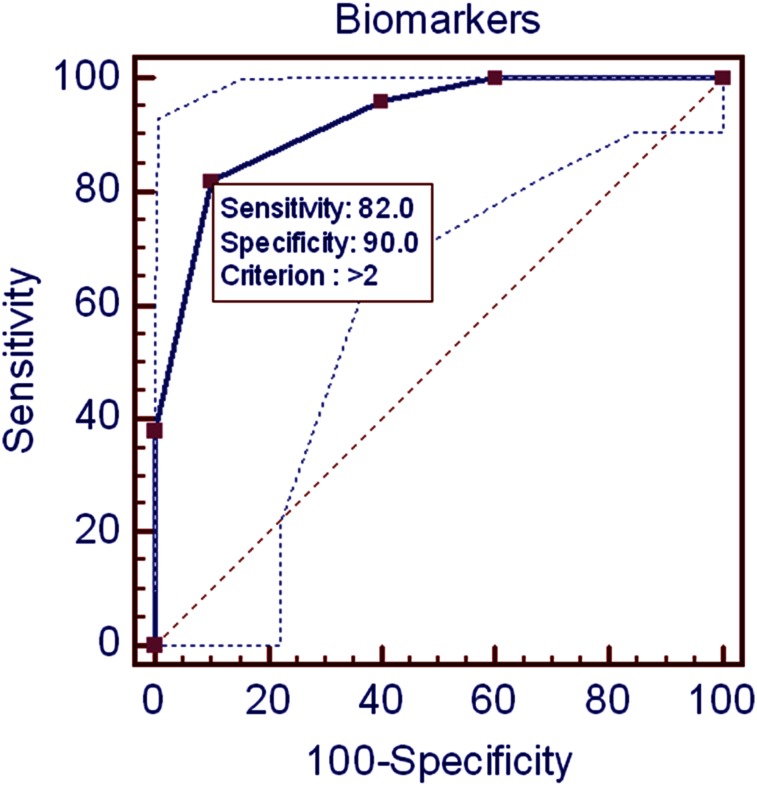
Significant overnight increases in creatinine-adjusted epinephrine and norepinephrine urinary levels were recorded in children with OSA (Tables 1, 2). Significant increases in GABA urinary concentrations also were apparent. Furthermore, larger decreases in urinary taurine levels were present; there were no significant differences in any of the other measured neurotransmitters (Tables 1, 2). ROC scrutiny of individual urinary neurotransmitters did not enable adequate prediction of OSA (sensitivity: 56%-76%; specificity: 38%-49%). However, use of the four urinary neurotransmitters showing statistically significant changes in children with OSA, and their expression as specific, cutoff, evening to morning changes, namely epinephrine (cutoff overnight change: > 47%), norepinephrine (cutoff overnight change: > 68%), GABA (cutoff overnight change: > 20%), and taurine (cutoff overnight change: < 65%), enabled relatively favorable predictions of OSA when three or more of these biomarkers fulfilled the cutoff criteria (area under the curve: 0.923; P < .0001) (Fig 1).
Table 2.
