Abstract
Recent studies that have evaluated the immunologic factors which mediate the development of the two forms of IBD, namely Crohn’s disease and ulcerative colitis, have suggested that these diseases are due to disparate immune responses. While Crohn’s disease has been characterized as a dysregulation of the Th1/Th17 pathways more recent evidence has emerged that ulcerative colitis pathogenesis is associated with a nonclassical NK T cell producing an atypical Th2 (IL-13) response. In the following review the insights gained from both animal models and human studies as to the role that IL-13 and NK T cells play in the pathogenesis of UC will be discussed.
Keywords: Ulcerative colitis, Natural Killer T cells, IL-13
Introduction
In contrast to Crohn’s disease, the immunopathogenesis of ulcerative colitis (UC) has been a more difficult disease to ascertain. This owing to the fact that it did not fit neatly into a niche of the Th1/Th2 paradigm. In particular, it was evident that cells from this disease produced neither excessive IFN-γ (Th1), nor IL-4, the major Th2 cytokine (1). Indeed, IL-4 production was found to be decreased in cells extracted from ulcerative colitis tissue and only the increased presence of an additional Th2 cytokine IL-5, gave reason that the disease may be Th2 mediated.
Oxazolone Colitis
To understand better the immune responses that may play a role in the pathogenesis of human UC, initial investigations turned to the analysis of murine models of intestinal inflammation. One such model is that of the hapten-induced oxazolone colitis. In initial studies, it was found that administration of oxazolone led to a short-lived colitis (lasting less than 5 days in duration) which had features of human UC -- in particular superficial inflammation of the gut wall, associated with edema, ulceration of the epithelial cell layer and neutrophil accumulation (2). This pathological picture differed greatly from the other type of hapten induced colitis (TNBS-colitis) which was characterized by a densely packed, transmural lesion.
A second difference between TNBS-colitis and oxazolone-colitis also emerged from the studies of the cytokine secretion patterns in these inflammations. These studies revealed that CD4+ T cells present in oxazolone-colitis lesions produce initially a large amounts of Th2 type cytokines (IL-4 and IL-5) (2) in contrast to the increased amount of Th1 cytokines (IFN-γ) found in TNBS-colitis. Furthermore, it was evident that this Th2 cytokine response played an initial role in the immune pathogenesis of oxazolone-colitis since administration of anti-IL-4 to mice at the time of disease induction prevented the development of colitis. However, as this form of oxazolone colitis was short-lived in nature, further studies investigating a more prolonged form of oxazolone-colitis, lasting 1–2 weeks was examined (3). This was accomplished using a pre-sensitization regimen in which the abdomen was pre-coated with oxazolone 1 week prior to the administration of a low dose intra-rectal oxazolone challenge. This more chronic model demonstrated that the initial IL-4 response was superceded after about 4–5 days with an increasing IL-13 response. This latter response was intrinsic to disease pathogenesis since the colitis could be prevented by administration of IL-13R2α-Fc, a soluble receptor for IL-13 that blocks IL-13 interaction with its signaling receptor.
