Abstract
Background:
CT scanning is increasingly used to characterize COPD. Although it is possible to obtain CT scan-measured lung lobe volumes, normal ranges remain unknown. Using COPDGene data, we developed reference equations for lobar volumes at maximal inflation (total lung capacity [TLC]) and relaxed exhalation (approximating functional residual capacity [FRC]).
Methods:
Linear regression was used to develop race-specific (non-Hispanic white [NHW], African American) reference equations for lobar volumes. Covariates included height and sex. Models were developed in a derivation cohort of 469 subjects with normal pulmonary function and validated in 546 similar subjects. These cohorts were combined to produce final prediction equations, which were applied to 2,191 subjects with old GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage II to IV COPD.
Results:
In the derivation cohort, women had smaller lobar volumes than men. Height positively correlated with lobar volumes. Adjusting for height, NHWs had larger total lung and lobar volumes at TLC than African Americans; at FRC, NHWs only had larger lower lobes. Age and weight had no effect on lobar volumes at TLC but had small effects at FRC. In subjects with COPD at TLC, upper lobes exceeded 100% of predicted values in GOLD II disease; lower lobes were only inflated to this degree in subjects with GOLD IV disease. At FRC, gas trapping was severe irrespective of disease severity and appeared uniform across the lobes.
Conclusions:
Reference equations for lobar volumes may be useful in assessing regional lung dysfunction and how it changes in response to pharmacologic therapies and surgical or endoscopic lung volume reduction.
COPD is characterized by emphysematous destruction of the lung parenchyma and remodeling of the distal small airways.1‐3 Emphysema is associated with hyperinflation, and the combination of emphysema and airway disease leads to gas trapping on exhalation. The degree to which these abnormalities exist for the whole lung can be easily assessed by either plethysmographic- or helium dilution-based measures of lung volume and regression models for predicted values derived from populations of healthy nonsmokers.4 However, given that the abnormalities associated with COPD pathology are not homogeneous, measures capable of assessing regional lung volumes and dysfunction rather than providing “averages” over the entire lung may provide useful information for developing and monitoring targeted therapy.
CT scanning is increasingly used for clinical, epidemiologic, and genetic investigations of COPD.5,6 Using readily available tools, investigators can now report regional and lobe-specific measures of emphysema (ie, percent of lobe that is emphysematous) and volume.7,8 These measures are commonly reported in clinical investigation and have been shown to correlate with spirometric and clinical measures of disease.9‐12 Little attention has been paid, however, to using chest CT scans to determine the normal range of lobe volumes at full inflation and relaxed exhalation. Once such models have been developed, investigators could then determine the absolute amount of lobe-specific hyperinflation present on the inspiratory scan (ie, how much lobe volume exceeds predicted at full inflation) and the lobe-specific volume of gas trapped on an expiratory CT image.
Using data from the COPDGene study, we developed lobe-specific models of CT scan-measured volume at both maximal inflation (total lung capacity [TLC]13,14) and relaxed exhalation (approximating functional residual capacity [FRC]) in normal subjects. We then applied these models to subjects with COPD as a means of quantifying the extent of lobar disease.
Materials and Methods
COPDGene (www.copdgene.org) is a National Heart, Lung, and Blood Institute-funded, multicenter, observational study designed to identify genetic factors associated with COPD and characterize the disease process using high-resolution CT scanning.15 Current and former smokers between the ages of 45 and 80 years with at least a 10 pack-year history of smoking were recruited at 21 clinical centers in the United States. Subjects were non-Hispanic white (NHW) or African American (AA). Additionally, a small cohort of nonsmoking control subjects was recruited as part of the study. All subjects underwent pre- and postbronchodilator spirometry according to American Thoracic Society standards16 and whole-lung volumetric inspiratory (full inspiration) and expiratory (at end of relaxed exhalation) CT scanning as previously described.15,17,18 Briefly, images were acquired using multidetector CT scanners (at least 16 detector channels) and reconstructed using submillimeter slice thickness and a smooth kernel. The following analyses were performed on segmented lung images using Pulmonary Workstation 2 software (VIDA Diagnostics, www.vidadiagnostics.com): total lung inspiratory and expiratory volumes, lobar inspiratory and expiratory volumes, percentage of total lung and lobar emphysema (% of lung volume below an attenuation threshold of −950 Hounsfield units [HU] on inspiratory CT scan [%LAA-950]), and percentage of total lung gas trapping (% of lung volume below −856 HU on expiratory CT scan [%LAA-856]). Demographic information and anthropometrics were also collected. The COPDGene study was approved by the institutional review board at each center, and all participants provided written informed consent. The current analysis was approved by the Partners HealthCare Institutional Review Board (2007P-000554).
Model Development Cohort (Normal Subjects)
The derivation cohort consisted of 92 nonsmoking control subjects with no known lung disease19 and 377 smokers from the first 2,500 COPDGene subjects (January 2011 COPDGene data set) with normal spirometry (postbronchodilator FEV1/FVC > 0.7 and FEV1 > 80% predicted), a race-adjusted CT scan-measured TLC % predicted between 90% and 110%, no self-reported physician diagnosis of asthma, no symptoms consistent with chronic bronchitis,20 and no interstitial abnormalities on CT scan.21
The validation cohort was composed of 546 smokers, from the final COPDGene cohort (10,300 subjects, April 2012 COPDGene data set) who had complete CT scan-measured lobar volume data and who were not part of the derivation group. Subjects in this cohort also met the previously described criteria for normality.