—Overnight Changes in Urinary Neurotransmitter Levels in 50 Children With OSA, 20 Control Subjects, 20 Children With OSA and Preserved GCA Scores, and 16 Children With OSA and Low GCA Scores
| OSA Group | OSAab | OSAn | Control Group | p Value | ||||||||||
| Neurotransmitter | Overnight Percent Change | Evening | Morning | Overnight Percent Change | Evening | Morning | Overnight Percent Change | Evening | Morning | Overnight Percent Change | Evening | Morning | Control Group vs OSA Group | OSAab vs OSAn |
| Norepinephrine | 114.3 ± 23.8 | 0.16 ± 0.08 | 0.33 ± 0.20 | 152.3 ± 69.6 | 0.13 ± 0.07 | 0.30 ± 0.13 | 90.3 ± 15.7 | 0.18 ± 0.08 | 0.35 ± 0.13 | −9.3 ± 3.3 | 0.14 ± 0.07 | 0.13 ± 0.07 | <.0001 | .06 |
| Epinephrine | 132.7 ± 10.5 | 0.91 ± 0.47 | 2.20 ± 0.53 | 120.3 ± 33.8 | 0.89 ± 0.39 | 1.88 ± 0.67 | 143.7 ± 30.8 | 0.91 ± 0.53 | 2.24 ± 0.87 | 0.8 ± 2.1 | 0.76 ± 0.57 | 0.76 ± 0.64 | <.0001 | NS |
| Dopamine | 13.2 ± 2.9 | 4.78 ± 1.88 | 4.90 ± 1.91 | 12.6 ± 4.1 | 4.80 ± 2.06 | 5.07 ± 2.22 | 13.4 ± 5.2 | 4.67 ± 2.35 | 4.81 ± 2.74 | 1.4 ± 0.2 | 4.26 ± 2.21 | 4.27 ± 2.4 | NS | NS |
| DOPAC | −30.5 ± 4.8 | 19.51 ± 7.6 | 14.72 ± 5.8 | −32.8 ± 6.6 | 19.77 ± 7.8 | 14.89 ± 6.8 | −28.7 ± 7.3 | 18.56 ± 8.1 | 14.31 ± 7.6 | −23.2 ± 3.9 | 18.7 ± 9.4 | 15.72 ± 9.8 | NS | NS |
| Serotonin | 51.6 ± 6.9 | 2.36 ± 1.23 | 3.13 ± 1.37 | 57.6 ± 10.2 | 2.32 ± 1.54 | 3.32 ± 1.76 | 49.2 ± 9.7 | 2.41 ± 1.62 | 3.02 ± 1.93 | 38.7 ± 5.6 | 2.22 ± 1.08 | 2.78 ± 1.43 | NS | NS |
| 5-HIAA | −13.8 ± 2.7 | 50.5 ± 22.7 | 45.3 ± 23.8 | −15.2 ± 3.5 | 52.3 ± 24.9 | 46.7 ± 26.2 | −11.4 ± 4.1 | 50.3 ± 23.3 | 44.3 ± 21.8 | −7.8 ± 2.6 | 48.3 ± 27.4 | 44.7 ± 24.1 | NS | NS |
| Glycine | −23.7 ± 3.8 | 18.99 ± 6.85 | 14.26 ± 7.21 | −25.4 ± 4.9 | 20.32 ± 9.43 | 14.86 ± 10.32 | −22.5 ± 8.76 | 17.87 ± 8.65 | 13.54 ± 9.32 | −16.6 ± 3.2 | 21.55 ± 5.8 | 18.87 ± 9.4 | NS | NS |
| Taurine | −105.2 ± 11.0 | 11.78 ± 4.31 | 4.83 ± 2.87 | −170.3 ± 29.1 | 12.65 ± 5.38 | 2.57 ± 2.98 | −45.8 ± 5.7 | 11.32 ± 6.43 | 6.65 ± 5.43 | 9.8 ± 1.7 | 13.21 ± 4.98 | 14.08 ± 5.76 | <.0001 | <.0001 |
| GABA | 45.2 ± 8.0 | 0.09 ± 0.03 | 0.14 ± 0.05 | 60.2 ± 5.7 | 0.08 ± 0.04 | 0.13 ± 0.05 | 38.6 ± 8.0 | 0.11 ± 0.06 | 0.14 ± 0.07 | −7.8 ± 1.8 | 0.07 ± 0.03 | 0.06 ± 0.03 | <.0001 | <.0001 |
| Glutamate | 9.2 ± 3.6 | 0.92 ± 0.21 | 1.02 ± 0.26 | 8.7 ± 4.7 | 0.87 ± 0.33 | 0.91 ± 0.36 | 10.8 ± 4.2 | 0.94 ± 0.44 | 1.07 ± 0.47 | −17.8 ± 4.7 | 0.88 ± 0.32 | 0.76 ± 0.43 | NS | NS |
| PEA | −51.4 ± 10.6 | 1.03 ± 0.37 | 0.58 ± 0.23 | −66.9 ± 5.3 | 1.13 ± 0.45 | 0.45 ± 0.42 | −32.4 ± 5.1 | 0.99 ± 0.46 | 0.67 ± 0.37 | −29.5 ± 9.8 | 0.89 ± 0.28 | 0.65 ± 0.34 | NS | <.001 |
| Histamine | −40.4 ± 9.3 | 0.55 ± 0.17 | 0.32 ± 0.23 | −45.3 ± 12.3 | 0.57 ± 0.27 | 0.35 ± 0.29 | −38.7 ± 17.8 | 0.52 ± 0.34 | 0.30 ± 37 | −44.2 ± 9.6 | 0.56 ± 0.31 | 0.37 ± 0.28 | NS | NS |
Data are expressed as ratios of corresponding individual urinary creatinine levels. GCA = General Conceptual Ability score; OSAab = children with OSA and low GCA; OSAn = children with OSA and normal GCA. See Table 1 legend for expansion of other abbreviations.
