Abstract
The pathophysiology of delirium remains elusive though neurotransmitters and their precursor large neutral amino acids (LNAAs) may play a role. This pilot study investigated whether alterations of tryptophan (Trp), phenylalanine (Phe), and tyrosine (Tyr), plasma levels were associated with a higher risk of transitioning to delirium in critically ill patients.
Methods
Plasma LNAA concentrations were determined on days 1 and 3 in mechanically ventilated (MV) patients from the MENDS randomized controlled trial (dexmedetomidine vs. lorazepam sedation). Three independent variables were calculated by dividing the plasma concentrations of Trp, Phe, and Tyr by the sum of all other LNAA concentrations. Delirium was assessed daily using the Confusion Assessment Method in ICU (CAM-ICU). Markov regression models were used to analyze the independent associations between plasma LNAA ratios and transition to delirium after adjusting for important covariates.
Results
The 97 patients included in the analysis had a high severity of illness (median APACHE II, 28; IQR, 24 to 32). Patients with either high or very low tryptophan to LNAA ratios (p=0.0003), and tyrosine to LNAA ratios (p=0.02) were at increased risk of transitioning to delirium, after adjusting for potential confounders. Phenylalanine levels were not associated with transition to delirium (p=0.27). Older age and exposure to fentanyl were also associated with a higher probability of transitioning to delirium.
Conclusions
In this pilot study, plasma tryptophan/LNAA (via serotonin or tryptophan metabolites) and tyrosine/LNAA ratios (via dopamine or its downstream neurotransmitter norepinephrine) were associated with transition to delirium in MV patients, suggesting that alterations of amino acids may be important in the pathogenesis of ICU delirium. Future studies studying the role of amino acid precursors of neurotransmitters are warranted in critically ill patients.
Keywords: delirium, amino acids, tryptophan, phenylalanine, tyrosine, large neutral amino acids, blood brain barrier, LAT-1 transporter, risk factor for delirium
Introduction
Delirium is highly prevalent among patients in the intensive care unit (ICU) with reported rates varying from approximately 20–80%, depending upon the severity of illness and the diagnostic method [1–3]. Delirium in the ICU setting is associated with prolonged hospitalization [4–6], increased costs [7], mortality [8–10], and potentially long term cognitive impairment [11, 12]. Despite previous efforts, a firm understanding of the pathophysiology of delirium remains elusive, and delirium is thought to occur due to alterations in neurotransmission [13–15], inflammation [16, 17], and/or cerebral blood flow [18, 19].
Theories associating changes in neurotransmission with delirium focus upon the effects of serotonin (5HT), dopamine (DA), acetylcholine (ACh) and norepinephrine (NE) pathways [14, 15, 20–22]. No technique currently exists to measure neurotransmitter concentrations in vivo in critically ill patients without the performance of invasive procedures such as lumbar punctures. However, the rate limiting step for synthesis and release of these neurotransmitters is the availability of the respective plasma precursor large neutral amino acids (LNAA), which can easily be measured [23]. Serotonin synthesis depends upon the availability of tryptophan (Trp). In contrast, DA and NE production require tyrosine (Tyr) and phenylalanine (Phe), as both are part of the same metabolic pathway. Cerebral uptake of these circulating amino acids involves transport through two membranes: the brain capillary endothelial wall, forming the blood brain barrier (BBB) in vivo, and the brain cell plasma membrane [23]. The entry of the LNAA across the BBB occurs via the sodium independent LNAA transporter type 1 (LAT1) [24, 25]. The LAT1 transporter in the BBB has a much higher affinity for the LNAAs than similar transporters in peripheral tissue [24], underlying the brain’s selective vulnerability to pathological effects of hyper/hypo-aminoacidemias. Given this high affinity, the LAT1 transporters are normally highly saturated, so a selective increase in one amino acid will reduce the entry of the other LNAAs (tryptophan, phenylalanine, tyrosine, lysine, methionine, valine, leucine and isoleucine) [23–25]. Thus, if the plasma Trp concentration increases in comparison to other LNAAs, an increased Trp to other LNAA ratio will result, and more Trp will pass through the BBB via the LAT-1 transporter to provide the opportunity for increased serotonin synthesis. Alternatively, an increase in the plasma concentration of Phe or Tyr would result in an increase in central DA and NE.