Study Cohort (Subjects With COPD)
This cohort consisted of 2,191 subjects enrolled in the final COPDGene cohort (April 2012 COPDGene data set). Subjects had (1) old GOLD (Global Initiative for Chronic Obstructive Lung Disease)22 stage II or higher fixed airflow obstruction (postbronchodilator FEV1/FVC < 0.7 and FEV1 < 80% predicted), and (2) complete quantitative CT scan measurements of lobar volumes.
Statistical Analysis
Using linear regression, we examined the effects of various demographic and anthropometric factors on CT scan-measured lobar volumes in our derivation cohort. Next, using the same cohort, we used linear regression to create race-specific reference equations for lobar volumes—left lower lobe (LLL), left upper lobe (LUL), right lower lobe (RLL), right middle lobe (RML), and right upper lobe (RUL)—at TLC and FRC. We examined the distributions of CT scan-measured total lung volume at FRC, CT scan-measured total lung volume at TLC, and the ratio of these two values (FRC/TLC). The distributions of both total lung volume at FRC and FRC/TLC had outliers at the upper end of their distributions. To guarantee that outliers did not influence the results, we only used subjects with the middle 95% of FRC/TLC observations to estimate regression models for lobar volumes at FRC.23 Model covariates were chosen based on factors previously shown to impact lung volumes,4,24‐27 their possible biologic connection to lobar volume, and their empirical association with lobar volumes. We assessed the reliability of our reference equations in the validation cohort using a cross-validation analysis.28 For consistency, in this cohort we also trimmed subjects with the upper and lower 2.5% of FRC/TLC values from our lobar volume assessments at FRC. Because the models estimated from the derivation cohort resulted in predicted values for the validation cohort that agreed with the observed values, and subsequent residual diagnostics showed the derivation cohort models were appropriate in the validation cohort, we pooled the data from these two cohorts and report the coefficient estimates based on the combined data.28 Finally, using these lobar volume reference equations, we determined the degree and distribution of lobar hyperinflation and lobar gas trapping in the study cohort by comparing the predicted and CT scan-measured values for lobar volumes at TLC and FRC, respectively. For all analyses, a P value < .05 was considered significant. Data analysis was performed using SAS, version 9.2 (SAS Institute Inc).
Results
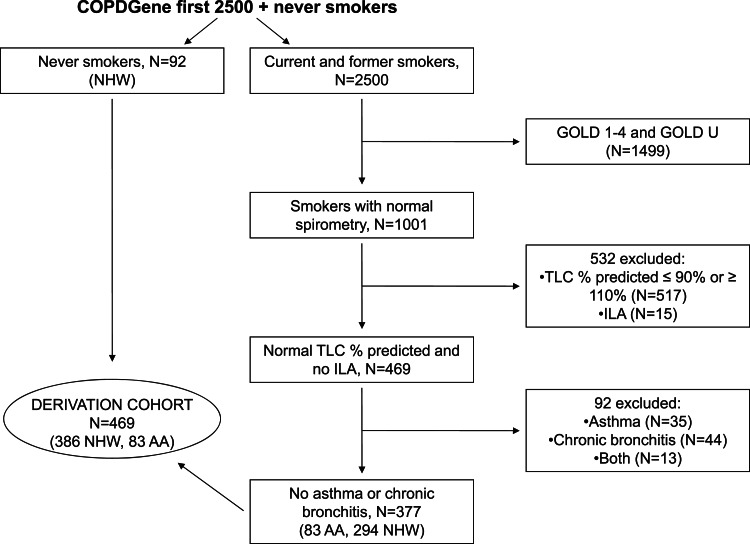
Out of the first 2,500 COPDGene subjects, 469 formed the derivation cohort (Fig 1). Table 1 summarizes the characteristics of the derivation cohort used to create our initial reference equations. Importantly, both races in this cohort had minimal low attenuation areas (%LAA-950) and gas trapping (%LAA-856 on expiratory CT scan) well within the normal range.29
Figure 1.
Consort diagram detailing selection of the model derivation cohort. AA = African American; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD U = FEV1 < 80% predicted with a preserved FEV1/FVC ratio (FEV1/FVC ≥ 0.7); ILA = interstitial lung abnormality; NHW = non-Hispanic white; TLC = total lung capacity (measured on CT scan).
Table 1.
—Characteristics of Subjects in the Derivation Cohort
| Characteristic | NHW (n = 386) | AA (n = 83) |
| Age, y | 61 (54-67) | 51 (48-55) |
| Female sex, No. (%) | 216 (56) | 35 (42) |
| Height, cm | 168.7 (161.5-175.3) | 170.4 (162.9-177.8) |
| Weight, kg | 80.0 (67.0-91.0) | 83.0 (71.0-90.5) |
| BMI, kg/m2 | 27.8 (24.1-31.5) | 27.0 (24.7-30.7) |
| Current smoker, No. (%) | 92 (24) | 73 (88) |
| Pack-y | 29 (11-44) | 33 (21-42) |
| FEV1 % predicted | 97 (90-106) | 101 (92-114) |
| %LAA-950 | 2.5 (0.9-4.9) | 1.7 (0.6-2.9) |
| CT scan-measured TLC, % predicted | 99 (94-105) | 96 (92-102) |
| %LAA-856 on expiratory CT scana | 10.7 (5.8-15.6) | 10.0 (3.9-17.6) |
Data presented as medians and interquartile ranges unless otherwise specified. AA = African American; NHW = non-Hispanic white; %LAA-856 = percentage of low attenuation area (< −856 Hounsfield units) on expiratory CT scan; %LAA-950 = percentage of low attenuation area (< −950 Hounsfield units) on inspiratory CT scan; TLC = total lung capacity.