Figure 1.
Receiver operating characteristic curve using cutoff values of overnight changes for four urinary neurotransmitters in the prediction of obstructive sleep apnea in children. Dotted lines indicate 95% CIs.
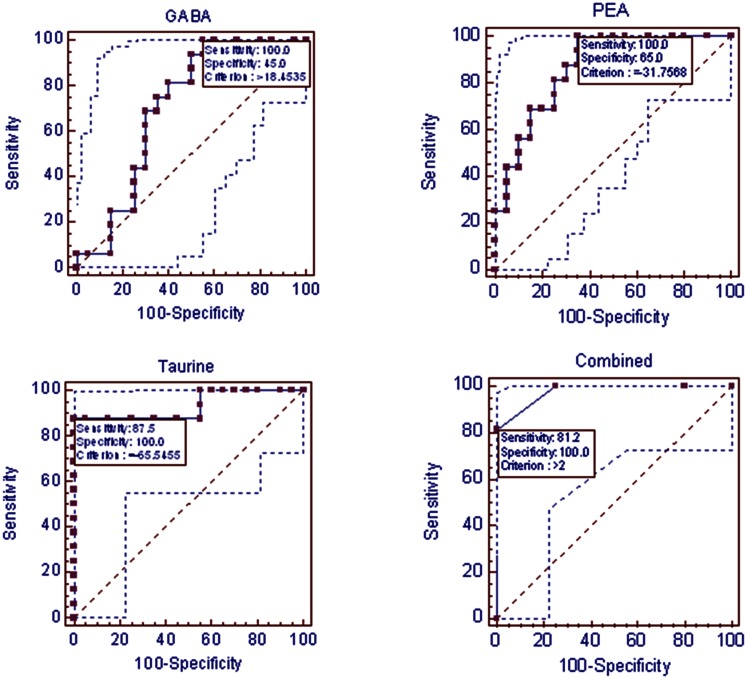
Of note, GABA and taurine alterations, as well as reduced PEA levels, were significantly more prominent in children with OSA who had OSAab than in those with OSAn (P < .01) (Tables 2, 3). Such differences between the two OSA subgroups were not present for urinary catecholamine levels. ROCs were constructed for each of these three urinary neurotransmitters and then used in combination for prediction of low general GCA scores in the context of OSA (Tables 2, 3) (Fig 2). Indeed, an area under the curve of 0.977, with a sensitivity of 82% and a specificity of 90%, was achieved (P < .0001).
Table 3.