Plasma levels of Phe have been implicated in delirium in febrile hospitalized patients and cardiac surgery patients [20–22, 26], while Trp increases have been seen in patients with hepatic encephalopathy [27]. No prior study, however, has evaluated the relationship of amino acid precursors for neurotransmitters and delirium in critically ill mechanically ventilated patients. This pilot investigation was designed to study the temporal association of Trp, Phe, and Tyr levels as risk factors for transitioning into delirium, in order to offer insight into potential pathophysiological mechanisms of delirium among ICU patients.
Materials and methods
The institutional review board (IRB) at Vanderbilt University Medical Center, Nashville, Tennessee approved this study. Plasma levels of Trp, Tyr, Phe and the other LNAAs (lysine, methionine, valine, leucine and isoleucine) were prospectively collected from subjects enrolled in the MENDS double blind, randomized controlled trial comparing sedation with dexmedetomidine versus lorazepam [28]. The patient population has previously been described in detail: 103 adult mechanically ventilated medical and surgical ICU patients from two tertiary care centers, enrolled between August 2004 and April 2006, excluding those with neurological disease (previous stroke, cerebral palsy, etc), active seizure disorders, Child-Pugh class B or C cirrhosis, alcohol abuse, active myocardial ischemia, second- or third-degree heart block, severe dementia, pregnancy, and severe hearing disabilities or inability to understand English, which would prevent delirium evaluations [28].
At enrollment, baseline cognitive abilities were assessed through surrogate interview using the validated Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [29], and demographics were collected via data accessed from the computerized medical record.
Delirium was assessed until hospital discharge or for up to 12 days using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) [1, 2]. The sedation level was measured via the Richmond Agitation-Sedation Scale (RASS) [30, 31]. Patients were categorized as delirious if they had a RASS score of −3 or greater (i.e. −3 to + 4) and a positive CAM-ICU score. Coma was defined as a RASS score of −4 (responsive only to physical stimulus) or −5 (unresponsive to physical stimulus). A more comprehensive description and training manual for the CAM-ICU and RASS scale are available at: http://www.icudelirium.org.
Patient blood samples were collected and centrifuged on study days 1 and 3. Plasma was separated and stored at −80° Celsius (C). Plasma amino acids were analyzed using high performance liquid chromatography (HPLC) [32]. To examine the independent relationship of the amino acid precursors for serotonin, DA and NE, plasma ratios of Trp, Phe, and Tyr were calculated by dividing the plasma concentration for each by the sum of the concentrations of all the remaining LNAAs (e.g the Trp/LNAA ratio is the concentration of Trp divided by the sum of tyrosine, phenylalanine, lysine, methionine, valine, leucine, and isoleucine), as previously reported [20–22]. Plasma ratios for these precursors were used as peripheral indices of their cerebral availability for neurotransmitter synthesis following transport across the BBB.
Statistical Analysis
Patients’ baseline demographic and clinical variables are presented using medians and interquartile ranges for continuous variables, and proportions for categorical variables. This study’s primary outcome was to determine the temporal association of Trp, Phe and Tyr in transition to delirium. Markov regression models [33] were used to determine the probability of transitioning to delirium as a function of the LNAA ratios from the previous 24 hours and pre-determined clinically relevant covariates: age, IQCODE, APACHE II at enrollment, sedation regimen (dexmedetomidine, lorazepam, or fentanyl) and mental status on the previous day. Generalized Estimation Equations (GEE) [34] were used to account for correlations among patient observations. Three separate models were evaluated, with Trp/LNAA, Phe/LNAA and Tyr/LNAA studied as the independent variables of interest. Given that sedatives are so closely tied with coma, we excluded all transitions to coma in our Markov model, so that the model examined transitions from normal, delirious or comatose mental state to either normal or delirious states. For this analysis, the outcome variable was binary; thus, the logit link function and a binomial distribution were specified in GEE. Since amino acids samples were collected on study days 1 and 3 and cognitive status data was recorded more frequently, we only used those cognitive assessments that had amino acid levels measured the previous day as the dependent variables. Restricted cubic splines were used to assess any nonlinear effect of amino acids. All statistical analyses were performed using R version 2.7 (www.r-project.org).