Missing data for 23 NHW, 5 AA.
In this cohort of normal subjects, men had significantly larger lungs and diffusely larger lobar volumes than women at both TLC and FRC. To determine whether these findings were due to a true sex effect on lobar volumes rather than a height effect, as men tend to be taller than women, we adjusted for height and still found that men had significantly larger total and lobar lung volumes than women at TLC and FRC (P < .0001 for all lobes at TLC, P ≤ .003 for all lobes at FRC) (e-Table 1 (365.7KB, pdf) ).
In normal subjects at TLC, NHW subjects had significantly larger total lung, lower lobe, and RML volumes than AA subjects. Upper lobe volumes were similar between the races. At FRC, NHW subjects tended to have larger lower lobes than AA subjects (relationship significant in the RLL, P = .08 in the LLL), whereas AA subjects tended to have larger upper lobes (relationship significant in the RUL, P = .06 in the LUL). There was no significant difference in total lung volume at FRC between the races. After adjusting for height, total lung volume and all lobes were significantly larger in NHW subjects at TLC. At FRC, the lower lobes were significantly larger in NHW subjects, but the upper lobes and middle lobe were not significantly different between the races (e-Table 2 (365.7KB, pdf) ).
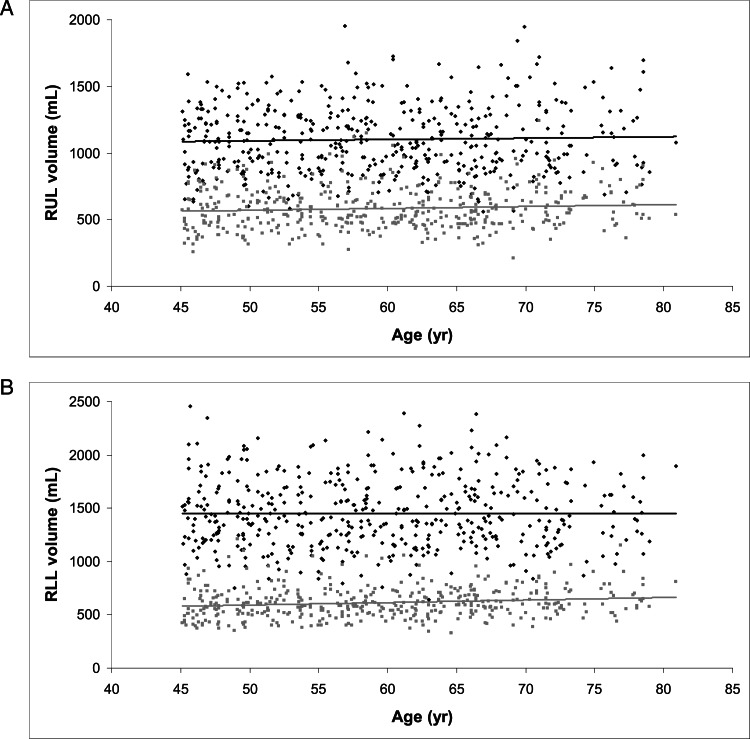
Figure 2 demonstrates the relationship between age and CT scan-measured lobar volume in normal subjects at TLC and FRC for the RUL (Fig 2A) and the RLL (Fig 2B). Age had no effect on lobar volume at TLC in either lobe. At FRC, older age was associated with a higher RLL volume, although the effect was very small (2.3 mL/y increase in RLL volume, P = .002); the relationship between age and RUL volume was nonsignificant. Similar relationships were seen for the LLL and LUL at both TLC and FRC.
Figure 2.
A, Relationship between age and RUL volume at TLC (■, β=1.01, P = .43) and FRC (gray squares, β=1.42, P = .09). B, Relationship between age and RLL volume at TLC (■, β=0.11, P = .95) and FRC (gray squares, β=2.35, P = .002). FRC = functional residual capacity; RLL = right lower lobe; RUL = right upper lobe. See Figure 1 legend for expansion of other abbreviation.
