Abstract
Background:
[18F]-2-fluoro-2-deoxyglucose (FDG)-PET scan uptake is increased in areas of fibrosis and honeycombing in patients with idiopathic pulmonary fibrosis (IPF). Glucose transporter-1 (Glut-1) is known to be the main transporter for FDG. There is a paucity of data regarding the distribution of Glut-1 and the cells responsible for FDG binding in fibrotic lung diseases.
Methods:
We applied immunofluorescence to localize Glut-1 in normal, IPF, and Hermansky-Pudlak syndrome (HPS) pulmonary fibrosis lung tissue specimens as well as an array of 19 different lung neoplasms. In addition, we investigated Glut-1 expression in inflammatory cells from BAL fluid (BALF) from healthy volunteers, subjects with IPF, and subjects with HPS pulmonary fibrosis.
Results:
In normal lung tissue, Glut-1 immunoreactivity was seen on the surface of erythrocytes. In tissue sections from fibrotic lung diseases (IPF and HPS pulmonary fibrosis), Glut-1 immunoreactivity was present on the surface of erythrocytes and inflammatory cells. BALF inflammatory cells from healthy control subjects showed no immunoreactivity; BALF cells from subjects with IPF and HPS pulmonary fibrosis showed Glut-1 immunoreactivity associated with neutrophils and alveolar macrophages.
Conclusions:
Glut-1 transporter expression in normal lung is limited to erythrocytes. In fibrotic lung, erythrocytes and inflammatory cells express Glut-1. Together, these data suggest that FDG-PET scan uptake in IPF could be explained by enhanced inflammatory and erythrocytes uptake due to neovascularization seen in IPF and not an upregulation of metabolic rate in pneumocytes. Thus, FDG-PET scan may detect inflammation and neovascularization in lung fibrosis.
Fibrotic lung diseases, such as idiopathic pulmonary fibrosis (IPF) and Hermansky-Pudlak syndrome (HPS) pulmonary fibrosis, are generally progressive and lead to death from respiratory failure.1,2 The molecular and cellular mechanisms responsible for the initiation and development of lung fibrosis are complex and incompletely understood. Pulmonary fibrosis is associated with abnormal interstitial accumulations of extracellular matrix proteins and mesenchymal cells.3 Usual interstitial pneumonia, the pathologic hallmark of IPF, is characterized by heterogeneity, with areas of normal lung alternating with abnormal fibrotic areas. Fibroblastic foci, type 2 cell hyperplasia, and honeycombing are found within fibrotic regions in lung tissue from patients with IPF.4 Abnormal blood vessels and lymphatics resulting from angiogenesis and lymphangiogenesis, respectively, within fibrotic areas of the lung have also been reported in IPF.5
HPS is an autosomal recessive disorder characterized by improper biogenesis of lysosome-related organelles.6 Patients with any of the nine known subtypes of HPS exhibit oculocutaneous albinism and platelet dysfunction of varying severity.7,8 Some patients with HPS manifest granulomatous colitis, and interstitial lung disease and pulmonary fibrosis may develop in children and young adults with HPS-2.6,9 Pulmonary fibrosis is more commonly found in adults with HPS-1 and HPS-4, which are HPS subtypes associated with abnormal biogenesis of lysosome-related organelles complex-3.9 HPS pulmonary fibrosis and IPF share some clinical, radiographic,1 and pathologic features.10 Notably, however, HPS pulmonary fibrosis usually leads to death at a much earlier age than IPF.11