Results
Among the available 103 patients, 96 were included in this study’s analyses, following exclusions based upon the lack of sufficient amino acid specimens or at least two cognitive status evaluations for the computation of Markov model transitions (total 7 patients). Baseline demographic characteristics are described in Table 1. Patients presented a high severity of illness with a median (interquartile range, IQR) APACHE-II score of 28 (24, 32), with sepsis and acute respiratory distress syndrome being the most common admission diagnosis.
Table 1.
Demographics of patients.a
| Variable | (N= 96) |
|---|---|
| Age, y | 60 (47 to 66) |
| Men, No. (%) | 51 (53) |
| APACHE II | 28 (24 to 32) |
| IQCODE at enrollment | 3 (3 to 3) |
| ICU type, No. (%) | |
| Medical ICU | 70 (72) |
| Surgical ICU | 27 (28) |
| Admission diagnoses, No. (%) | |
| Sepsis/acute respiratory distress syndrome | 41 (42) |
| Pulmonary (other)b | 22 (23) |
| Malignancies | 8 (8) |
| Chronic obstructive pulmonary disease | 4 (4) |
| Cardiogenic shock | 2 (2) |
| Hemorrhagic shock | 1 (1) |
| Renal Failure | 1 (1) |
| Otherc | 34 (35) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Median (interquartile range) unless otherwise noted
Pulmonary (other) included admissions due to pulmonary hypertension, cystic fibrosis, hemoptysis, pulmonary embolism, and pulmonary fibrosis.
Included admission diagnoses due to gastric and colonic surgery, urological surgery, vascular surgery, cardiac surgery, transplant surgery (except liver); neuromuscular disease; reasons other than sepsis.
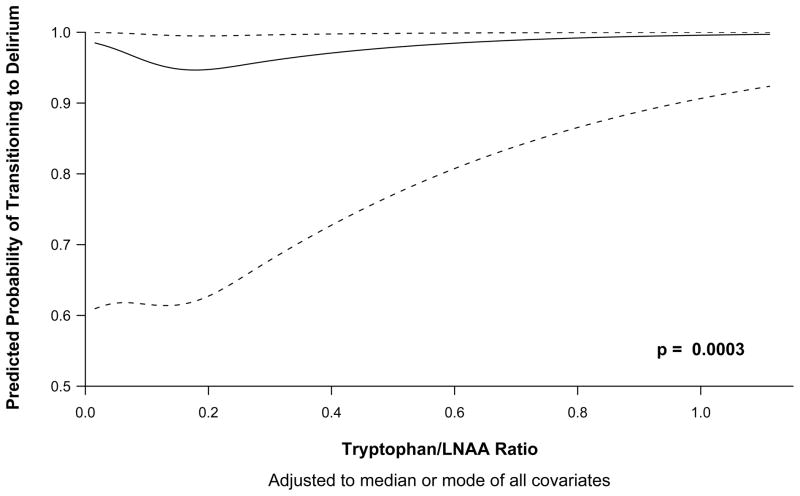
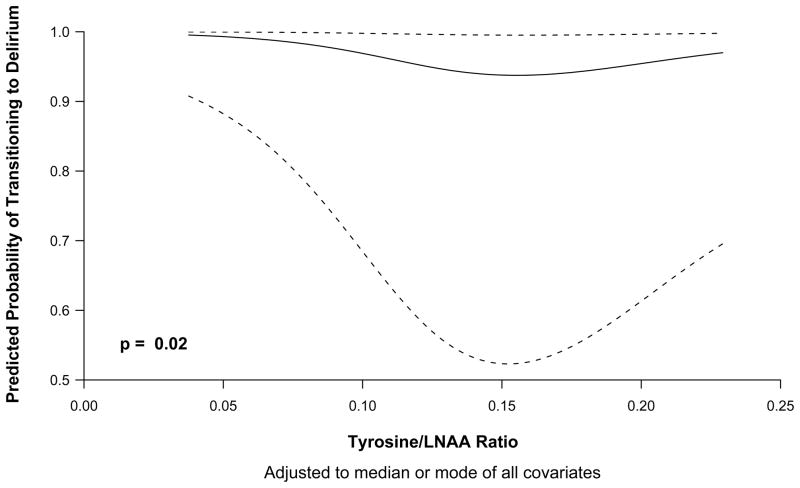
In the Markov model, both Trp/LNAA (p=0.0003) and Tyr/LNAA (p=0.02) ratios were independent risk factor for transitioning to delirium (Tables 2 and 3). The relationship between Trp/LNAA ratios and Tyr/LNAA ratios and transitioning to delirium are graphically shown in Figures 1 and 2 respectively, and indicate that both low and high levels of tryptophan and tyrosine were associated with an increased risk of delirium. The Phe/LNAA ratio was not associated with an increased risk of transitioning to delirium (p=0.27) (Tables 4). In keeping with results of our previous studies [35, 36], increasing age, APACHE II scores and exposure to fentanyl was associated with an increased probability of transitioning to delirium in all 3 models (Trp/LNAA, Tyr/LNAA and Phe/LNAA) (Tables 2, 3 and 4).