—Demographic, Polysomnographic, and Overnight Changes in Urinary Neurotransmitter Levels in 20 Children With OSA and Preserved GCA Scores and 16 Children With OSA and Low GCA Scores
| Characteristics | OSAab (n = 16) | OSAn (n = 20) | P Value |
| Age, y | 6.1 ± 1.8 | 6.2 ± 1.7 | NS |
| Sex, male, % | 50% | 50% | NS |
| White ethnicity, % | 68.7 | 65 | NS |
| BMI Z score | 1.30 ± 0.91 | 1.25 ± 0.87 | NS |
| Apnea-hypopnea index (events/h) | 12.24 ± 3.2 | 8.93 ± 4.87 | NS |
| SaO2 nadir, % | 83.5 ± 5.1 | 85.6 ± 4.7 | NS |
| TAI/hrTST | 18.2 ± 8.2 | 17.1 ± 7.9 | NS |
| GCA score | 87.3 ± 13.9 | 101.2 ± 14.5 | <.01 |
| Overnight percent change in | |||
| Norepinephrine | 152.3 ± 69.6 | 90.3 ± 15.7 | 0.06 |
| Epinephrine | 120.3 ± 33.8 | 143.7 ± 30.8 | NS |
| Taurine | −170.3 ± 29.1 | −45.8 ± 5.7 | <.0001 |
| GABA | 60.2 ± 5.7 | 38.6 ± 8.0 | <.0001 |
| PEA | −66.9 ± 5.3 | −32.4 ± 5.1 | <.0001 |
Data for changes in urine neurotransmitter level changes are shown as mean ± SEM; all other values are shown as mean ± SD. See Table 1 legend for expansion of abbreviations.
Figure 2.
Receiver operating characteristic curves using three urinary-neurotransmitter-defined cutoff values corresponding to individual overnight changes for prediction of neurocognitive dysfunction in children with obstructive sleep apnea. Dotted lines indicate 95% CIs. GABA = γ-aminobutyric acid; PEA = β-phenylethylamine.
Discussion
This study shows that pediatric OSA is associated with significant nocturnal alterations in a selected number of neurotransmitters when the latter are assayed as overnight changes in the urine. Furthermore, when used in combination, defined cutoffs of overnight changes among the urinary neurotransmitters with significantly altered concentrations appear to enable relatively favorable prediction of OSA. Similar approaches using a slightly different set of urinary neurotransmitters also permits relatively accurate identification of those children with OSA who manifest reduced neurocognitive function when tested using the DAS battery.
Before we discuss the potential implications of our findings, some comments regarding biomarker discovery in pediatric OSA and on the potential limitations of this study are needed. First, our study is only one of several critical steps required for the exploration of biomarker discovery in human-derived biologic samples17; therefore, the current findings should be viewed as preliminary. In our initial attempt to explore the presence of diagnostic biomarkers in pediatric OSA, we identified three differentially expressed proteins that were associated with OSA in plasma.18 However, although these three proteins displayed relatively favorable predictive values, the proteomic methodologies used by Shah et al18 did not allow for accurate identification of the proteins, and such putative biomarkers could not be confirmed. In a subsequent study using urine samples, we examined the urine proteome in children with OSA, in children with primary snoring, and in control subjects using two-dimensional gel electrophoresis.19 In this 2009 study,19 we identified 16 unique proteins that were differentially expressed in the OSA group, and validated four of these proteins (kallikrein-1, urocortin-3, orosomucoid-1, and uromodulin) using enzyme-linked immunosorbent assays. Using a combinatorial-biomarker, discriminative approach similar to the one implemented in the present study, highly predictive ROC were generated. The present study undertook a different strategic approach using an a priori hypothesis positing that concentrations of some neurotransmitters would be differentially affected by the presence of OSA, and that some of such altered, overnight trajectories would reflect underlying neurocognitive outcomes.
