Summary
Background
Beta-2 adrenergic receptor (ADRB2) is the primary target of both short- and long-acting beta-agonist asthma medications. ADRB2 5'-UTR methylation changes in blood have the potential to act as a surrogate biomarker of responsiveness to beta-agonist treatment and childhood asthma severity.
Objective
To study the association between ADRB2 5'-UTR methylation, NO2 exposure and childhood asthma severity.
Methods
We compared ADRB2 5'-UTR methylation levels in blood between 60 children with mild asthma and 122 children with severe asthma using methylation-specific PCR. We also investigated potential joint effects between NO2 exposure and ADRB2 5'-UTR methylation.
Results
We found a significant association between intermediate (OR: 4.11, 95% CI: 1.58–10.73) and high levels (OR: 7.63, 95% CI: 3.02–19.26) of ADRB2 methylation and severe childhood asthma. In addition, we found a significant association between indoor exposure to NO2, an air pollutant and known asthmogen, and severe asthma among children exhibiting high ADRB2 methylation (OR: 4.59, 95% CI: 1.03–20.55) but no association among children exhibiting low levels of ADRB2 methylation (OR: 0.35, 95% CI: 0.01–14.13).
Conclusions and Clinical Relevance
These findings support the potential use of ADRB2 5'-UTR methylation as a biomarker of both asthma severity and risk for NO2-associated asthma exacerbations in children, and present the first evidence of an epigenetic link between an important environmental exposure and childhood asthma severity.
Keywords: ADRB2, methylation, asthma severity, epigenetic, NO2
Introduction
Asthma has long been recognized as a complex genetic disease mediated by exposures to a variety of environmental triggers and is the most common chronic illness among children [1]. While the incidences of most chronic illnesses have decreased over the last several decades, the prevalence and severity of childhood asthma have increased over the same time period [2–5]. This does not represent enough time to realize a change in genetic composition capable of explaining this increase. Recent studies have suggested that, in addition to genetic variations, epigenetic alterations, such as aberrant DNA methylation patterns, may play a role in the development of asthma and asthma-related phenotypes [6–9]. However, little research effort has been made to understand the role of epigenetic changes in asthma pathogenesis.
The current study examines the potential of using epigenetic variations of asthma-relevant genes as biomarkers of childhood asthma severity by measuring the 5'-UTR methylation of beta-2 adrenergic receptor (ADRB2) in human blood. ADRB2 is a member of the G protein-coupled receptor superfamily and plays a key role in both the regulation of airway smooth muscle tone and lung fluid clearance [10, 11]. The intronless ADRB2 gene covers a 3.45 kb stretch on chromosome 5 and is one of the best studied asthma candidate genes. ADRB2 polymorphisms have been found to be associated with several asthma-related phenotypes, including responsiveness to exogenous beta-agonist challenge and asthma severity [12–16].
Of particular therapeutic interest is the localization of these receptors on airway epithelial cells and on airway smooth muscle. ADRB2 represents the primary target of short- and long-acting beta agonists, the two leading classes of drugs used for the treatment and control of asthmatic symptoms [12, 17, 18]. When expressed on airway smooth muscle, ADRB2 mediates relaxation and may reduce inflammatory cytokine production in asthma [12]. However, the question of whether epigenetic regulation of ADRB2 expression can alter the clinical course of childhood asthma has yet to be examined. Similarly, we know of no previous study that has examined the epigenetics of interactions between asthma-relevant genes and environmental pollutants in the context of asthma severity.
In the current study, we test the hypothesis that increased ADRB2 5'-UTR methylation may increase asthma severity in a study population of asthmatic children. We further explored the joint impact of residential indoor NO2 (nitrogen dioxide) exposure (most commonly from use of gas stoves), a respiratory irritant known to be associated with childhood asthma [19], and ADRB2 5'-UTR methylation on risk for severe asthma. Given that NO2 is a common indoor exposure and ADRB2's preponderance as the target of asthma medications, our study represents a novel examination of the role of epigenetic alterations of an asthma-relevant gene and its interplay with a key environmental exposure in the pathogenesis of severe childhood asthma.