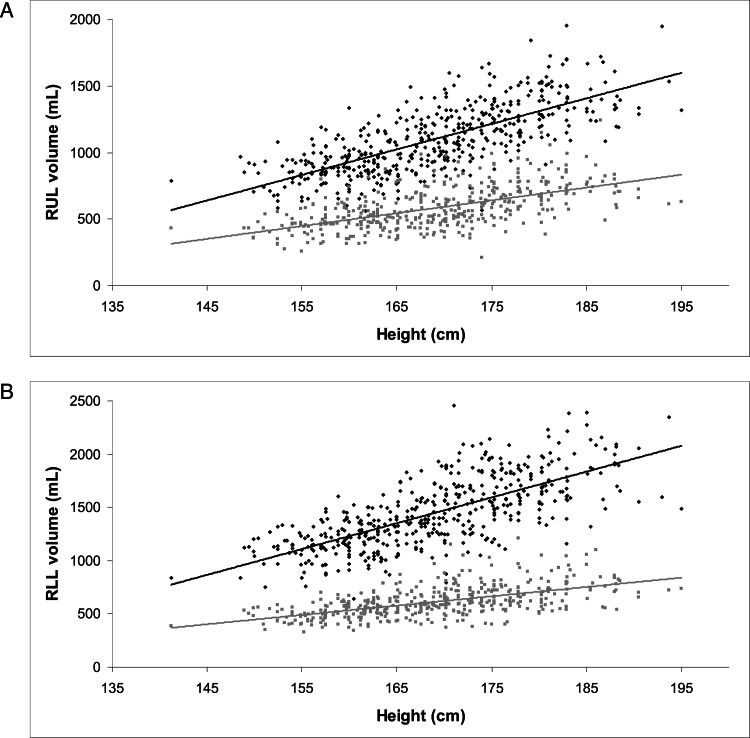
Height was positively correlated with all lobar volumes at both TLC and FRC (Fig 3); height had a greater effect on lobar volumes at TLC than FRC. On univariate analysis, heavier weight was associated with larger volumes in all lobes at both TLC and FRC. After adjusting for height (to ensure that heavier subjects were not simply taller), weight did not have a significant effect on any lobar volume at TLC. At FRC, however, after adjusting for height, increased weight was significantly associated with decreased lobar volume (effect estimates ranged from −0.8 to −1.8 mL/kg) in all lobes except the RML and the LLL.
Figure 3.
A, Relationship between height and RUL volume at TLC (■, β=19.2, P < .0001) and FRC (gray squares, β=9.6, P < .0001). B, Relationship between height and RLL volume at TLC (■, β=24.2, P < .0001) and FRC (gray squares, β=8.8, P < .0001). See Figure 1 and 2 legends for expansion of abbreviations.
Using the derivation cohort, race-specific prediction equations for lobar volumes at TLC and FRC were created (e-Tables 3, 4 (365.7KB, pdf) , respectively). Covariates for TLC equations included height and sex. For NHW subjects, height explained the majority of the variance in lobar volumes. For AA subjects, height explained the majority of the variance in volume only in the lower lobes, whereas sex explained the majority of the variance in the other lobes. Covariates for the FRC equations included height and sex. Although age and weight were associated with some lobar volumes at FRC, they were not included in the prediction equations because they explained very little of the variance (0.15%-3%) in lobar volume. As for TLC, height explained the majority of the variance in lobar volumes for NHW subjects, whereas it explained the majority of variance only in lower lobe volumes for AA subjects.
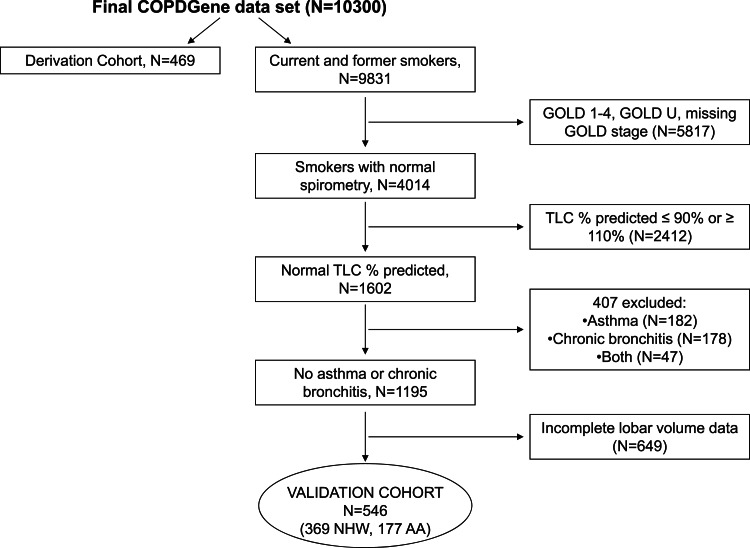
Assembly of the validation cohort is outlined in Figure 4. After confirming the reliability of our lobar volume regression models in this group, we combined the derivation and validation cohorts to produce our final reference equations (Tables 2, 3).
Figure 4.
Consort diagram detailing selection of the model validation cohort. See Figure 1 legend for expansion of abbreviations.
Table 2.
—Prediction Equations for Lobar Volume at Full Inflation by Race: Derivation Cohort and Validation Cohort
| Lobe | Prediction Equation | Model R2 |
| LLL | ||
| NHW (n = 755) | = −1897.26 + 18.82(ht) + 208.18(male) | 0.68 |
| AA (n = 260) | = −1752.02 + 16.43(ht) + 226.84(male) | 0.64 |
| LUL | ||
| NHW (n = 755) | = −1459.08 + 15.90(ht) + 297.56(male) | 0.74 |
| AA (n = 260) | = −1496.77 + 15.53(ht) + 253.84(male) | 0.76 |
| RLL | ||
| NHW (n = 755) | = −1667.14 + 18.20(ht) + 189.14(male) | 0.64 |
| AA (n = 260) | = −1581.19 + 16.07(ht) + 180.09(male) | 0.59 |
| RML | ||
| NHW (n = 755) | = −827.34 + 7.60(ht) + 87.95(male) | 0.55 |
| AA (n = 260) | = −679.79 + 6.30(ht) + 95.56(male) | 0.52 |
| RUL | ||
| NHW (n = 755) | = −858.36 + 11.06(ht) + 248.28(male) | 0.65 |
| AA (n = 260) | = −700.25 + 9.74(ht) + 203.98(male) | 0.56 |
ht = height in cm; LLL = left lower lobe; LUL = left upper lobe; male = 1 for male and 0 for female; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe. See Table 1 legend for expansion of other abbreviations.