A known limitation of urinary neurotransmitter assessment is that neurotransmitters, in any medium, are not recognized as diagnostic for any particular disease or condition with the exception of pheochromocytoma.20 Another limitation of such assessments concerns the insufficient data available regarding the origin of urinary neurotransmitters. Neurotransmitters are synthesized in organs of the CNS and in many other organs. Therefore, multiple systems may contribute to the total urinary pool of neurotransmitters being assayed, presenting a challenge in the interpretation of urinary neurotransmitter data.21 It also is unclear how factors other than OSA (eg, medications and supplements or even other concomitant disorders such as attention-deficit/hyperactivity disorder) that alter neurotransmitter levels in the CNS will affect neurotransmitters in the urine and vice versa.22‐24 Further limitations of the present study include a relatively small number of subjects, particularly among those who underwent neurocognitive testing; the fact that none of the control subjects were tested for cognitive function; the absence of a post hoc validation cohort for confirmatory purposes; and, finally, the need for determination of the effects of treatment on the changes reported in this study.
The overnight increases in urinary catecholamine levels reported herein were anticipated based on the several previously published studies in children with OSA, all of which indicated that morning urinary catecholamine levels were elevated.5‐7,25 However, the present study further indicates that the changes in urinary-catecholamine levels indeed occur during sleep, since urinary levels in the evening prior to the sleep study were similar between children with OSA and control subjects (data not shown). Thus, the recurrent hypoxemic events and the episodic arousals that characterize sleep in patients with OSA likely enhance sympathetic tonic outflow, and result in increased recovery of catecholamines in the urine.4 Taken together, we surmise that the changes in evening-to-morning epinephrine and norepinephrine levels are probably better suited for detection of OSA and the response to specific therapeutic interventions for pediatric OSA. Notwithstanding, the individual ROCs for overnight changes in urinary catecholamine levels were not sufficiently adequate predictors of OSA to enable their use as single biomarkers. The addition of two other urinary neurotransmitters, namely GABA (with its overnight increases) and taurine (with its overnight decreases), was required to more predictably identify OSA using urinary neurotransmitter levels in evening and morning samples (Fig 1).
Although the organ sources of increases in urinary GABA levels and declines in taurine urinary levels are unclear, changes in levels of these two neurotransmitters were not unanticipated. Indeed, animal studies suggest that the presence of intermittent hypoxia is associated with significant alterations in GABAergic neurotransmission.26‐28 Similarly, taurine is known to play important roles against reactive oxygen species and hypoxia in several organs, including the those of the CNS and the vasculature.29‐33 Both intermittent hypoxia and increased oxidative stress are hallmark characteristics of OSA,34 and the degree of oxidative stress associated with OSA has emerged as an important determinant of cognitive dysfunction in both animal models and in children.35‐37
A unique and intriguing observation in the present study involves the differential and predictable changes in urinary neurotransmitters in children with OSA and without evidence of neurocognitive dysfunction. In this context, the patterns of overnight changes in levels of three neurotransmitters, namely GABA, taurine, and PEA, allowed accurate prediction of cognitive deficits in this small cohort (Fig 2). The potential contributory roles of GABA and taurine have already been discussed, and their contributions to cognition will not be further expanded. Furthermore, monoaminergic systems are firmly established as playing critical roles in mood, cognition, emotion, reward, learning, and attention,38 and PEA has been shown to affect the monoamine transporter function in brain synaptosomes, whereby aberrant levels of PEA and other monoaminergic receptor ligands have been associated with various neuropsychiatric disorders, including depression and attention-deficit/hyperactivity disorder.39 Thus, the putative biomarkers for cognitive deficits appear as scientifically plausible. Of note, an association between a marker for systemic inflammation (ie, high-sensitivity C-reactive protein) and the presence of cognitive deficits has been previously reported in pediatric OSA,40 lending further credence to the use of biomarkers in the detection of patients at higher risk for end-organ morbidity, particularly considering the labor-intensive nature of such seldom-performed assessments during evaluation of children with suspected OSA.
In summary, predictable overnight changes in the concentrations of specific neurotransmitters in urine of children with OSA offer the opportunity to potentially develop screening approaches for this condition in children who habitually snore, particularly considering the inability to reliably diagnose the disease when relying on history and physical examination alone.41 Moreover, similar strategies may provide reliable indicators of children at risk for the presence of specific end-organ morbidities, such as neurocognitive dysfunction, associated with pediatric OSA.