PET scan using [18F]-2-fluoro-2-deoxyglucose (FDG) is useful for diagnostic purposes to detect metabolic activity of tissues and for assessment of responses to therapy in several lung diseases, including sarcoidosis12 and pulmonary hypertension.8 FDG-PET scan has more limited usefulness in other pulmonary diseases, such as lymphangioleiomyomatosis.13 FDG-PET scan was evaluated in interstitial lung diseases, pulmonary fibrosis in particular.14 Specifically, increased radionuclide uptake occurs in areas of honeycombing compared with areas of ground-glass infiltrates, which suggests that regions of honeycombing and fibrosis may be more biologically active than previously believed.14
Glucose transporter-1 (Glut-1), the predominant glucose transporter in the lung, is responsible for FDG uptake.14,15 To determine the cause of increased FDG uptake in pulmonary fibrosis, we undertook an immunohistochemical analysis of normal and fibrotic lung disease. We show that Glut-1 is predominantly expressed on the surface of erythrocytes and inflammatory cells, such as neutrophils and macrophages. These findings suggest that the enhanced FDG-PET scan uptake in pulmonary fibrosis is a function of an increase in inflammatory cells as well as an increase in lung parenchymal vasculature due to angiogenesis in fibrotic areas, rather than upregulation of the metabolic rate of fibrotic lung as previously suggested.14
Materials and Methods
Subject Selection
Subjects with IPF, subjects with HPS, and healthy research volunteers were enrolled in protocols approved by the institutional review boards of the National Heart, Lung, and Blood Institute (96-H-0100) and National Human Genome Research Institute (04-HG-0211). The diagnosis of IPF was established according to published criteria.4 Subjects with HPS were diagnosed by platelet electron microscopy and genotyping; pulmonary fibrosis was diagnosed by characteristic chest CT scan findings.1 Healthy volunteers had normal pulmonary function tests and chest radiographs and no clinical evidence of lung disease. Characteristics of subjects enrolled in these studies are listed in Table 1. BAL was performed as previously described.5 Cytospins of BAL fluid (BALF) cells were stained with Diffquik (Siemens Healthcare Diagnostics Inc), and differential cell counts were performed (Table 1). Lung tissue specimens were obtained from clinically indicated procedures. Lung sections from subjects with IPF were obtained from explanted lungs (n = 6) or from surgical biopsies (n = 6), including a subject with IPF who underwent a medically indicated PET-CT scan and an open lung biopsy. Lung sections from subjects with HPS pulmonary fibrosis (n = 3) were obtained from explanted lungs. An array of normal lung tissue and 19 different lung tumors was purchased from US Biomax Inc (Table 2).
Table 1.
—Subject Characteristics for BALF Cell Analyses
| Characteristics | Healthy Volunteers (n = 5) | IPF (n = 5) | HPSPF (n = 3) | P Value |
| Age, mean (SD), y | 55.8 (4.8) | 65.2 (12.0) | 40.6 (9.7) | .015a |
| Male (female) | 4 (1) | 4 (1) | 0 (3) | … |
| FVC % predicted | 99.6 (8.42) | 87.7 (25.6) | 90 (18) | .599 |
| TLC % predicted | 99.6 (9.79) | 82.3 (19.0) | 75.3 (24.6) | .165 |
| Dlco % predicted | 105 (9.1) | 53.4 (18.4) | 79.3 (16.3) | .001b |
| FEV1/FVC | 77 (6.4) | 82.4 (8.15) | 85.5 (9.0) | .324 |
| BAL Neutrophil, % | 1.26 (0.8) | 5.7 (7.4) | 3.4 (2.1) | .379 |
BALF = BAL fluid; Dlco = diffusing capacity of the lung for carbon monoxide; HPSPF = Hermansky-Pudlak syndrome pulmonary fibrosis; IPF = idiopathic pulmonary fibrosis; TLC = total lung capacity.
HPSPF compared with IPF and healthy volunteers.
IPF compared with HPSPF and healthy volunteers.
Table 2.
—Lung Tissue and Cytospins Used in These Studies
| Material | Lung Tissue | Cytospins |
| IPF lung explants | 6 | … |
| IPF biopsy | 6 | 5 |
| HPSPF explants | 3 | 5 |
| Normal lung | 3 | 5 |
| Lung malignancy | 19 | … |
See Table 1 legend for expansion of abbreviations.