Table 2.
Transition to Delirium and Tryptophan/Large Neutral Amino Acids Ratio.
| Variables | Chi-Square | P Value |
|---|---|---|
| Tryptophan/LNAA ratio | 16.1 | 0.0003 |
| Age | 8.1 | 0.005 |
| Modified APACHE II at enrollment | 4.9 | 0.02 |
| IQCODE | 0.1 | 0.73 |
| Previous day’s mental status | 15.6 | 0.0004 |
| Dexmedetomidine on previous day | 0.2 | 0.67 |
| Lorazepam on previous day | 0.3 | 0.58 |
| Fentanyl on previous day | 5.23 | 0.02 |
Abbreviations: LNAA, Large Neutral Amino Acids; Modified APACHE II, Acute Physiology and Chronic Health Evaluation II excluding Glasgow coma scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Table 3.
Transition to Delirium and Tyrosine/Large Neutral Amino Acids Ratio.
| Variables | Chi-Square | P Value |
|---|---|---|
| Tyrosine/LNAA ratio | 8.3 | 0.02 |
| Age | 7.3 | 0.007 |
| Modified APACHE II at enrollment | 8.2 | 0.004 |
| IQCODE | 0.2 | 0.65 |
| Previous day’s mental status | 18.8 | <0.0001 |
| Dexmedetomidine on previous day | 0.72 | 0.39 |
| Lorazepam on previous day | 1.71 | 0.19 |
| Fentanyl on previous day | 3.3 | 0.07 |
Abbreviations: LNAA, Large Neutral Amino Acids; Modified APACHE II, Acute Physiology and Chronic Health Evaluation II excluding Glasgow coma scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Figure 1.
Odds of transitioning to delirium according to tryptophan/large neutral amino acids ratio. The odds of transitioning from any mental state (normal, delirious, or comatose) to delirium are higher at very small Trp/LNAA ratios, lower when the ratio is approximately 0.2 and then increase as the Trp/LNAA ratio increases.
Figure 2.
Odds of transitioning to delirium according to tyrosine/large neutral amino acids ratio. The odds of transitioning from any mental state (normal, delirious, or comatose) to delirium are higher at very small Tyr/LNAA ratios, lower when the ratio is approximately 0.15 and then increase as the Tyr/LNAA ratio increases.
Table 4.