Acknowledgments
Author contributions: Dr Gozal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Kheirandish-Gozal: contributed to the conception and design of the study, recruited subjects to the study, scored and interpreted the sleep studies, performed components of the data analysis, drafted the initial manuscript, and approved the final manuscript as submitted.
Ms McManus: carried out the initial urinary neurotransmitter analyses, reviewed the manuscript, and approved the final manuscript as submitted.
Dr Kellermann: designed urinary neurotransmitter methodologies, supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Dr Samiei: recruited subjects to the study, collected and processed urinary samples, constructed the database, and approved the final manuscript as submitted.
Dr Gozal: contributed to the conception and design of the study, assisted with data analysis, provided critical input into the drafting of the manuscript, and approved the final manuscript as submitted.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- AHI
apnea-hypopnea index
- DAS
Differential Abilities Scale
- GABA
γ-aminobutyric acid
- GCA
General Conceptual Ability
- hrTST
hours of total sleep time
- OSA
obstructive sleep apnea
- OSAab
obstructive sleep apnea and low general cognitive ability
- OSAn
obstructive sleep apnea and normal general cognitive ability
- PEA
β-phenylethylamine
- ROC
receiver operating characteristic curve
Footnotes
Funding/Support: This study was supported in part by US National Institutes of Health [grants HL-65270 and HL107160 to Dr Gozal].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol. 2010;108(2):436-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5(2):274-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185(1):177-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snow AB, Khalyfa A, Serpero LD, et al. Catecholamine alterations in pediatric obstructive sleep apnea: effect of obesity. Pediatr Pulmonol. 2009;44(6):559-567 [DOI] [PubMed] [Google Scholar]
- 6.Kaditis AG, Alexopoulos EI, Damani E, et al. Urine levels of catecholamines in Greek children with obstructive sleep-disordered breathing. Pediatr Pulmonol. 2009;44(1):38-45 [DOI] [PubMed] [Google Scholar]
- 7.O’Driscoll DM, Horne RS, Davey MJ, et al. Increased sympathetic activity in children with obstructive sleep apnea: cardiovascular implications. Sleep Med. 2011;12(5):483-488 [DOI] [PubMed] [Google Scholar]
- 8.Marc DT, Ailts JW, Campeau DC, Bull MJ, Olson KL. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: validity and clinical applicability. Neurosci Biobehav Rev. 2011;35(3):635-644 [DOI] [PubMed] [Google Scholar]
- 9.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741-753 [DOI] [PubMed] [Google Scholar]
- 10. Iber C A-IS, Chesson A, Quan S, American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
- 11.Grigg-Damberger M, Gozal D, Marcus CL, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3(2):201-240 [PubMed] [Google Scholar]
- 12.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3(2):169-200 [PubMed] [Google Scholar]
- 13.Elliott CD. Differential Abilities Scale: Handbook. San Antonio, TX: The Psychological Corporation; 1990 [Google Scholar]
- 14.Nichkova MI, Huisman H, Wynveen PM, Marc DT, Olson KL, Kellermann GH. Evaluation of a novel ELISA for serotonin: urinary serotonin as a potential biomarker for depression. Anal Bioanal Chem. 2012;402(4):1593-1600 [DOI] [PubMed] [Google Scholar]
- 15.Huisman H, Wynveen P, Nichkova M, Kellermann G. Novel ELISAs for screening of the biogenic amines GABA, glycine, β-phenylethylamine, agmatine, and taurine using one derivatization procedure of whole urine samples. Anal Chem. 2010;82(15):6526-6533 [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845 [PubMed] [Google Scholar]
- 17.Mischak H, Allmaier G, Apweiler R, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. 2010;2(46):46ps42. [DOI] [PubMed] [Google Scholar]
- 18.Shah ZA, Jortani SA, Tauman R, Valdes R, Jr, Gozal D. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res. 2006;59(3):466-470 [DOI] [PubMed] [Google Scholar]