Glut-1 Immunostaining and Confocal Microscopy
Lung sections were deparaffinized and rehydrated in graded alcohol. Sections or cytospins were fixed with 4% paraformaldehyde and then incubated with 1% goat serum or 1% human serum, respectively, to block nonspecific antibody binding. After blocking, sections (or cytospins) were incubated with either anti-Glut-1 antibody (1:100 dilution; Abcam plc) overnight at 4°C or with phosphate-buffered saline replacing the primary antibody. After washing, the slides were incubated with the secondary fluorescein isothiocyanate-labeled goat anti-rabbit antibody (1:100 dilution; Vector Laboratories, Inc) for 1 h at room temperature. Slides were then prepared with mounting medium containing 4,6-diamidino-2-phenylindole-2-HCl (Vector Laboratories, Inc), and fluorescence was examined using a 40× oil objective. Images were collected using a Zeiss 510 laser-scanning confocal microscope (Carl Zeiss Microscopy GmbH). Consecutive lung sections were stained with hematoxylin and eosin to visualize the same fields that were imaged with laser scanning confocal microscopy. Composite figures were assembled with Adobe Photoshop software (Adobe Systems Incorporated).
Quantification of Fluorescence Intensity
To quantify fluorescence intensities, confocal images were collected using the same instrument settings for all experimental conditions and imported in Imaris (v7.1). A 100-pixel-square box was cropped from each image over erythrocytes only. Mean Glut-1 fluorescence intensity per pixel was measured and exported in Excel (Microsoft Corporation). Fluorescence intensity is expressed in arbitrary units.
Results
Increased Glucose Uptake in the Lung in IPF
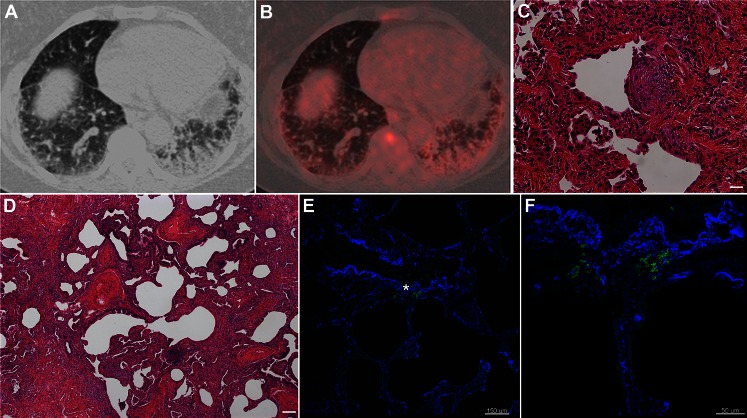
A subject with a history of interstitial lung disease, suggestive of IPF, underwent a PET-CT scan because of concern about possible malignancy of a small subpleural mass. The PET-CT scan showed increased FDG uptake in the regions of honeycombing and fibrosis (Figs 1A, 1B). The patient subsequently underwent a video-assisted thoracoscopic biopsy, and the pathologic diagnosis was consistent with usual interstitial pneumonia (Figs 1C, 1D). Tissue sections from this open lung biopsy were incubated with anti-Glut-1 antibody, and immunofluorescence showed the presence of Glut-1 in erythrocytes and inflammatory cells (Fig 1E). Mesenchymal cells in fibroblastic foci were negative for Glut-1, and areas of honeycombing showed Glut-1 positivity only in erythrocytes (Fig 1F).
Figure 1.
Increased glucose uptake in the lung in idiopathic pulmonary fibrosis. A, CT scan showing increased uptake in the areas of honeycombing and fibrosis. B, Axial attenuation corrected [18F]-2-fluoro-2-deoxyglucose PET scan showing increased uptake in the areas of honeycombing and fibrosis. C, Hematoxylin and eosin (H&E) staining of a lung biopsy tissue section from the same patient shows a fibroblastic focus (magnification × 20, size bar 20 μm). D, H&E staining of a lung biopsy tissue section from the same patient shows areas of honeycombing (magnification × 10, size bar 100 μm). E, Immunofluorescence staining of paraffin-embedded sections of the same biopsy presented as a merged image of glucose transporter-1 (Glut-1; green) and 4,6-diamidino-2-phenylindole-2-HCl (blue) of an area of honeycombing shows Glut-1 staining associated with erythrocytes. F, High-power magnification of selected area in E (*).