Transition to Delirium and Phenylalanine/Large Neutral Amino Acids Ratio.
| Variables | Chi-Square | P Value |
|---|---|---|
| Phenylalanine/LNAA ratio | 2.6 | 0.27 |
| Age | 8.1 | 0.004 |
| Modified APACHE II at enrollment | 3.9 | 0.05 |
| IQCODE | 0.08 | 0.78 |
| Previous day’s mental status | 19.4 | <0.0001 |
| Dexmedetomidine on previous day | 0.24 | 0.62 |
| Lorazepam on previous day | 1.2 | 0.28 |
| Fentanyl on previous day | 3.7 | 0.05 |
Abbreviations: LNAA, Large Neutral Amino Acids; Modified APACHE II, Acute Physiology and Chronic Health Evaluation II excluding Glasgow coma scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly.
Discussion
To our knowledge, this is the first study evaluating the relationship between plasma amino acid ratios and transitioning to delirium in a cohort of mechanically ventilated critically ill patients. In this pilot study, extremely low and high levels of Trp/LNAA and Tyr/LNAA were significantly associated with transitioning to delirium. Furthermore, our study was consistent with previous studies in that it showed that age, APACHE II scores and exposure to fentanyl were risk factors for delirium [35, 36].
Our findings of the association of high levels of Trp with delirium are in accordance with clinical studies that have suggested that excess serotonin activity is responsible for the development of psychosis associated with hepatic encephalopathy and serotonin syndrome [20, 37]. Our data also suggest that very low levels of tryptophan are associated with an increased risk of transitioning into delirium. Low Trp levels (thus presumably low serotonin) have been associated with neuropsychiatric complications among patients with severe alcohol withdrawal, individuals treated with levodopa for Parkinson’s disease, and postoperative patients [20]. Potentially these low Trp levels, however, must be sufficiently decreased past a threshold value in order to decrease serotonin efflux [38], which could possibly explain the lack of increased risk among patients with intermediate Trp ratio levels. Unfortunately, it is unclear whether the symptoms of delirium associated with alterations in Trp concentrations are due to the production of neurotoxic metabolites of tryptophan, fluctuations of serotonin or its down steam neurotransmitter, melatonin, or a combination of both.
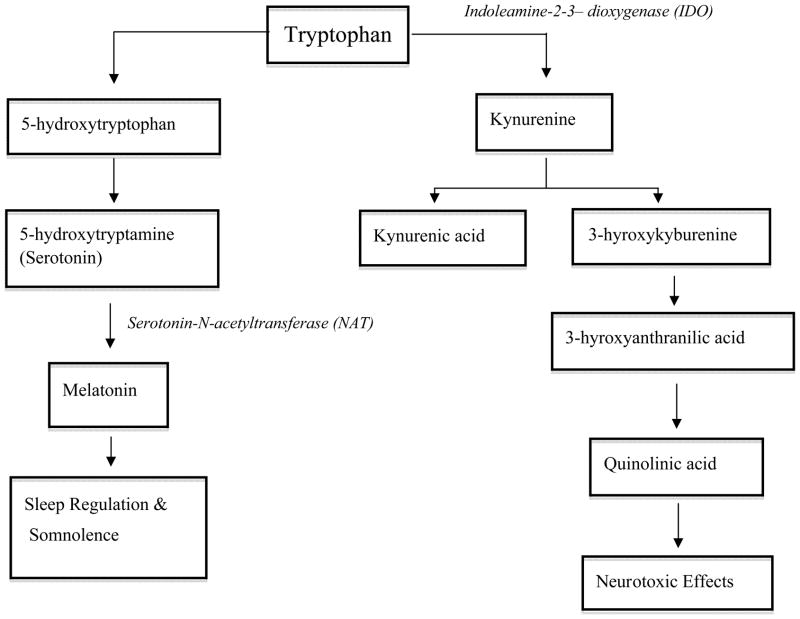
A possible mechanism for Trp associated delirium is through the production of metabolites that are neurotoxic. Following activation of the immune and inflammatory response systems as seen in critical illness, Trp undergoes metabolism via the kynurenine pathway through the induction of indoleamine-2,3-dioxygenase (IDO) (Figure 3). This leads to an increased production of kynurenine and additional metabolites in both central [38] and peripheral tissues [39]. In the presence of inflammation, kynurenine is further metabolized to quinolinic acid, an N-methyl-D-aspartate (NMDA) receptor agonist and neurotoxin, while to a lesser extent Trp is metabolized to the neuroprotective NMDA antagonist, kynurenic acid. Potentially enhancing the detrimental effects of inflammation, kynurenine also makes the blood brain barrier more susceptible to the neurotoxic effects of quinolinic acid [40]. Of note, the kynurenine pathway of Trp metabolism is quantitatively more prominent in comparison to the pathways for serotonin and melatonin production, and it has even been described as occurring ten times more frequently [41], with approximately only 1% of Trp metabolism undergoing the serotonin pathway [42]. Potentially, such metabolites in increased amounts could lead to the excitatory neuropsychiatric complications found among patients with delirium [38, 40, 41].