Methods
Study population
Subjects for the current analysis (N=182) were selected from a larger study of 1,401 asthmatic children living in Connecticut and the Springfield and Worcester areas of Massachusetts enrolled in a prospective, one-year follow-up study designed to investigate the effect of NO2 on asthma severity. To be eligible for enrollment, children had to be 5 years of age or older but younger than 12 years of age, and have at least two of the following: physician-diagnosed asthma, active asthma symptoms during the 12 months prior to enrollment, or asthma prescription medication use during the 12 months prior to enrollment. In addition, enrolled children had to agree to provide a blood sample for allergy testing. Each child was followed for 12 months for symptoms and medication use recorded by the child's mother using a provided calendar. Daily symptoms and medication use were reported for 4, one-month periods during the year of follow-up (one month during each season). Each one-month daily reporting period was separated by 2 months for which monthly symptoms and medication use were reported. Health information was collected in telephone interviews at the end of each reporting period. The study began in April, 2006, and was completed in August, 2009. Yale University Human Investigation Committee approved the study, and all respondents (mothers of study subjects) gave informed consent prior to participation. Separate consent was also obtained for the use of blood samples for asthma genetic studies.
A 5-level asthma severity score was created by using a combination of recorded symptoms and medication use. The severity score has possible values of 0 (no symptoms or medication use) 1 (mild transient), 2 (mild persistent), 3 (moderate persistent), or 4 (severe persistent), and is adapted from the Global Initiative for Asthma (GINA) guidelines (U.S. Department of Health and Human Services; February, 2002). The severity scores were calculated for each of 8 monitoring periods (4, 1-month periods and 4, 2-month periods) for the year-long study. The mean severity was the arithmetic mean of all monitoring period severity scores. Subjects for the current analysis were selected from among Caucasian children including 2 self-identified as white/Hispanic (N=506, or 36% of all subjects) whose asthma were classified as “mild” for the year prior to the study as well as the study year (asthma severity score of 0 or 1; N = 77) and children classified as “severe” for the study year (severity score of 3 or 4; N = 249). Of these 326 children, 264 both consented to have blood samples used for genetic analysis and had viable samples available. A total of 182 samples (60 mild and 122 severe cases matched for age and gender) were used in the current analysis.
Blood draw and data collection
Blood samples were collected from each study participant by trained phlebotomists at the time of the enrollment home visit. Blood clots from blue top tubes were stored at −80 °C, and were used as the source of DNA for methylation analysis following DNA extraction. Integrated NO2 measurements were collected in each subject's home using passive monitors (Palmes tubes [19, 20]) for four, one-month periods, once per season. An annual mean NO2 level was calculated as the arithmetic average over all monitoring periods.
5'-UTR CpG island identification and methylation analysis
A CpG island in the 5'-UTR region of ADRB2 was identified using the CpG Island Searcher web tool (www.cpgislands.com). Methylation-specific PCR primers for this region were then designed using the MethPrimer program (www.urogene.org/methprimer), with one pair designed to amplify methylated DNA and one pair designed to amplify unmethylated DNA. The two methylated primer sequences were L:5'-GTATATAACGGGTAGAACGTATTGC-3' and R:5'-GTCCTACACACTCAACTTATCGA-3', and the two unmethylated primer sequences were L:5'-TGTATATAATGGGTAGAATGTATTGTG-3' and R:5'-TCATCCTACACACTCAACTTATCAA-3'. Genomic DNA was extracted from frozen blood clots using the QIAamp Blood Kit (Qiagen) according to the manufacturer's protocol. Extracted DNA was then bisulfite-treated using the EZ DNA Methylation Kit (Zymo Research) according to the manufacturer's protocol, which converts unmethylated cytosines into uracil and leaves methylated cytosines unchanged. Following treatment, quantitative methylation-specific PCR was performed using the primers described above and the SYBR Fast Universal qPCR Kit (Kapa Biosystems), according to the protocols of the manufacturer, to distinguish methylated from unmethylated DNA sequences. A percent methylation was calculated for each sample using the formula: % methylation = 1/(1 + 2−(CTu − CTme)) × 100%, as previously described [21], where CTu is the average cycle threshold (CT) obtained from duplicate quantitative PCRs using the unmethylated primer pair, and CTme is the average CT obtained using the methylated primer pair.