Glut-1 Staining in Normal and Fibrotic Lung
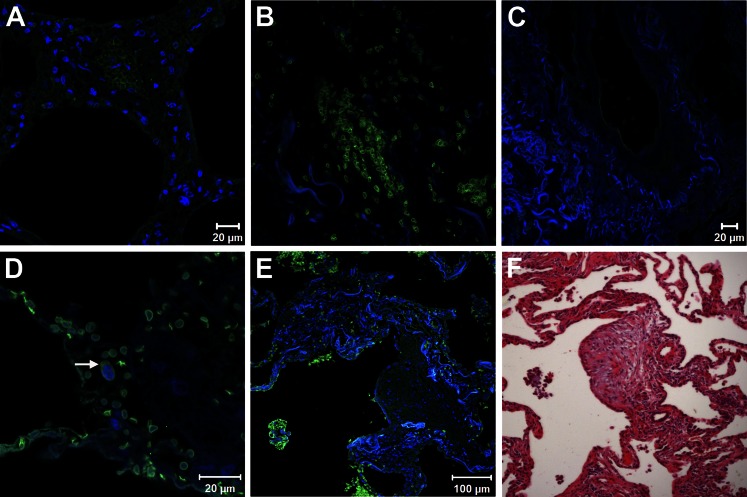
In normal lung tissue, Glut-1 immunofluorescence was limited to erythrocytes; no other cells were reactive with anti-Glut-1 antibody (Fig 2A). In IPF lung sections, erythrocytes were the predominant cells expressing Glut-1; some inflammatory cells were also positive for Glut-1 immunofluorescence (Fig 2D). In HPS pulmonary fibrosis lung tissue, erythrocytes and some inflammatory cells expressed Glut-1 (Fig 2B); no immunostaining was found in control tissue sections (Fig 2C). To examine the possibility that erythrocytes from patients with IPF and HPS expressed more Glut-1 on cell surface compared with erythrocytes from healthy volunteers, we measured mean fluorescence intensity of Glut-1 transporters over erythrocytes and did not find any significant difference (P = .7). Fibroblastic foci, which are devoid of blood vessels,5,16 did not show any reactivity with anti-Glut-1 antibodies (Figs 2E, 2F).
Figure 2.
Glucose transporter-1 (Glut-1) expression in normal and fibrotic lung. A, B, Immunofluorescence staining of paraffin-embedded sections of lung tissue shown as merged image of Glut-1 (green) and 4,6-diamidino-2-phenylindole-2-HCl (blue) demonstrates Glut-1 expression in erythrocytes. A, Normal lung (n = 3; representative shown). B, Hermansky-Pudlak syndrome (HPS) pulmonary fibrosis lung (n = 3; representative shown). C, Omission of the first antibody shows complete absence of fluorescence signal in an HPS pulmonary fibrosis lung. D, Idiopathic pulmonary fibrosis (IPF) (n = 12; representative shown) lung shows Glut-1 expression associated with erythrocytes and weaker staining of neutrophils (arrow). E, Fibroblastic focus from IPF lung section demonstrates absence of Glut-1 staining of mesenchymal cells and positive Glut-1 staining of surrounding erythrocytes. F, hematoxylin and eosin image of consecutive lung sections showing the fibroblastic focus.
In marked contrast, sections of different lung neoplasms without fibrosis showed pronounced immunoreactivity with anti-Glut-1 antibodies. Tissues from adenosquamous carcinoma, squamous cell carcinoma, and adenocarcinoma showed increased reactivity with anti-Glut-1 antibody associated with neoplastic cells (Figs 3A‐3C, respectively). Further, lung tissue from bronchoalveolar carcinoma, a neoplasm that typically does not show increased PET scan uptake, exhibited anti-Glut-1 antibody reactivity with erythrocytes and not with malignant cells (Fig 3D). These findings support the contention that PET scan uptake is associated with cells expressing Glut-1.