Table 3.
—Prediction Equations for Lobar Volume at End of Normal Exhalation by Race: Derivation Cohort and Validation Cohort
| Lobe | Prediction Equation | Model R2 |
| LLL | ||
| NHW (n = 669) | = −842.05 + 8.27(ht) + 45.32(male) | 0.47 |
| AA (n = 210) | = −1020.98 + 8.84(ht) + 70.96(male) | 0.38 |
| LUL | ||
| NHW (n = 669) | = −781.80 + 8.44(ht) + 140.30(male) | 0.47 |
| AA (n = 210) | = −1049.09 + 9.87(ht) + 156.25(male) | 0.40 |
| RLL | ||
| NHW (n = 669) | = −654.55 + 7.53(ht) + 38.26(male) | 0.40 |
| AA (n = 210) | = −1024.50 + 9.26(ht) + 41.34(male) | 0.31 |
| RML | ||
| NHW (n = 669) | = −419.26 + 4.06(ht) + 56.74(male) | 0.41 |
| AA (n = 210) | = −445.56 + 4.05(ht) + 72.68(male) | 0.46 |
| RUL | ||
| NHW (n = 669) | = −434.68 + 5.80(ht) + 109.55(male) | 0.41 |
| AA (n = 210) | = −331.72 + 5.05(ht) + 126.25(male) | 0.29 |
* Fifty-one of 755 NHWs and 39 of 260 AAs did not have lobar volume measurements at the end of normal exhalation; 35 of 755 NHWs and 11 of 260 AAs were excluded because of total lung volume at functional residual capacity to total lung volume at TLC ratios that were outside the middle 95% of the distribution.
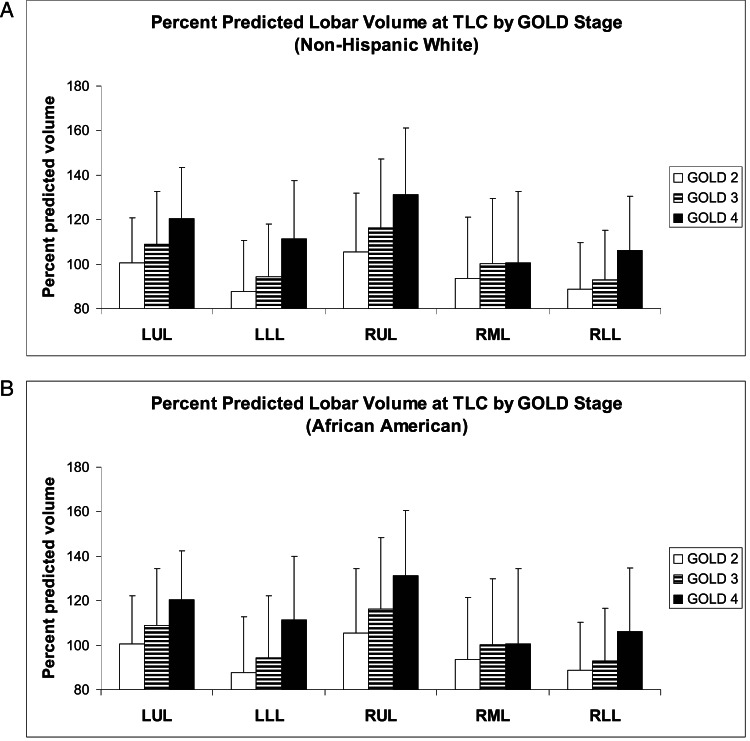
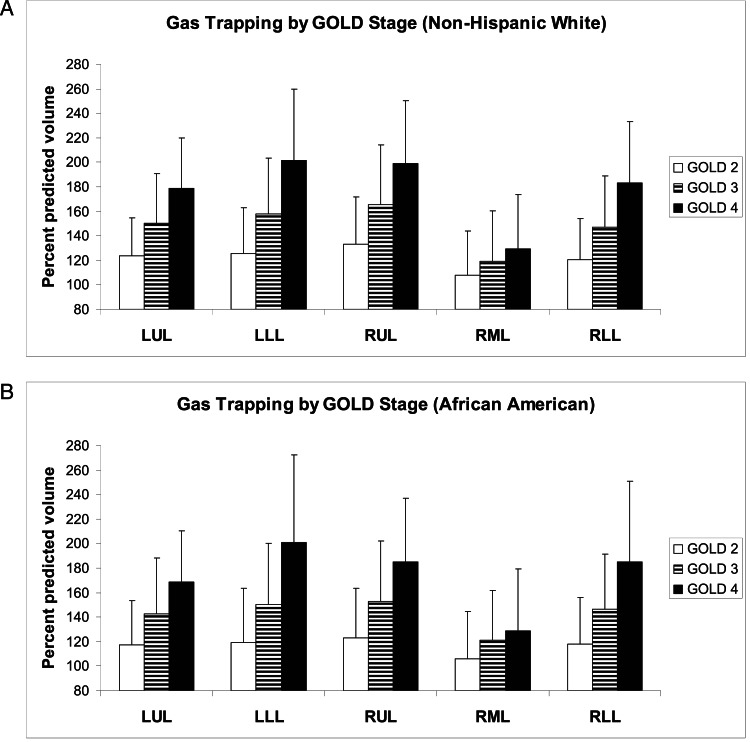
Finally, we compared predicted with measured lobar volumes at TLC and FRC in this study cohort. This cohort was composed of 1,069 subjects with GOLD II (860 NHW, 209 AA), 727 subjects with GOLD III (617 NHW, 110 AA), and 395 subjects with GOLD IV (348 NHW, 47 AA). Figure 5 shows lobar hyperinflation ([measured lobar volume at TLC/predicted lobar volume at TLC] × 100) by GOLD stage for NHWs (Fig 5A) and AAs (Fig 5B). As expected, with increasing GOLD stage, both the upper lobes and the lower lobes progressively enlarge. In both races, the upper lobes exceed 100% of the predicted volume even in subjects wtih GOLD II, whereas in the lower lobes only subjects with GOLD IV exceed 100% of the predicted volume. Overall, the pattern of hyperinflation appears relatively consistent between the races. Additional data available in the online supplement demonstrate a statistically significant correlation between lobar volume and lobar percent emphysema at each GOLD stage (e-Tables 5, 6 (365.7KB, pdf) ). Figure 6 shows lobar gas trapping ([measured lobar volume at FRC/predicted lobar volume at FRC] × 100) by GOLD stage for NHWs (Fig 6A) and AAs (Fig 6B). There is progressive gas trapping with subsequent GOLD stages; however, even GOLD II subjects exhibit significant gas trapping. As with hyperinflation, the pattern of gas trapping with progressive disease appears similar in both races.