The next question to be addressed was the cellular origin of the cytokine response, particularly IL-13 production and its targets. Interestingly, this turned out to be a natural killer T cell (NK T cell), i.e., a CD4+ T cell bearing NK markers that take part in innate immune responses through their capacity to function as cytotoxic cells that can produce either Th1 (IFN-γ) or Th2 (IL-4/IL-13) cytokines under various circumstances. In order to understand the significance of how NK T cells may be intrinsic to the inflammation of oxazolone-colitis, we need to discuss the nature and function of these cells. An NK T cell has characteristics of both an NK cell and a cell with T cell receptors (TCRs) that nevertheless bears surface NK markers (such as NK1.1). In addition to its distinctive surface markers, the NK T cell can be defined by the fact that it recognizes glycolipid antigens presented to it by a highly conserved MHC class I-like molecule, (CD1 in mice and CD1d in humans) and in most cases it expresses an invariant TCR that is made up of a particular TCRα chain (Vα14-Jα281 in mice and Vα24-Jα18 in humans) linked to a restricted repertoire of Vβ chains, hence the fact that it can be stimulated by a particular prototype glycolipid, α-galactosyl-ceramide (4). However, it is important to recognize that while the great majority of NK T cells have these attributes, a minority of NK T cells (“non-classical” NK T cells), while still recognizing antigen restricted by CD1, bear non-invariant TCRs (5). An additional characteristic of NK T cells is their ability to act as cytotoxic T cells and it is this property that accounts for the fact that NK T cells have anti-tumor cell activity and act as effector cells in certain inflammatory states such as experimental hepatitis (6). In the latter regard, it has been shown that NK T cells in hepatic lesions secrete perforin and granzymes that mediate cellular injury (7). Finally, it is important to emphasize that NK T can be activated by CD1-bearing antigen presenting cells in the absence of added antigen (8). This latter point indicates that NK T cells probably recognize self-antigens present in the groove of CD1 derived antigen-presenting cells; in other words, NK T cells can be self-antigen specific. Thus, NK T cells are important components of the innate immune system since they do not require exogenous antigens to begin secreting cytokines.
Evidence for the identification of the NK T cell as the source of IL-13 in oxazolone-colitis drew on these NK T cell characteristics and included the fact that administration of anti-NK T cell antibodies to mice prevented development of oxazolone-colitis. In addition, the latter could not be induced in mice deficient in CD1 (3). Finally and perhaps most importantly, stimulation of lamina propria T cells extracted from oxazolone-colitis tissue with antigen-presenting cells bearing CD1 resulted in IL-13 secretion. Therefore, these studies of a murine model of UC strongly suggested that the pathogenesis of the colitis was due to the generation of NK T cells that secrete IL-13. From these data it was inferred that oxazolone-colitis could be due to either a direct effect of IL-13 on epithelial cells or a (cytolytic) effect of NK T cells on epithelial cells.
Taken together, this information concerning NK T cells, it is possible to construct a mechanism of how these cells mediate oxazolone-colitis (see Figure 1). First, oxazolone may act as a unique kind of haptenating agent that has the particular ability to induce NK T cell development, presumably by giving rise to a lipid moiety that is presented to a small but expandable population of NK T cells in the context of CD1 on antigen presenting cells. In the intestinal tract, the latter would include not only antigen presenting cells, but also gut epithelial cells, which have been shown to express CD1 (9). Second, these NK T cells then proliferate and differentiate into Th2 type cells. Initially, they begin to produce IL-4 and IL-13, the latter a cytokine which may act as an autocrine factor that causes the NK T cells to become cytotoxic effector cells with the capacity to cause tissue injury. The immediate target of possible NK T cell activity in oxazolone colitis may in fact be the epithelial cell since, as mentioned, this cell bears CD1 and can present antigens to NK T cells. Such cytotoxicity could be responsible for the ulceration of the mucosa and observed alteration of the epithelial barrier. Third, once this occurs, there is the possibility of penetration of the mucosa by bacteria in the mucosal microflora and thus further NK T cell stimulation by antigens in the flora that are cross-reactive with self antigens. However, these latter points concerning changes in epithelial barrier function may be brought about not only by NK T cells but by IL-13 itself.
Figure 1.