Figure 3.
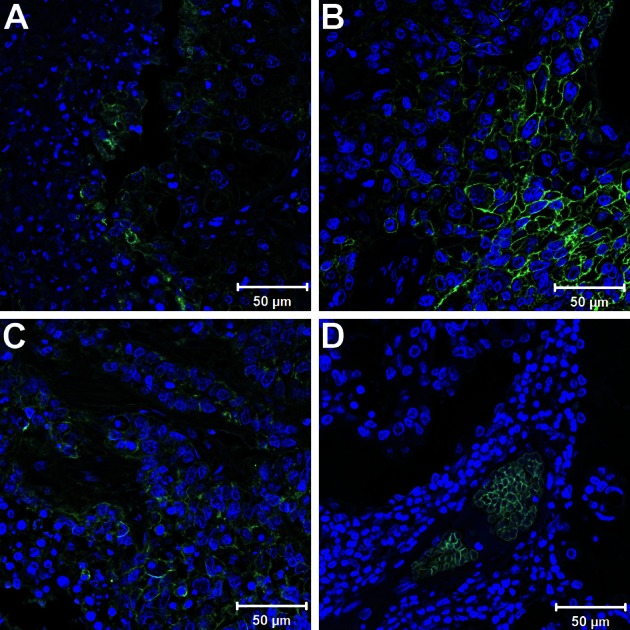
Glucose transporter-1 (Glut-1) staining of different lung neoplasms. A-C, Immunofluorescence staining of paraffin-embedded sections shown as merged image of Glut-1 (green) and 4,6-diamidino-2-phenylindole-2-HCl (blue) demonstrates positive surface Glut-1 lung tumor staining in adenosquamous carcinoma (A), squamous cell carcinoma (B), and adenocarcinoma (C). D, In contrast, sections from bronchoalveolar carcinoma show Glut-1 staining in erythrocytes, but not in malignant cells.
Glut-1 Staining in BALF Cells
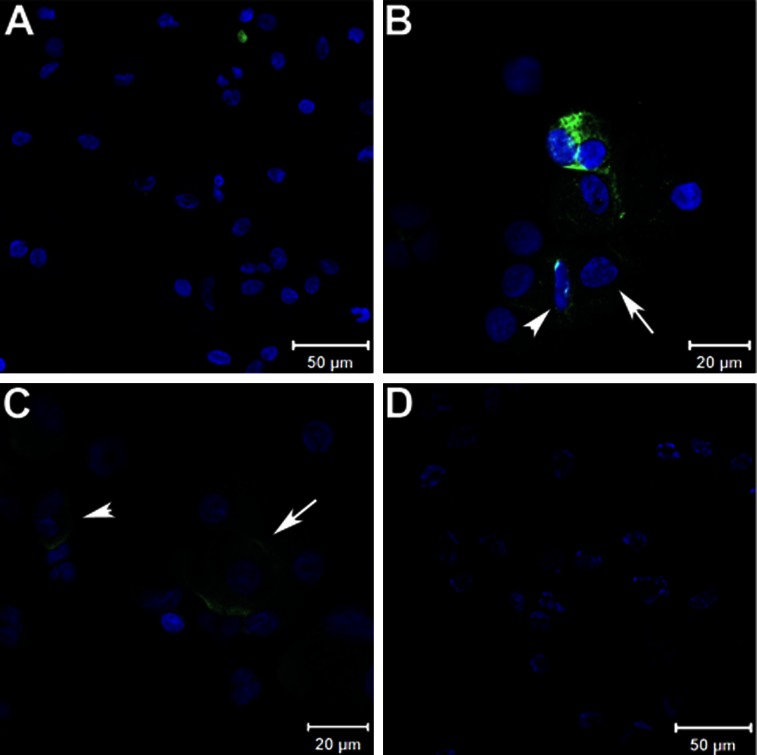
To determine which inflammatory cells in the lung express Glut-1, cytospin preparation of cells isolated from BALF were immunostained using anti-Glut-1 antibody. BALF cells from healthy volunteers showed evidence of Glut-1 staining associated with erythrocytes and not with inflammatory cells (Fig 4A). In contrast, cells isolated from BALF from subjects with HPS pulmonary fibrosis (Fig 4B) and IPF (Fig 4C) showed reactivity with anti-Glut-1 antibodies associated with alveolar macrophages and neutrophils.