Figure 3.
Schematic of tryptophan metabolism via the kynurenine pathways or through the synthesis of serotonin. Note that this is not representative of all intermediates and enzymes for the purpose of simplicity.
A second possible mechanism by which tryptophan may lead to delirium is via alteration of melatonin production secondary to changes in serotonin levels. The production of melatonin from serotonin is controlled by serotonin-N-acetyltransferase (NAT), which is the rate limiting step (Figure 3). Melatonin is responsible for sleep-wake cycle modulation and may similarly increase or decrease in concentration depending upon Trp levels [26]. A high Trp concentration (or increased serotonin breakdown) can potentially lead to increased production of melatonin, thereby causing the somnolent symptoms of hypoactive delirium, while a low Trp ratio could be responsible for decreased melatonin, sleep dysregulation, and the development of hyperactive delirium [43]. This pattern of abnormal plasma melatonin levels has been noted among peri- and post-operative patients with delirium [43]. In support of these theories, Balan et al. [44] have found high levels of the melatonin metabolite 6-sulphatoxymelatonin (6-SMT) in the urine of patients with hypoactive delirium, with much lower levels in those with hyperactive delirium [44]. We were not able to evaluate the relationship between tryptophan levels and the hypoactive and hyperactive subtypes of delirium, because pure hyperactive delirium occurs in <5% of ICU patients [45, 46], limiting the ability to study this relationship.
The other notable finding in our study was that the Tyr/LNAA ratio was also an independent risk factor for transitioning to delirium in critically ill mechanically ventilated patients, such that both low and high levels were associated with increased risk of developing delirium. It can be hypothesized that patients with high levels of tyrosine are more likely to have excess dopamine or its down stream neurotransmitter, norepinephrine, both of which have been implicated in the pathogenesis of delirium [23]. On the other hand given that both tryptophan and tyrosine compete to cross the blood brain barrier, it is also plausible that the reason low levels of both tyrosine and tryptophan are associated with delirium is because this infact is a reflection of higher levels of the other amino acid. [23–25].
In concordance with our previous investigation [35], both age and APACHE II scores were risk factors for development of delirium. Additionally the previous day’s mental status (delirium or normal) was a strong predictor of the mental status the next day. Exposure to fentanyl was also associated with transition to delirium in the three models. These findings are consistent with the fact that delirium is often due to multiple risk factors. In our study, exposure to the alpha2 agonist, dexmedetomidine, was not associated with delirium in all three models. The mechanisms via which dexmedetomidine alters delirium risk are not known, though it possibly modulates neurotransmission (by noradrenergic blockage at the locus ceruleus), attenuates inflammation and may improve natural sleep. Therefore the relationships between LNAA levels, sedative choice and delirium risk should remain an important component of this line or research, as they have not been clearly elucidated in this pilot investigation.