Figure 5.
A, Percent predicted lobar volume at TLC ([CT scan-measured lobar volume/predicted lobar volume at TLC] × 100) by GOLD stage for NHW subjects. B, Percent predicted lobar volume at TLC by GOLD stage for AA subjects. The pattern of hyperinflation appears to be relatively consistent between the races: the upper lobes exceed 100% of the predicted volume even in moderate airflow obstruction (GOLD II), whereas the lower lobes only exceed 100% of the predicted volume in very severe disease (GOLD IV). Data are presented as means and SDs. LLL = left lower lobe; LUL = left upper lobe; RML = right middle lobe. See Figure 1 legend for expansion of other abbreviations.
Figure 6.
A, Lobar gas trapping ([CT scan-measured lobar volume/predicted lobar volume at FRC] × 100) by GOLD stage for NHW subjects. B, Lobar gas trapping by GOLD stage for AA subjects. For both races, there is progressive gas trapping with increasing airflow obstruction, and even GOLD II subjects have significant gas trapping. Data are presented as means and SDs. See Figure 1 legend for expansion of abbreviations.
Discussion
Using data from the multicenter COPDGene study, we developed novel reference equations to predict normal CT scan-measured lung lobe volumes at full inflation and relaxed exhalation in NHW and AA without COPD. We then considered a cohort of subjects with COPD and compared measured anatomic CT scan-measured lobar volumes with equation-derived nominal values, thereby allowing physiologic (hyperinflation and gas trapping) assessment. We showed the ability to define regional lung dysfunction and how it correlates with disease severity.
Our analyses relating epidemiologic and anthropometric variables with CT scan-measured lobar volumes in normal subjects yielded results consistent with previously published data correlating these variables with pulmonary function and roentgenographic measures of whole lung volumes.4,24‐26,30 We confirmed a sex effect on lung volumes, with women having universally smaller lobar volumes than their male counterparts. We also confirmed a significant effect of height on all lobar volumes.
Not all variables affected lung lobes uniformly. Similar to prior studies,25,30 after height adjustment, NHWs had larger total lung and lobar volumes at full inspiration than did AAs. However, at FRC, after adjusting for height, only the lower lobes were significantly larger in NHWs. Although the literature suggests several reasons for racial differences in lung volumes (for example, differences in the ratio of trunk length to standing height and chest dimensions),4 lobar heterogeneity has not been described, and its mechanisms and implications require further investigation. In keeping with the TLC and FRC data presented at the American Thoracic Society workshop on lung volume measurements,4 we also found that although age had no effect on lobar volumes at TLC, it did have a small effect at FRC, but only in some lobes. Although this finding may be due to age-related emphysema, we would expect senile emphysema to affect all lobes at FRC equally. Instead, we only found a significant effect of age on lower lobe volumes; to the best of our knowledge, there are no data reporting a regional predilection for development of senile emphysema. Similarly, after adjusting for height, weight was not associated with lobar volumes at TLC but did have a significant effect on some lobar volumes at FRC. Again, although this finding is consistent with prior reports of decreased FRC in overweight subjects attributable to altered chest wall compliance, the reason for lobar heterogeneity is unknown and merits further study.
For the first time to our knowledge, we developed reference equations for lobar volumes at TLC and FRC. Model R2 values were much higher for the equations predicting lobar volumes at TLC than at FRC. This is likely in part because the CT scans were not spirometrically gated, and subjects can likely get to TLC more reliably than FRC. We attempted to account for this by excluding those subjects with FRC/TLC ratios in the tails of the distribution; however, despite this, the reference group still included subjects with FRC values that were likely inaccurate. Adjusting for clinical center improved the predictive ability of some of our equations for lobar volume at FRC. However, doing so had no significant effect on the prediction equations for lobar volume at TLC (data not shown).