Figure 4.
Glucose transporter-1 (Glut-1) staining of BAL cell cytospins. A, Representative field of cytospin slide stained for Glut-1 from a healthy volunteer, showing positive staining of an erythrocyte but not inflammatory cells. B, Representative field of cytospin slide stained for Glut-1 from a patient with Hermansky-Pudlak syndrome pulmonary fibrosis, showing positive alveolar macrophages (arrow) and neutrophil (arrowhead). C, Representative field of cytospin slide stained for Glut-1 from a patient with idiopathic pulmonary fibrosis (IPF), showing positive alveolar macrophages (arrow) and neutrophils (arrowhead) (n = 5 for each group; representative case shown). D, A negative control from a patient with IPF.
Discussion
In this report, we show that Glut-1 immunoreactivity in fibrotic lung disease localizes to erythrocytes and inflammatory cells and not fibroblasts, epithelial cells, or endothelial cells. Our data suggest that increased FDG uptake on PET scans in fibrotic lung disease is associated with Glut-1 expression by immune cells and erythrocytes and not with enhancement of the metabolic rate of epithelial cells as previously postulated.
Evidence from lung tissue and BALF cell analyses show that in fibrotic lung disease, inflammatory cells express Glut-1, which could contribute to increased PET scan uptake. In contrast, Glut-1 immunofluorescence in healthy volunteers was found to be associated with erythrocytes and not with immune cells in the lung. The absence of Glut-1 expression by normal lung inflammatory cells is consistent with previous reports of inherent differences between the immune cells of patients with lung fibrosis and those of healthy control subjects.5,17,18 In agreement with our data generated using diseased human tissue, FDG localized to lung neutrophils after IV administration in bleomycin-induced pulmonary fibrosis.19 Taken together, these results suggest that inflammatory cells and erythrocytes are critical for FDG-PET scan uptake in fibrotic lung diseases.
Angiogenesis, or the formation of new blood vessels, is a key process in tissue repair and results from the net effects of antiangiogenic and proangiogenic factors.20 The complex molecular signaling pathways stimulating and inhibiting angiogenesis in lung fibrosis have recently been reviewed.21 The net result is that fibroblastic foci and areas of intense fibrosis are nearly devoid of blood vessels, and areas of honeycombing show increased vascularity.16,22 This distribution mirrors that of vascular endothelial growth factor-A22,23 and hypoxia inducible factor-1α,23 which were shown to be increased in alveolar epithelial cells and decreased in fibroblastic foci.
Erythrocytes, which express Glut-1 in normal and fibrotic lung, are not reported to express vascular endothelial growth factor-A or hypoxia inducible factor-1α.24 Glut-1 is the only glucose transporter expressed at the surface of erythrocytes.25 Further, Glut-1 in erythrocytes is not translocated and is consistently present on the cell surface.3 Our data showing similar Glut-1 fluorescence intensity on the cell surface of erythrocytes in healthy and fibrotic lungs suggest that increased Glut-1 expression in fibrotic lung disease is due to changes in vascular development and increased erythrocyte availability. Overall, these changes lead to enhanced FDG uptake in the areas of honeycombing seen on PET-CT scans in patients with pulmonary fibrosis.