An additional real-time methylation-specific PCR was performed using the SYBR Fast Universal qPCR Kit (Kapa Biosystems) and universally methylated and universally unmethylated human DNA (Zymo Research) with the same primer pairs and PCR conditions used for subject DNA samples. DNA amount was adjusted to be equal between methylated and unmethylated samples. Correct fragment length and purity of PCR products were analyzed by agarose gel electrophoresis and subsequent DNA staining with ethidium bromide. Amplificates of both methylated (M) and unmethylated (U) primers showed a single band at roughly 110 bp, which matches the expected length of our PCR amplicon (105 bp). The contrast in band intensities between primer-specific amplificates for both methylated and unmethylated samples suggested specificity of methylated and unmethylated primers for methylated and unmethylated sequences, respectively (see Supplemental Figure 1).
Statistical analyses
All statistical analyses were performed using the SAS statistical software, version 9.1 (SAS Institute). To assess the association between ADRB2 5'-UTR methylation status and asthma severity, percent methylation values were divided into tertiles of “low,” “intermediate,” and “high” according to the methylation distribution among the mild group, and comparisons were made using “low” methylation as the referent category. Odds ratios (OR) and 95% confidence intervals (CI) were determined using multivariate logistic regression (adjusted for age and gender). As the inclusion of allergy status did not alter the results of the main effects model (data not shown), it was removed as a covariate in order to arrive at the most parsimonious model. To assess the existence of joint effects between NO2 exposure and ADRB2 5'-UTR methylation on asthma severity, stratified comparisons were performed using logistic regression while controlling for age and gender. A Breslow-Day test for homogeneity of ORs was performed to ascertain whether the observed differences in ORs across strata are statistically significant at an α of 0.05. In addition, a Spearman's rank correlation test was conducted to measure the degree of statistical correlation between NO2 exposure level and ADRB2 methylation (α = 0.05). Power calculations for OR and independent t-test estimates were performed using the PS: Power and Sample Size Calculation software (Department of Biostatistics, Vanderbilt University). For OR estimates, inter-tertile comparisons had approximately 80% power to detect an OR of 3.0 at an α of 0.05. Our independent t-test had 80% power to detect a difference in percent methylation of 0.18% at an α of 0.05, assuming a within group standard deviation of 0.5%.
Results
Our study included 182 children with active asthma. A borderline significant trend was found for more severe cases to be in homes with annual indoor NO2 levels above 11 ppb versus homes with annual indoor levels below 11 ppb (P = 0.084). Distributions of selected characteristics by asthma severity status are shown in Table 1.
Table 1.
Distribution of selected characteristics by asthma severity status (N=182 children with asthma, CT and MA, 2006–2009)
| Variable | Mild cases (N = 60) N (%) | Severe cases (N = 122) N (%) | χ2 P-value |
|---|---|---|---|
| Racial background | |||
| Caucasian | 60 (100.0) | 122 (100.0) | N/A |
| Gender | |||
| Male | 37 (61.7) | 77 (63.1) | |
| Female | 23 (38.3) | 45 (36.9) | 0.849 |
| Age at enrollment (years) | |||
| <7 | 19 (31.7) | 44 (36.1) | |
| 7–9 | 26 (43.3) | 53 (43.4) | |
| >9 | 15 (25.0) | 25 (20.5) | 0.742 |
| NO2 exposure level (ppb) | |||
| ≤11 | 55 (91.7) | 100 (82.0) | |
| >11 | 5 (8.3) | 22 (18.0) | 0.0835 |