Our study presents several strengths and limitations. First, the samples used to test the levels of the LNAA were drawn on study days 1 and 3, and therefore differences in the relationship between transitioning to delirium and LNAA might have been altered had we collected these samples daily, especially if major dietary changes occurred during this period, such as the start of parenteral nutrition. If important dietary changes did occur, it is unlikely that they were influenced by delirium status. Therefore, any misclassification resulting from dietary changes and the lack of daily sample collection would be non-differential and would bias our results toward showing less of an association than truly exists. To further reduce the potential for any systematic bias, we utilized only those cognitive assessments on the day following the plasma amino acid levels. This limitation highlights the difficulty in conducting research involving daily blood collections from critically ill patients. Second, we did not monitor dietary information that may have had a role in altering the levels of LNAA. While dietary supplementation may be a method by which to manipulate these levels towards therapeutic goals when these relationships are better understood, the main purpose of this study was to assess whether Trp, Phe, and Tyr levels were associated with increased risk of the development of delirium, regardless of the source of excess or depletion. Future studies will be needed to further characterize the etiology of Trp or Tyr excess (or significant insufficiency, as evident with our patient data) and to design interventional trials to ascertain if optimizing LNAA ratio levels of Trp and Tyr improve delirium outcomes. Third, we did not detect an association between levels of Phe with delirium, as seen in previous studies in cardiac surgery patients and long-term care patients [20, 22, 47]. This may be due to the different patient population utilized (ICU mechanically ventilated critically ill patients versus elective cardiac surgical/hospitalized), which is also reflected with the higher rate of delirium found in our patient population compared to the non-ICU cohort (80% versus 13.5%). Addressing these limitations in future work will provide opportunities by which to build on the knowledge gained via this pilot study in order to advance our understanding of the role of amino acids and neurotransmitters in delirium in the critically ill.
Conclusions
This pilot investigation found that high and extremely low levels of plasma tryptophan/LNAA (via serotonin or tryptophan metabolites) and tyrosine/LNAA ratios (via dopamine or its downstream neurotransmitter norepinephrine) were associated with transition to delirium in MV patients, suggesting that alterations of amino acids may be important in the pathogenesis of ICU delirium. The mechanisms postulated in this report provide insight into potential pathogenesis of delirium and indicate potential areas for future research into the modifiable causes and treatments for delirium during critical illness.
Acknowledgments
Funding/Support: Dr. Pandharipande is supported by the VA Clinical Science Research and Development Service (VA Career Development Award), the ASCCA-FAER-Abbot Physician Scientist Award, and the Vanderbilt Physician Scientist Development Award. Dr. Girard is supported by the Hartford Geriatrics Health Outcomes Research Scholars Award Program and the Vanderbilt Physician Scientist Development Program. Dr. Ely is supported by the VA Clinical Science Research and Development Service (VA Merit Review Award) and the National Institutes of Health (AG027472). The amino acid analysis was supported by the Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH.
Footnotes
Potential financial conflict of interest: Dr. Pandharipande has received research grant and honoraria from Hospira Inc. Dr. Ely has received research grant and honoraria from Hospira, Inc, Pfizer, Eli Lilly, GSK, and a research grant from Aspect Medical Systems. Dr. Girard has received honoraria from Hospira, Inc The other authors report no financial disclosures.