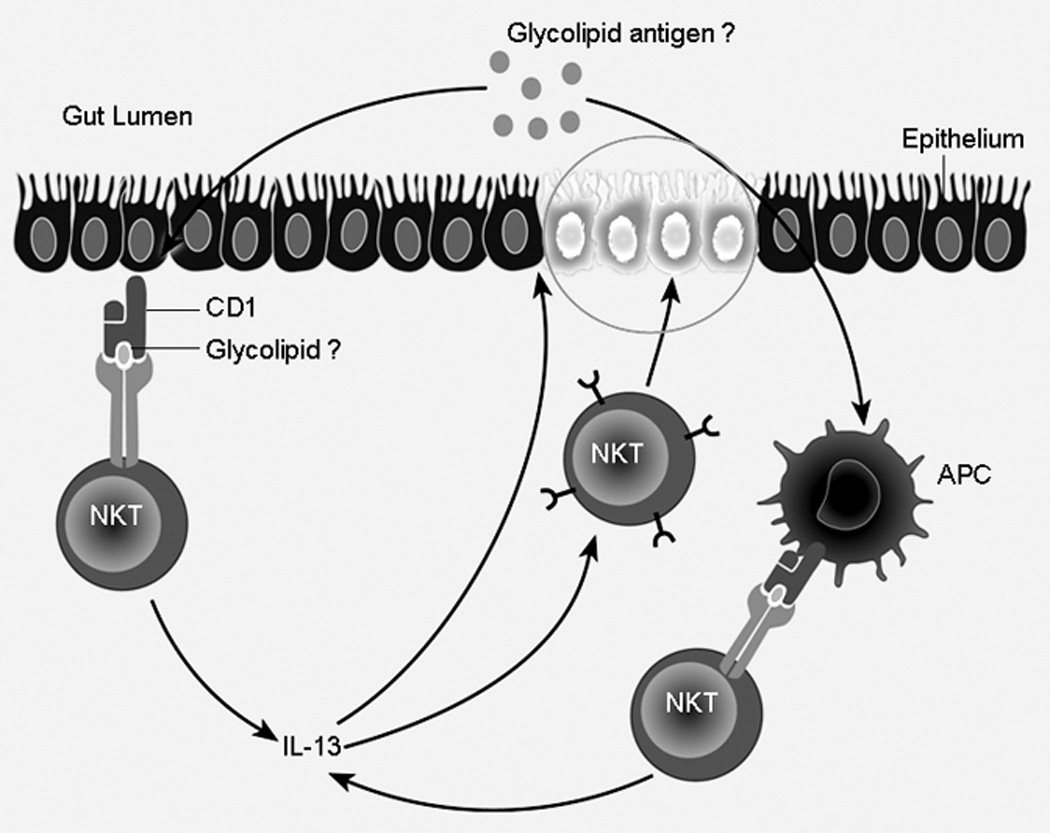
Proposed Mechanism for experimental and human ulcerative colitis. Innate antigen is presented to and taken up by LP antigen presenting cells bearing CD1, which in turn presents to NK T cells. These NK T cells are then activated to secrete IL-13. IL-13 may bring about changes in epithelial barrier function leading to further antigen exposure. In addition, NK T cells upon activation may be directly cytotoxic to epithelial cells.
In further studies, investigating the role of IL-13 and epithelial barrier function, Heller et al. demonstrated that IL-13 can have a direct effect on epithelial barrier function in a multitude of ways (10). Concerning effects on epithelial cells, when IL-13 was added in vitro, a dose-dependent decrease in electrical resistance of HT-29 epithelial cell monolayers was observed. Concomitantly, an increase in cellular epithelial apoptotic rate was also found. These changes in epithelial cell function both could give rise to a change in barrier function.
Furthermore, the addition of IL-13 in vitro to these monolayer cultures led to an increased expression of the pore-forming tight junction protein claudin-2. This protein when found at increased levels is associated with increased epithelial barrier permeability. More significantly, examination of the lamina propria tissue from human ulcerative colitis patients also demonstrated similar changes in claudin-2 expression. These results suggest that the changes in barrier function in human UC may not only be due to cytolytic destruction of the epithelial layer by NK T cells but concomitantly due to a more subtle alteration secondary to effects from IL-13. Thus, this cytokine may have a dual pathogenic role – one directly affecting epithelial cells and a second as a stimulant of NK T cell cytotoxicity.
Ulcerative Colitis
Given the above findings, the next series of studies focused on the human disease counterpart, ulcerative colitis (UC) to ascertain if similar mechanisms played a significant role. Here it was found that lamina propria cells isolated from inflamed UC tissue stimulated in vitro did indeed produce increased amounts of IL-13 as compared to cells from both control individuals and patients with Crohn’s disease (11). Moreover, depletion of cells bearing a marker found on NK T cells (CD161) from the lamina propria cell population extracted from UC tissue, followed by stimulation in vitro led to greatly decreased IL-13 production.