Increased ADRB2 5'-UTR methylation in blood is associated with increased risk for severe asthma
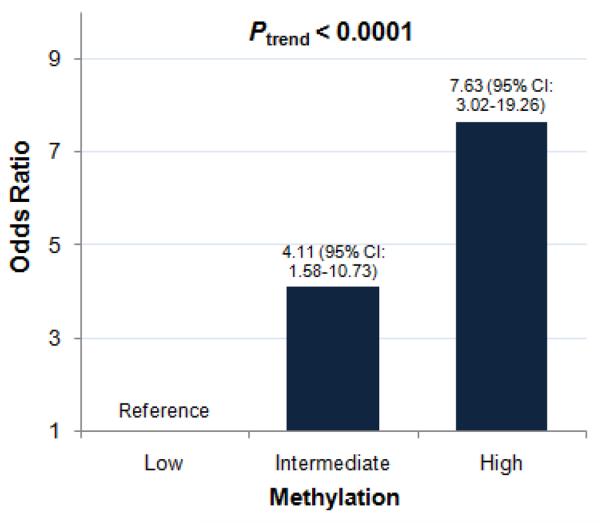
Methylation of three CpG sites located 1,357, 1,366, and 1,373 bases downstream of the transcriptional start site within the ADRB2 5'-UTR were interrogated for methylation differences between mild and severe asthmatic children (Supplemental Figure 2). Mean methylation was found to be higher in children with severe asthma (1.19%) than in children with mild asthma (0.89%). Similarly, median percent methylation values were higher in severe asthmatics (0.81%) than in mild asthmatics (1.14%). These subtle inter-group methylation differences were found to be highly statistically significant by both the independent t-test and the nonparametric Wilcoxon rank-sum test (P < 0.0001 and P < 0.001, respectively). The frequency distribution of ADRB2 methylation values among both mild and severe asthmatic children can be found in Supplemental Figure 3. When all methylation levels were categorized based on tertiles of the distribution of the mild group, we found a positive association between severe asthma and intermediate (OR: 4.11, 95% CI: 1.58–10.73) and high (OR: 7.63, 95% CI: 3.02–19.26) compared to low levels of ADRB2 methylation (Figure 1). Additional details on the distribution of ADRB2 methylation status by asthma severity status are included in the Supplemental Material (see Supplemental Table 1). No significant correlation was observed between NO2 exposure level and ADRB2 methylation in our study sample (data not shown), suggesting that the observed associations between ADRB2 methylation and asthma severity were unlikely to have been confounded by exposure to NO2, a known asthmogen.
Figure 1.
Associations between ADRB2 methylation and asthma severity. Percent methylation values were divided into tertiles (from the mild group distribution) of “low,” “intermediate,” and “high”. The odds for having severe asthma increase with increasing ADRB2 methylation in a linear, dose-dependent manner (Cochran-Armitage trend test (Ptrend < 0.0001)). The odds for having severe as opposed to mild asthma is 4.11 times as likely in children exhibiting an intermediate level of ADRB2 methylation and is over 7 times as likely among children exhibiting a high degree of methylation (N=182 children with asthma, CT and MA, 2006–2009).
Joint effects of NO2 exposure and ADRB2 5'-UTR methylation on asthma severity
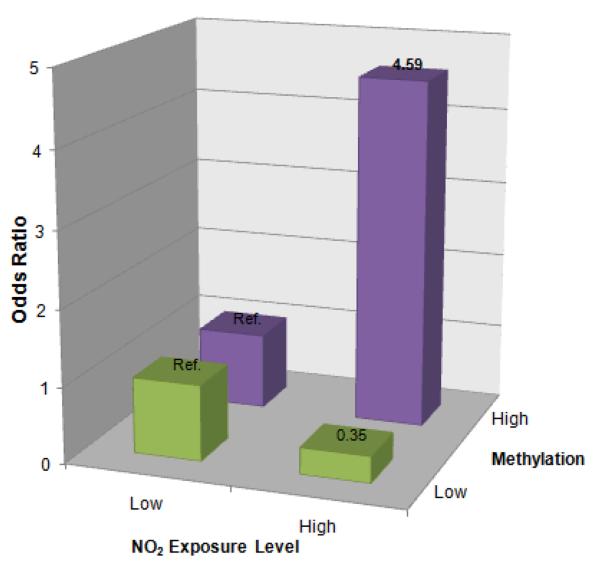
To assess the existence of joint effects between NO2 exposure and ADRB2 5'-UTR methylation on asthma severity, we stratified 60 mild and 122 severe asthma cases into “high” and “low” levels of ADRB2 methylation, with “high” as the combination of the top two methylation tertiles and “low” as the bottom methylation tertile. Separate stratified analyses were performed to test for associations between NO2 exposure and asthma severity in children exhibiting “high” ADRB2 methylation and children exhibiting “low” ADRB2 methylation. Stratified comparisons were performed using binary annual NO2 exposure levels above and below 11 ppb. While no significant association was observed between NO2 exposure and asthma severity in children with low ADRB2 methylation (OR: 0.35, 95% CI: 0.01–14.13), children exposed to high levels of NO2 are 4.59 (95% CI: 1.03–20.55) times more likely than children exposed to low levels to have severe asthma in the high methylation group (Figure 2). A Breslow-Day test for homogeneity of ORs yielded a statistically significant P-value of 0.029. Supplemental Table 2 describes the distribution of NO2 exposure levels by asthma severity status following stratification by ADRB2 methylation level.