Glut-1 expression was reported in hepatic fibrosis. In cirrhosis, Glut-1 staining was similar to normal liver. Specifically, immunoreactivity for Glut-1 was found in endothelial cells of portal arteries and veins and not hepatocytes.26 Our finding of increased Glut-1 immunostaining associated with inflammatory cells and not with the endothelial or epithelial cells of lung fibrosis contrasts markedly with a published report of Glut-1 expression in pulmonary hypertension.8 In that disorder, Glut-1 is found in pulmonary artery endothelial cells and smooth muscle cells but not inflammatory cells.8 Thus, although the glycolytic rate in pulmonary hypertension may be increased, and PET scans may be a useful method to follow disease activity and response to therapy,8 the usefulness of PET scans in managing patients with pulmonary fibrosis remains unclear and should be addressed.
It is possible that other Glut transporters could be involved in FDG uptake.27 We found that lung malignancies known to be PET scan avid (eg, adenocarcinoma, squamous cell carcinoma) show increased Glut-1 expression, and a cancer that is recognized as non-PET avid (ie, bronchoalveolar carcinoma)28‐30 shows a marked absence of Glut-1 expression. Moreover, our results suggest that Glut-1 is an important determinant of FDG uptake in PET imaging of the fibrotic lung. In addition, FDG-PET uptake in fibrotic lung diseases appears to be driven by inflammatory cells and erythrocytes within regions of neovascularization and not by an increased metabolic rate of epithelial cells. Further studies are needed to determine the usefulness of FDG-PET in the clinical management of patients with fibrotic lung diseases. As the role of blood vessel formation in pulmonary fibrosis is further defined, FDG-PET may be a potential modality to noninvasively measure inflammation and/or erythrocyte binding as a surrogate for monitoring abnormal blood vasculature formation in fibrotic disorders of the lung.
Acknowledgments
Author contributions: Drs El-Chemaly and Gochuico are the guarantors of the paper and take responsibility for the integrity of the work as a whole, from inception to published article.
Dr El-Chemaly: contributed to designing and performing the research, analyzing data, and writing the manuscript.
Dr Malide: contributed to designing and performing the research, analyzing data, and writing the manuscript.
Dr Yao: contributed to analyzing the data and revision of the manuscript.
Dr Nathan: contributed new reagents and revision of the manuscript.
Dr Rosas: contributed new reagents and revision of the manuscript.
Dr Gahl: contributed new reagents and revision of the manuscript.
Dr Moss: contributed to designing the research, analyzing data, and writing of the manuscript.
Dr Gochuico: contributed to designing the research, analyzing data, contributing new reagents, and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- BALF
BAL fluid
- FDG
[18F]-2-fluoro-2-deoxyglucose
- Glut-1
glucose transporter-1
- HPS
Hermansky-Pudlak syndrome
- IPF
idiopathic pulmonary fibrosis
Footnotes
Funding/Support: This research was supported in part by the Intramural Research Program of the National Institutes of Health (National Heart, Lung, and Blood Institute and National Human Genome Research Institute) and the National Institutes of Health [Grant 1K22HL092223, National Heart, Lung, and Blood Institute].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Brantly M, Avila NA, Shotelersuk V, Lucero C, Huizing M, Gahl WA. Pulmonary function and high-resolution CT findings in patients with an inherited form of pulmonary fibrosis, Hermansky-Pudlak syndrome, due to mutations in HPS-1. Chest. 2000;117(1):129-136 [DOI] [PubMed] [Google Scholar]
- 2.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140(1):221-229 [DOI] [PubMed] [Google Scholar]