Further studies investigating the link between NK T cells and IL-13 production in UC came from flow cytometric analysis that revealed that whereas both UC and CD lamina propria mononuclear cells contained increased numbers of cells bearing a surface marker found on NK T cells (CD161), those from UC specimens were producing IL-13 whereas those from Crohn’s disease specimens were producing IFN-γ (11). Perhaps more importantly, it was observed that antigen presenting cells bearing a CD1d construct (and thus expressing CD1d on its surface) could only induce lamina propria mononuclear cells from UC patients but not that of Crohn’s disease to produce any cytokine (in particular, IL-13). Thereby, establishing that, in fact, the cytokine secretion profile seen in UC was produced from a non-classical CD1 dependent NK T cell whereas the cytokines produced in Crohn’s disease were from that of an activated classical Th1 CD4+ T cell.
One final important finding in the study of UC patients only hinted in the animal studies, was that lamina propria cells enriched for NK T cells from the patients could be shown to be cytotoxic for epithelial cells and such cytotoxicity was further enhanced by IL-13 (11), suggesting an associated link between IL-13 and NK T cells in the pathogenic mechanisms of this inflammation. On this basis, an additional possibility for the immunopathologic mechanism operating in UC is that antigens in the mucosal microflora activate NK T cells due to aforementioned changes in barrier function that, in turn, cause cytolysis of epithelial cells and the characteristic ulcerations associated with the disease. However, additional factors other than that of the mucosal flora may activate NK T cells. As suggested, enhancement of cytolytic activity was observed in vitro in the presence of IL-13. IL-13, therefore, may play an autocrine role in the activation of these cells. How IL-13 may function in this capacity may be inferred from a recent study (12). IL-13 was shown to have direct effects on activation of cytokine transcription. These studies demonstrated that TGF-β transcription was dependent upon IL-13 acting through a receptor previously thought to function as a decoy (IL-13Rα2). Whether IL-13 can indeed induce transcription of other cytokines (namely itself) or activation of NK T cells through a similar manner remains to be determined.
In summary, these considerations bring us back to the opening comments of this discussion. The point to make is that we have made many inroads to the understanding of inflammatory bowel disease in all its complexity. The information obtained point towards new avenues of treatment. However, the final proof that IL-13 and NK T cells play a significant role in human UC awaits study in a human trial in which these components may be blocked or depleted.
Footnotes
Disclosure
Ivan J. Fuss is a patent holder for the use of anti-IL-12 mAb in the treatment of Crohn’s disease.
References
- 1.Fuss IJ, Neurath M, Boirivant M, Klein JS, et al. Disparate CD4+ lamina propria (LP) lymphokines secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-γ, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J. Immunol. 1996;153(3):1261–1270. [PubMed] [Google Scholar]
- 2.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazalone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 1998;188(10):1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller F, Fuss IJ, Niewenhuis EE, Blumberg RS, Strober W. Oxazalone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17(5):629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2002;2(8):557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 5.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 6.Baron JL, Gardiner L, Nishimura S, Shinkai K. Activation of a nonclassic NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16(4):583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 7.Dao T, Mehal WZ, Crispe IN. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J. Immunol. 1998;161(5):2217–2222. [PubMed] [Google Scholar]
- 8.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent beta 2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182(6):1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Wal Y, Corazza N, Allez M, Mayer LF, Iijima H, et al. Delineation of a CD1d-restricted antigen presentation pathway associated with human and mouse intestinal epithelial cells. Gastroenterology. 2003;124(5):1420–1431. doi: 10.1016/s0016-5085(03)00219-1. [DOI] [PubMed] [Google Scholar]
- 10.Heller F, Florian P, Bojarski C, Richter J, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Fuss IJ, Heller F, Boirivant M, Leon F, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, et al. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat. Med. 2006;12(1):99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]