Figure 2.
Joint effect between ADRB2 5'-UTR methylation and NO2 exposure on risk for asthma severity. 60 mild and 122 severe asthma cases were stratified into “high” and “low” levels of ADRB2 methylation, where “high” corresponds to top two methylation tertiles and “low” the bottom methylation tertile. Among children exhibiting a high level of ADRB2 methylation, those with high NO2 exposure had 4.59 (95% CI: 1.03–20.55) times the odds of severe asthma as those with low NO2 exposure. However, no significant association was found between high NO2 exposure and severe asthma among children exhibiting a low level of ADRB2 methylation (OR: 0.35, 95% CI: 0.01–14.13). These observations suggest that ADRB2 methylation may help to modify the effect of NO2 exposure on the progression from mild to severe asthma (N = 182 children with asthma, CT and MA, 2006–2009).
Discussion
Our results suggest that risk for asthma severity differs by levels of ADRB2 5'-UTR methylation in the blood, and that ADRB2 methylation and associated biological events may modify the effect of NO2 on asthma severity. These findings represent the successful identification of a possible epigenetic biomarker of asthma severity. They also indicate that seemingly very subtle changes in ADRB2 methylation within the blood may reflect large differences in phenotypic outcome. The observation of a dose-dependent response between ADRB2 methylation and risk for severe asthma suggests its potential utility in providing important information on susceptibility to more severe asthma at multiple levels of methylation.
NO2, a byproduct of fuel-burning appliances such as gas stoves and gas or oil furnaces, is an indoor trigger of asthma [22] and exposure to even low levels of NO2 may cause increased bronchial reactivity and make young children more susceptible to respiratory infections [23]. Our findings support the hypothesis that asthma development and severity may be affected by interactions between environmental and epigenetic factors. Our findings also suggest that relatively increased methylation of ADRB2 or biological processes associated with this altered rmethylation may be required for NO2-associated asthma exacerbations. However, a recent study found no significant associations between NO2 and asthma for any of the four ADRB2 tag SNPs among a population of 2,920 adults [24]. Possibly, the effect of NO2 on asthma severity is modified by ADRB2 methylation, but may not depend upon genetically induced alterations to ADRB2 expression or function. It is also plausible that the pathologic consequences of the joint effect of NO2 exposure and ADRB2 5'-UTR methylation are specific to asthma severity, as opposed to asthma development, and that this process might differ in children compared to adults.
In airway smooth muscle, increased methylation of ADRB2 may lead to the reduced expression of the ADRB2 gene, which may compromise its ability to act as a mediator of airway relaxation and the body's response to both endogenous and synthetic β2-agonists. Previous molecular and genetic studies have found that both ADRB2 expression and at least one ADRB2 genetic variant can alter bronchoreactivity to β2-agonists [12, 13]. Furthermore, studies of the effect of the use of the short-acting β2-agonist albuterol as monotherapy have shown that asthma control may deteriorate over time with regular use as opposed to intermittent use [12]. Reduced response to β2-agonist challenge as a result of the epigenetic downregulation of ADRB2 expression may prompt the more frequent use of short-acting β2-agonists, which in turn may further compromise the effectiveness of β2-agonist treatment and lead to the exacerbation of asthma symptoms. Among our subjects, 76 (42%) reported using β2-agonists in the month prior to the blood draw (75 subjects reported using short acting inhalers and 1 used long-acting medication). Although the mean difference in percent methylation was not significant (p=0.14), levels were higher in samples from the users of β2-agonists (mean [SD] 1.16 [0.56]%) compared to non-users (1.04 [0.42] %).