- 19.Gozal D, Jortani S, Snow AB, et al. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180(12):1253-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westphal SA. Diagnosis of a pheochromocytoma. Am J Med Sci. 2005;329(1):18-21 [DOI] [PubMed] [Google Scholar]
- 21.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331-349 [DOI] [PubMed] [Google Scholar]
- 22.Kusaga A, Yamashita Y, Koeda T, et al. Increased urine phenylethylamine after methylphenidate treatment in children with ADHD. Ann Neurol. 2002;52(3):372-374 [DOI] [PubMed] [Google Scholar]
- 23.Montoya A, Escobar R, García-Polavieja MJ, et al. Changes of urine dihydroxyphenylglycol to norepinephrine ratio in children with attention-deficit hyperactivity disorder (ADHD) treated with atomoxetine. J Child Neurol. 2011;26(1):31-36 [DOI] [PubMed] [Google Scholar]
- 24.Moriarty M, Lee A, O’Connell B, Kelleher A, Keeley H, Furey A. Development of an LC-MS/MS method for the analysis of serotonin and related compounds in urine and the identification of a potential biomarker for attention deficit hyperactivity/hyperkinetic disorder. Anal Bioanal Chem. 2011;401(8):2481-2493 [DOI] [PubMed] [Google Scholar]
- 25.Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33(9):1185-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darnall RA, Schneider RW, Tobia CM, Zemel BM. Arousal from sleep in response to intermittent hypoxia in rat pups is modulated by medullary raphe GABAergic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302(5):R551-R560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pae EK, Yoon AJ, Ahuja B, et al. Perinatal intermittent hypoxia alters γ-aminobutyric acid: a receptor levels in rat cerebellum. Int J Dev Neurosci. 2011;29(8):819-826 [DOI] [PubMed] [Google Scholar]
- 28.Pozdnyakova N, Yatsenko L, Parkhomenko N, Himmelreich N. Perinatal hypoxia induces a long-lasting increase in unstimulated gaba release in rat brain cortex and hippocampus. The protective effect of pyruvate. Neurochem Int. 2011;58(1):14-21 [DOI] [PubMed] [Google Scholar]
- 29.Ørtenblad N, Young JF, Oksbjerg N, Nielsen JH, Lambert IH. Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol. 2003;284(6):C1362-C1373 [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Zhang Q, Wang J, et al. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res. 2009;1279:131-138 [DOI] [PubMed] [Google Scholar]
- 31.Amano H, Maruyama K, Naka M, Tanaka T. Target validation in hypoxia-induced vascular remodeling using transcriptome/metabolome analysis. Pharmacogenomics J. 2003;3(3):183-188 [DOI] [PubMed] [Google Scholar]
- 32.Mankovskaya IN, Serebrovskaya TV, Swanson RJ, Vavilova GL, Kharlamova ON. Mechanisms of taurine antihypoxic and antioxidant action. High Alt Med Biol. 2000;1(2):105-110 [DOI] [PubMed] [Google Scholar]
- 33.Abebe W, Mozaffari MS. Role of taurine in the vasculature: an overview of experimental and human studies. Am J Cardiovasc Dis. 2011;1(3):293-311 [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174(3):307-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair D, Zhang SX, Ramesh V, et al. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am J Respir Crit Care Med. 2011;184(11):1305-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE. 2011;6(5):e19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gozal D, Khalyfa A, Capdevila OS, Kheirandish-Gozal L, Khalyfa AA, Kim J. Cognitive function in prepubertal children with obstructive sleep apnea: a modifying role for NADPH oxidase p22 subunit gene polymorphisms?. Antioxid Redox Signal. 2012;16(2):171-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Z, Miller GM. Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol. 2009;78(9):1095-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandy DK. Trace amine-associated receptor 1-Family archetype or iconoclast?. Pharmacol Ther. 2007;116(3):355-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176(2):188-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576-584 [DOI] [PubMed] [Google Scholar]