- 3.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132(4):1311-1321 [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Chemaly S, Malide D, Zudaire E, et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A. 2009;106(10):3958-3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cullinane AR, Curry JA, Carmona-Rivera C, et al. A BLOC-1 mutation screen reveals that PLDN is mutated in Hermansky-Pudlak Syndrome type 9. Am J Hum Genet. 2011;88(6):778-787 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Gahl WA, Huizing M. Hermansky-Pudlak Syndrome. 2000 Jul 24 [updated 2010 Jul 08]. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, eds. GeneReviews [Internet]. Seattle WA: University of Washington, Seattle. http://www.ncbi.nlm.nih.gov/books/NBK1287/
- 9.Gochuico BR, Huizing M, Golas GA, et al. Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol Med. 2012;18(1):56-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas de Montpréville V, Mussot S, Dulmet E, Dartevelle P. Pulmonary fibrosis in Hermansky-Pudlak syndrome is not fully usual [in French]. Ann Pathol. 2006;26(6):445-449 [DOI] [PubMed] [Google Scholar]
- 11.Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76(3):234-242 [DOI] [PubMed] [Google Scholar]
- 12.Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging. 2008;35(8):1537-1543 [DOI] [PubMed] [Google Scholar]
- 13.Young LR, Franz DN, Nagarkatte P, et al. Utility of [18F]2-fluoro-2-deoxyglucose-PET in sporadic and tuberous sclerosis-associated lymphangioleiomyomatosis. Chest. 2009;136(3):926-933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groves AM, Win T, Screaton NJ, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med. 2009;50(4):538-545 [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Pascual JM, Yang H, et al. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15(7):1169-1179 [DOI] [PubMed] [Google Scholar]
- 16.Ebina M, Shimizukawa M, Shibata N, et al. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;169(11):1203-1208 [DOI] [PubMed] [Google Scholar]
- 17.El-Chemaly S, Ziegler SG, Calado RT, et al. Natural history of pulmonary fibrosis in two subjects with the same telomerase mutation. Chest. 2011;139(5):1203-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouhani FN, Brantly ML, Markello TC, et al. Alveolar macrophage dysregulation in Hermansky-Pudlak syndrome type 1. Am J Respir Crit Care Med. 2009;180(11):1114-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones HA, Clark RJ, Rhodes CG, Schofield JB, Krausz T, Haslett C. In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med. 1994;149(6):1635-1639 [DOI] [PubMed] [Google Scholar]
- 20.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117(3):549-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough?. Chest. 2012;142(1):200-207 [DOI] [PubMed] [Google Scholar]
- 22.Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170(3):242-251 [DOI] [PubMed] [Google Scholar]
- 23.Tzouvelekis A, Harokopos V, Paparountas T, et al. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am J Respir Crit Care Med. 2007;176(11):1108-1119 [DOI] [PubMed] [Google Scholar]
- 24.Roux-Dalvai F, Gonzalez de Peredo A, Simó C, et al. Extensive analysis of the cytoplasmic proteome of human erythrocytes using the peptide ligand library technology and advanced mass spectrometry. Mol Cell Proteomics. 2008;7(11):2254-2269 [DOI] [PubMed] [Google Scholar]
- 25.Zhang JZ, Ismail-Beigi F. Activation of Glut1 glucose transporter in human erythrocytes. Arch Biochem Biophys. 1998;356(1):86-92 [DOI] [PubMed] [Google Scholar]
- 26.Daskalow K, Pfander D, Weichert W, et al. Distinct temporospatial expression patterns of glycolysis-related proteins in human hepatocellular carcinoma. Histochem Cell Biol. 2009;132(1):21-31 [DOI] [PubMed] [Google Scholar]
- 27.Kaira K, Okumura T, Ohde Y, et al. Correlation between 18F-FDG uptake on PET and molecular biology in metastatic pulmonary tumors. J Nucl Med. 2011;52(5):705-711 [DOI] [PubMed] [Google Scholar]
- 28.Heyneman LE, Patz EF. PET imaging in patients with bronchioloalveolar cell carcinoma. Lung Cancer. 2002;38(3):261-266 [DOI] [PubMed] [Google Scholar]
- 29.Higashi K, Ueda Y, Seki H, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39(6):1016-1020 [PubMed] [Google Scholar]
- 30.Yap CS, Schiepers C, Fishbein MC, Phelps ME, Czernin J. FDG-PET imaging in lung cancer: how sensitive is it for bronchioloalveolar carcinoma?. Eur J Nucl Med Mol Imaging. 2002;29(9):1166-1173 [DOI] [PubMed] [Google Scholar]