The viability of ADRB2 methylation as an epigenetic surrogate biomarker may depend on whether methylation changes in blood reflect changes found in airway smooth muscle. However, without tissue from airway smooth muscle, it is not possible to determine the degree of inter-tissue correlation of methylation patterns. Lack of tissue-specific mRNA in our study precluded the possibility of ascertaining the degree of phenotypic agreement between ADRB2 methylation changes in blood and potential expression changes in the airway. Differences in raw methylation values, although highly statistically significant, were nonetheless confined to a narrow range. However, it is important to note that these subtle changes in the blood may reflect far greater changes within airway tissue. Other limitations of our study include its relatively small sample size and its restriction to Caucasian subjects, thus limiting the generalizability of our findings across disparate ethnic groups. In addition, although blood collection was initiated prior to ascertainment of asthma severity status, it is nonetheless not possible to determine whether observed methylation changes preceded the onset of disease symptoms. Despite these limitations, our preliminary findings are promising and warrant further investigation in a larger pediatric population.
In summary, our results provide the first evidence of an association between epigenetic changes in an established asthma candidate gene and risk for severe asthma among children. Increased ADRB2 5'-UTR methylation in blood was found to be associated with increased risk for severe asthma in a population of children with active asthma. A joint effect between NO2 exposure and ADRB2 methylation in blood was also found, suggesting the possibility that increased ADRB2 methylation may synergistically enhance the asthmogenic effects of NO2 in asthmatic children. Future investigations are needed to further elucidate the nature of this interaction, including whether the observed associations reflect current effects or are the byproducts of cumulative effects during gestation and early childhood. Altogether, our study suggests the potential use of ADRB2 methylation in blood as a prognostic biomarker of both asthma severity and risk for NO2-associated asthma exacerbations.
Supplementary Material
Acknowledgements
This work was supported by the NIEHS grants ES011013 and ES05410 and funds from Yale University.
Footnotes
Conflicts of interest The authors declare no conflicts of interest.
References
- [1].Newacheck PW, Budetti PP, Halfon N. Trends in Activity-Limiting Chronic Conditions among Children. American Journal of Public Health. 1986;76:178–184. doi: 10.2105/ajph.76.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gergen PJ, Mullally DI, Evans R. National Survey of Prevalence of Asthma among Children in the United-States, 1976 to 1980. Pediatrics. 1988;81:1–7. [PubMed] [Google Scholar]
- [3].Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, Ball LB, Jack E, Kang DS. Surveillance for asthma--United States, 1960–1995. MMWR CDCSurveill Summ. 1998;47:1–27. [PubMed] [Google Scholar]
- [4].Weitzman M, Gortmaker SL, Sobol AM, Perrin JM. Recent trends in the prevalence and severity of childhood asthma. JAMA. 1992;268:2673–2677. [PubMed] [Google Scholar]
- [5].Weiss KB, Gergen PJ, Wagener DK. Breathing better or wheezing worse? The changing epidemiology of asthma morbidity and mortality. Annu Rev Public Health. 1993;14:491–513. doi: 10.1146/annurev.pu.14.050193.002423. [DOI] [PubMed] [Google Scholar]
- [6].Perera F, Tang WY, Herbstman J, Tang DL, Levin L, Miller R, Ho SM. Relation of DNA Methylation of 5 '-CpG Island of ACSL3 to Transplacental Exposure to Airborne Polycyclic Aromatic Hydrocarbons and Childhood Asthma. Plos One. 2009;4 doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Strauch K, Bogdanow M, Fimmers R, Baur MP, Wienker TF. Linkage analysis of asthma and atopy including models with genomic imprinting. Genetic Epidemiology. 2001;21:S204–S209. doi: 10.1002/gepi.2001.21.s1.s204. [DOI] [PubMed] [Google Scholar]