Assessment of lobar hyperinflation and lobar gas trapping by degree of airflow limitation in patients with COPD showed that there is progressive expansion of lung lobes at both TLC and FRC with increasing disease severity. The pattern of lobar hyperinflation was similar between races but was nonuniform between lobes. The upper lobes hyperinflate at earlier disease stages and remain more hyperinflated than the lower lobes as disease progresses. This finding is consistent with hyperinflation being primarily due to loss of elastic recoil and smoking-related emphysema tending to be most prominent in upper lobes.31,32 The pattern of gas trapping was similar between the races and more uniform across the lobes, likely because gas trapping is influenced not only by emphysema but also by airway disease.
We believe that lobar volume assessments add information beyond that of densitometric analysis. First, unlike density, volume is not affected by the alterations in parenchymal perfusion that can be seen in advanced lung disease. Second, although parenchymal destruction is captured by densitometry, the extent to which this destruction causes loss of elastic recoil with subsequent hyperinflation and physiologic impairment is not. Indeed, our data suggest that lobar percent emphysema is an imperfect correlate to lobe volume and more importantly to lobe hyperinflation (ie, emphysema explains < 5% up to approximately 28% of the variability observed in lobar hyperinflation) (e-Table 6 (365.7KB, pdf) ). It has been shown that patients with upper lobe-predominant emphysema derive the most benefit from lung volume reduction surgery.33 Perhaps, however, outcomes from volume reduction would be further optimized by targeting those with the most hyperinflated upper lobes and/or those with the least hyperinflated lower lobes.
We acknowledge limitations in this study. To increase our sample size for developing reference equations, we included current and former smokers in the model development cohort. Although smoking could certainly affect lobar volumes, we attempted to minimize the effect of smoking by only including subjects with normal spirometry, a CT scan-measured TLC well within the normal range (90% to 110% predicted), and no significant pulmonary symptoms. On univariate analysis, there was a small (0.9-2.5 mL/pack-y at TLC; 0.6-1.7 mL/pack-y at FRC) but significant effect of pack-years on some lobar volumes. However, pack-years was either not significant on multivariate analysis or did not contribute significantly to the model R2. A second limitation is that this population only included NHWs and AAs; thus, our reference equations cannot be applied to other races. Likewise, the equations only apply to subjects aged 45 to 80 years with a height range of 151 to 187 cm for AA women, 140 to 181 cm for NHW women, 159 to 195 cm for AA men, and 159 to 198 cm for NHW men. A third limitation is that the CT scans were not spirometrically gated. However, examination of data from a single center demonstrated strong correlation between physiologic and CT scanning measures of TLC and FRC (n = 363, r = 0.92, P < .0001, and n = 348, r = 0.88, P < .0001, respectively). Finally, lobar volumes may be inaccurate in subjects with severe COPD with fissure disruption. Although this may have affected our findings in the study cohort, this should have had less of an effect in the model-development cohort assuming that loss of fissure integrity is a disease-related process. Additionally, the VIDA lobar segmentation algorithm includes a visual quality control step, which would have limited gross mislabeling of fissure location.34
In summary, this study provides novel reference equations for CT scan-measured lung lobe volumes, allowing physiologic information (hyperinflation and gas trapping) to be derived from anatomic metrics. As we show here, these tools may be useful for better assessing regional disease severity and progression, and they may have therapeutic implications. Although prior studies of bronchoscopic lung volume reduction examined anatomic changes in regional and lobar lung volumes,10,35 appreciation of regional physiologic changes was not possible. Improved understanding of local hyperinflation and gas trapping may lead to improved therapeutic targeting and may be useful in predicting response to surgical or bronchoscopic lung volume reduction. Additionally, measurements of lobar hyperinflation and gas trapping may be valuable for determining the location and effect of inhaled drug therapy.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Come takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Come: contributed to the creation and final approval of this manuscript.
Dr Diaz: contributed to the creation and final approval of this manuscript.
Dr Curran-Everett: contributed to providing important input for the statistical analyses and to the creation and final approval of this manuscript.
Ms Muralidhar: contributed to the creation and final approval of this manuscript.
Dr Hersh: contributed to the creation and final approval of this manuscript.
Mr Zach: contributed to processing the imaging data and to the creation and final approval of this manuscript.
Dr Schroeder: contributed to processing the imaging data and to the creation and final approval of this manuscript.
Dr Lynch: contributed to processing the imaging data and to the creation and final approval of this manuscript.
Dr Celli: contributed to the creation and final approval of this manuscript.