Reference List
- 1.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 4.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 6.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 8.McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51:591–598. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, Fang YF, Shieh MH, Kuo HP. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 12.Jackson JC, Gordon SM, Girard TD, Thomason JWW, Pun BT, Dunn J, Canonico AE, Light RW, Shintani AK, Thompson JL, Dittus RS, Bernard GR, Ely EW. Delirium as a risk factor for long term cognitive impairment in mechanically ventilated ICU survivors. Am J Respir Crit Care Med. 2007;175:A22. [Google Scholar]
- 13.Van Der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol. 1998;11:138–145. doi: 10.1177/089198879801100304. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1277–1280. doi: 10.1093/gerona/61.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008;24:45–65. viii. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. 2007;36:222–225. doi: 10.1093/ageing/afl121. [DOI] [PubMed] [Google Scholar]
- 17.Kudoh A, Takase H, Katagai H, Takazawa T. Postoperative interleukin-6 and cortisol concentrations in elderly patients with postoperative confusion. Neuroimmunomodulation. 2005;12:60–66. doi: 10.1159/000082365. [DOI] [PubMed] [Google Scholar]
- 18.Pfister D, Siegemund M, Kuster S, ll, Smielewski P, Ruegg S, Strebel SP, Marsch SC, Pargger H, Steiner LA. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong TG, Bogardus ST, Daftary A, Auerbach E, Blumenfeld H, Modur S, Leo-Summers L, Seibyl J, Inouye SK. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. Journal of Gerontology: Medical Sciences. 2007;61A:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 20.Van Der Mast RC, van den Broek WW, Fekkes D, Pepplinkhuizen L, Habbema JD. Is delirium after cardiac surgery related to plasma amino acids and physical condition? J Neuropsychiatry Clin Neurosci. 2000;12:57–63. doi: 10.1176/jnp.12.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Semin Clin Neuropsychiatry. 2000;5:125–131. doi: 10.153/SCNP00500125. [DOI] [PubMed] [Google Scholar]
- 22.Flacker JM, Lipsitz LA. Large neutral amino acid changes and delirium in febrile elderly medical patients. J Gerontol A Biol Sci Med Sci. 2000;55:B249–B252. doi: 10.1093/gerona/55.5.b249. [DOI] [PubMed] [Google Scholar]
- 23.Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23:635–644. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- 24.Wurtman RJ, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980;32:315–335. [PubMed] [Google Scholar]
- 25.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis MC, Barnett SR. Postoperative delirium: the tryptophan dyregulation model. Med Hypotheses. 2004;63:402–406. doi: 10.1016/j.mehy.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Mizock BA, Sabelli HC, Dubin A, Javaid JI, Poulos A, Rackow EC. Septic encephalopathy. Evidence for altered phenylalanine metabolism and comparison with hepatic encephalopathy. Arch Intern Med. 1990;150:443–449. doi: 10.1001/archinte.150.2.443. [DOI] [PubMed] [Google Scholar]
- 28.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 29.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 31.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 32.Fekkes D, van DA, Edelman M, Voskuilen A. Validation of the determination of amino acids in plasma by high-performance liquid chromatography using automated pre-column derivatization with o-phthaldialdehyde. J Chromatogr B Biomed Appl. 1995;669:177–186. doi: 10.1016/0378-4347(95)00111-u. [DOI] [PubMed] [Google Scholar]
- 33.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Clarendon Press; Oxford: 1994. [Google Scholar]
- 34.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 35.Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA, Jr, Dittus R, Ely EW. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 38.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115 (Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 39.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 41.Miuller N, Schwarz MJ. The immunological basis of glutamatergic disturbance in schizophrenia: towards an integrated view. J Neural Transm Suppl. 2007:269–280. doi: 10.1007/978-3-211-73574-9_33. [DOI] [PubMed] [Google Scholar]
- 42.Wolf H. The effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand J Clin Lab Invest. 1974;136:1–186. [PubMed] [Google Scholar]
- 43.Uchida K, Aoki T, Ishizuka B. Postoperative delirium and plasma melatonin. Med Hypotheses. 1999;53:103–106. doi: 10.1054/mehy.1998.0724. [DOI] [PubMed] [Google Scholar]
- 44.Balan S, Leibovitz A, Zila SO, Ruth M, Chana W, Yassica B, Rahel B, Richard G, Neumann E, Blagman B, Habot B. The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci. 2003;15:363–366. doi: 10.1176/jnp.15.3.363. [DOI] [PubMed] [Google Scholar]
- 45.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Truman PB, Dittus R, Ely EW. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33:1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 46.Marquis F, Ouimet S, Riker R, Cossette M, Skrobik Y. Individual delirium symptoms: Do they matter?*. Crit Care Med. 2007;35:2533–2537. doi: 10.1097/01.ccm.0000284506.43390.f3. [DOI] [PubMed] [Google Scholar]
- 47.Van Der Mast RC, van den Broek WW, Fekkes D, Pepplinkhuizen L, Habbema JD. Incidence of and preoperative predictors for delirium after cardiac surgery. J Psychosom Res. 1999;46:479–483. doi: 10.1016/s0022-3999(99)00002-1. [DOI] [PubMed] [Google Scholar]