- [8].Ho SM. Environmental epigenetics of asthma: An update. Journal of Allergy and Clinical Immunology. 2010;126:453–465. doi: 10.1016/j.jaci.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morales E, Bustamante M, Vilahur N, Escaramis G, Montfort M, de Cid R, Garcia-Esteban R, Torrent M, Estivill X, Grimalt JO, Sunyer J. DNA Hypomethylation at ALOX12 Is Associated with Persistent Wheezing in Childhood. American Journal of Respiratory and Critical Care Medicine. 2012;185:937–943. doi: 10.1164/rccm.201105-0870OC. [DOI] [PubMed] [Google Scholar]
- [10].Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis. 1985;132:541–547. doi: 10.1164/arrd.1985.132.3.541. [DOI] [PubMed] [Google Scholar]
- [11].Hamid QA, Mak JC, Sheppard MN, Corrin B, Venter JC, Barnes PJ. Localization of beta 2-adrenoceptor messenger RNA in human and rat lung using in situ hybridization: correlation with receptor autoradiography. Eur J Pharmacol. 1991;206:133–138. doi: 10.1016/0922-4106(91)90021-9. [DOI] [PubMed] [Google Scholar]
- [12].Anderson GP. Current issues with beta2-adrenoceptor agonists: pharmacology and molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2006;31:119–130. doi: 10.1385/CRIAI:31:2:119. [DOI] [PubMed] [Google Scholar]
- [13].Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G, Liggett SB. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carroll CL, Stoltz P, Schramm CM, Zucker AR. Beta2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations. Chest. 2009;135:1186–1192. doi: 10.1378/chest.08-2041. [DOI] [PubMed] [Google Scholar]
- [15].Selivanova PA, Kulikov ES, Kozina OV, Gereng EA, Freidin MB, Ogorodova LM. Morphological and molecular characteristics of “difficult” asthma. Journal of Asthma. 2010;47:269–275. doi: 10.3109/02770900903584001. [DOI] [PubMed] [Google Scholar]
- [16].Panebra A, Schwarb MR, Swift SM, Weiss ST, Bleecker ER, Hawkins GA, Liggett SB. Variable-length poly-C tract polymorphisms of the beta2-adrenergic receptor 3'-UTR alter expression and agonist regulation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L190–195. doi: 10.1152/ajplung.00277.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by beta-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway beta-agonist paradox. J Clin Invest. 2003;112:619–626. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bai TR. Beta 2 adrenergic receptors in asthma: a current perspective. Lung. 1992;170:125–141. doi: 10.1007/BF00174316. [DOI] [PubMed] [Google Scholar]
- [19].Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. American Journal of Respiratory and Critical Care Medicine. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Palmes ED, Gunnison AF, Dimattio J, Tomczyk C. Personal Sampler for Nitrogen-Dioxide. American Industrial Hygiene Association Journal. 1976;37:570–577. doi: 10.1080/0002889768507522. [DOI] [PubMed] [Google Scholar]
- [21].Lu L, Katsaros D, de la Longrais IAR, Sochirca O, Yu H. Hypermethylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-II expression and favorable prognosis. Cancer research. 2007;67:10117–10122. doi: 10.1158/0008-5472.CAN-07-2544. [DOI] [PubMed] [Google Scholar]
- [22].Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care Med. 2006;173:297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Linaker CH, Coggon D, Holgate ST, Clough J, Josephs L, Chauhan AJ, Inskip HM. Personal exposure to nitrogen dioxide and risk of airflow obstruction in asthmatic children with upper respiratory infection. Thorax. 2000;55:930–933. doi: 10.1136/thorax.55.11.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Castro-Giner F, Kunzli N, Jacquemin B, Forsberg B, de Cid R, Sunyer J, Jarvis D, Briggs D, Vienneau D, Norback D, Gonzalez JR, Guerra S, Janson C, Anto JM, Wjst M, Heinrich J, Estivill X, Kogevinas M. Traffic-Related Air Pollution, Oxidative Stress Genes, and Asthma (ECHRS) Environmental Health Perspectives. 2009;117:1919–1924. doi: 10.1289/ehp.0900589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.