Dr Washko: contributed to the creation and final approval of this manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hersh has received lecture fees from Novartis AG. Dr Lynch has received research support from Siemens AG and Janssen Biotech, Inc and consulting fees from InterMune, Gilead, and Perceptive Informatics, Inc. Dr Celli is on the advisory boards of GlaxoSmithKline plc, Boehringer-Ingelheim GmbH, AstraZeneca, and Almirall, SA. He also has a grant from AstraZeneca to study cardiac function in patients with COPD. Dr Washko has received consulting fees from Spiration, Inc, and his spouse is an employee of Merck & Co, Inc. Drs Come, Diaz, Curran-Everett, and Schroeder, Ms Muralidhar, and Mr Zach have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Tables can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- AA
African American
- FRC
functional residual capacity
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HU
Hounsfield Units
- LLL
left lower lobe
- LUL
left upper lobe
- NHW
non-Hispanic white
- %LAA-856
% of total lung volume falling below an attenuation threshold of −856 Hounsfield Units on expiratory CT scan
- %LAA-950
% of total lung volume falling below an attenuation threshold of −950 Hounsfield Units on inspiratory CT scan
- RLL
right lower lobe
- RML
right middle lobe
- RUL
right upper lobe
- TLC
total lung capacity
Footnotes
Funding/Support: The COPDGene study is funded by the National Institutes of Health (NIH) [Grants U01HL089897 and U01HL089856]. This work was supported by the NIH [Grant T32HL007633-26 to Dr Come and K23HL089353 to Dr Washko].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278(25):1355-1360 [DOI] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653 [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709-721 [DOI] [PubMed] [Google Scholar]
- 4.Stocks J, Quanjer PH; Official Statement of The European Respiratory Society Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8(3):492-506 [DOI] [PubMed] [Google Scholar]
- 5.Washko GR. Diagnostic imaging in COPD. Semin Respir Crit Care Med. 2010;31(3):276-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka S, Yamashiro T, Washko GR, Kurihara Y, Nakajima Y, Hatabu H. Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics. 2010;30(1):55-66 [DOI] [PubMed] [Google Scholar]
- 7.Revel MP, Faivre JB, Remy-Jardin M, et al. Automated lobar quantification of emphysema in patients with severe COPD. Eur Radiol. 2008;18(12):2723-2730 [DOI] [PubMed] [Google Scholar]
- 8.Brown MS, Kim HJ, Abtin F, et al. Reproducibility of lung and lobar volume measurements using computed tomography. Acad Radiol. 2010;17(3):316-322 [DOI] [PubMed] [Google Scholar]
- 9.Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172-1178 [DOI] [PubMed] [Google Scholar]
- 10.Coxson HO, Nasute Fauerbach PV, Storness-Bliss C, et al. Computed tomography assessment of lung volume changes after bronchial valve treatment. Eur Respir J. 2008;32(6):1443-1450 [DOI] [PubMed] [Google Scholar]
- 11.Sciurba FC, Ernst A, Herth FJ, et al. ; VENT Study Research Group A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233-1244 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo K, Iwano S, Okada T, Koike W, Naganawa S. 3D-CT lung volumetry using multidetector row computed tomography: pulmonary function of each anatomic lobe. J Thorac Imaging. 2012;27(3):164-170 [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD?. Chest. 2010;137(5):1108-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwano S, Okada T, Satake H, Naganawa S. 3D-CT volumetry of the lung using multidetector row CT: comparison with pulmonary function tests. Acad Radiol. 2009;16(3):250-256 [DOI] [PubMed] [Google Scholar]
- 15.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107-1136 [DOI] [PubMed] [Google Scholar]
- 17.Han MK, Kazerooni EA, Lynch DA, et al. ; COPDGene Investigators Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz AA, Come CE, Ross JC, et al. ; COPDGene Investigators Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest. 2012;141(3):736-744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zach JA, Newell JD, Jr, Schroeder J, et al. ; COPDGene Investigators Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47(10):596-602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim V, Han MK, Vance GB, et al. ; COPDGene Investigators The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140(3):626-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washko GR, Hunninghake GM, Fernandez IE, et al. ; COPDGene Investigators Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabe KF, Hurd S, Anzueto A, et al. ; Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555 [DOI] [PubMed] [Google Scholar]
- 23.Kotz SJN, Read CB. eds. D R. Trimming and Winsorization. In: Encyclopedia of Statistical Sciences New York, NY: John Wiley & Sons; 1988;348-353 [Google Scholar]
- 24.American Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144(5):1202-1218 [DOI] [PubMed] [Google Scholar]
- 25.Lapp NL, Amandus HE, Hall R, Morgan WK. Lung volumes and flow rates in black and white subjects. Thorax. 1974;29(2):185-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128(3):501-506 [DOI] [PubMed] [Google Scholar]
- 27.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130(3):827-833 [DOI] [PubMed] [Google Scholar]
- 28.Kleinbaum DG, Kupper LL, Nizam A, Muller KE. Applied Regression Analysis and Other Multivariable Methods. 4th ed. Belmont, CA: Duxbury Press, 1998;398-406. [Google Scholar]
- 29.Barr RG, Berkowitz EA, Bigazzi F, et al. ; COPDGene CT Workshop Group A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien RJ, Drizd TA. Roentgenographic determination of total lung capacity: normal values from a National Population Survey. Am Rev Respir Dis. 1983;128(5):949-952 [DOI] [PubMed] [Google Scholar]
- 31.Snider GL. Emphysema: the first two centuries—and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992;146(5 pt 1):1334-1344 [DOI] [PubMed] [Google Scholar]
- 32.Akuthota P, Litmanovich D, Zutler M, et al. An evidence-based estimate on the size of the potential patient pool for lung volume reduction surgery. Ann Thorac Surg. 2012;94(1):205-211 [DOI] [PubMed] [Google Scholar]
- 33.Fishman A, Martinez F, Naunheim K, et al. ; National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059-2073 [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Hoffman EA, Reinhardt JM. Atlas-driven lung lobe segmentation in volumetric X-ray CT images. IEEE Trans Med Imaging. 2006;25(1):1-16 [DOI] [PubMed] [Google Scholar]
- 35.Brown MS, Kim HJ, Abtin FG, et al. Emphysema lung lobe volume reduction: effects on the ipsilateral and contralateral lobes. Eur Radiol. 2012;22(7):1547-1555 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement