Abstract
Molecular mechanisms regulating TGF-β induction of Foxp3 expression and thus induction of iTregs has been the focus of a great deal of study in recent years. It has become clear that this process is influenced by a number of factors as perhaps might be predicted by the fact that there is an overarching need of the immune system to fine-tune response to environmental antigens. In this review we discuss these mechanisms, with the aim of presenting a broad picture of how the various observations fit together to form an integrated regulatory regime.
Introduction
The central role of the transcription factor Foxp3 in regulatory T cell (Treg) activity was initially identified by Sakaguchi’s group and others1–5 who showed that both mice and humans with genetic defects leading to defects in the expression of this factor were subject to autoimmune and inflammatory disease. In addition, they showed that transduction of naïve cells with a Foxp3-expressing retrovirus converted the latter into regulatory cells. However it is still quite unclear how Foxp3 orchestrates the Treg suppressor program.
Given the importance of Foxp3 to Treg function it becomes apparent that a critical step in Treg development is the induction of Foxp3. For such development to occur in the thymus, IL-2 appears to be a necessary factor since IL-2 deficient mice have reduced Foxp3+ Tregs and exhibit autoimmunity6. In contrast, for such development to occur in the peripheral immune system, TGF-β (in addition to IL-2) is a necessary factor; thus, in initial studies Horwitz and his colleagues7 showed that TGF-β induces naïve peripheral human T cells to become functional Tregs and Chen et al8 showed that TGF-β induced naive (TCR-activated) CD4+ T cells to express Foxp3. More recently, Coombs et al.9 and Sun et al.10 have shown that CD103+ dendritic cells in the lamina propria of the colon produce all-trans retinoic acid which then enhanced TGF-β-induced naïve CD4+ T cell conversion into Tregs and thereby contributed to the regulation of mucosal responses. This function of mucosal dendritic cells makes the process of TGF-β-induced Treg induction particularly relevant to the understanding of mucosal immunology.
TGF-β-induced Tregs (hereafter called induced Tregs, iTregs) are cells that participate in regulatory responses to environmental antigens and thus appear to be a way in which the immune system greatly expands its repertoire of regulatory cells. While they lack the complete molecular “signature” of natural (thymus-derived) Tregs and differ from the latter functionally as well11, they have been shown to be fully capable of regulatory function in all studies of murine cells and in most (but not all) studies of human cells8,11,12,13.
In the past half decade numerous studies have appeared attempting to define the molecular mechanisms governing TGF-β-mediated induction of Foxp3, the enhancement of such induction by retinoic acid and the positive and negative influence of various cytokines on this process. While our understanding of this area of immunoregulation has advanced as a result of these studies a number of unresolved and important issues remain. In this review we will discuss the salient features of this body of work with an eye to defining what is now known and what remains to be discovered.
The Basic Topography of the Foxp3 Gene and the Mechanism of TGF-β Induction of Foxp3
As initially shown by Mantel et al.14 in human CD4+ T cells, and then more completely by Tone et al.15 in mouse CD4+ T cells the Foxp3 gene ATG translation start site is >6 kb downstream of the transcription start site in exon -2b so only exons 1–11 are translated. Importantly, the intron between exon -2a and exon -1 (hereafter referred to as intron 2) contained two conserved noncoding sequences (CNS) which could represent enhancer regions (Figure 1).
Figure 1.
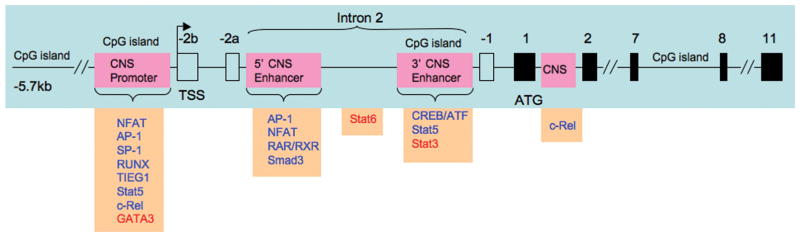
Structure of the murine Foxp3 gene. Locations of CNS (conserved non-coding sequences) in the promoter and intron 2 are shown in pink; the 5′ and 3′ CNS in intron 2 serve as enhancer sites. Location of CpG islands are also shown; these islands exhibit demethylation in the active gene. Transcription factors binding to the promoter and enhancer sites shown in orange boxes; positive factors shown in blue; negative factors shown in red. See text for further details.
Mantel et al. focused their attention on the human FOXP3 promoter and showed using a promoter-driven-luciferase assay in activated CD4+ T cells that the promoter contained TATA, GC and CAAT boxes that when mutated exhibited decreased activity. In addition they showed that: 1) the promoter contained three conserved NFAT binding sites within the 500 bp segment 5′ to the transcription start site, two of which were located near AP-1 sites; 2) mutation of one of the AP-1 sites (that at position -324 which was adjacent to an NFAT site) led to greatly decreased promoter-driven luciferase activity and 3) binding of Sp-1 to a GC site and NFATc2 to NFAT sites could be demonstrated. These findings correlated with the fact that anti-CD3 alone can upregulate Foxp3 expression in CD25− T cells, although not to the level seen in unstimulated CD25+ cells, and that Foxp3 expression was down-regulated in anti-CD3-stimulated cells cultured in the presence of cyclosporin A, a known inhibitor of NFAT activity. These studies thus suggested that, in humans, the FOXP3 promoter plays a role in anti-CD3-driven Foxp3 expression probably via its NFAT/AP-1 site(s). Moreover, this promoter function is independent of the inductive effect of TGF-β.
Tone et al. in initial studies of mouse Foxp3 expression similar to those of Mantel et al. showed with luciferase reporter assays in EL-4 cells (a cell line that supports TGF-β-induced Foxp3 expression) that while the mouse Foxp3 promoter contains NFAT and Sp-1 binding sites corresponding to those in the human promoter it exhibits negligible promoter activity; this corresponded to the fact that NFAT and Sp-1 binding to their target sequences in the promoter was quite weak. It should be noted however, that histone acetylation of the Foxp3 promoter of CD4+ T cells stimulated with anti-CD3 and TGF-β was elevated in proportion to the level of induced Foxp3 expression in spite of the fact that the promoter was devoid of Smad binding sites. This apparent contradiction was resolved in very recent studies from two groups16,17. These investigators showed first that the mouse Foxp3 promoter has c-Rel-NFAT binding sites and that c-Rel-deficient mice have greatly reduced numbers of Tregs. They then showed that these sites form an “enhanceosome” containing not only c-Rel and NFATc2, but also p65, Smad3 and pCREB, the latter two recruited from intronic enhancer sites discussed below. Such recruitment occurs in a sequential manner in the several hours after induction of Foxp3 in naïve cells with TGF-β so that initially cRel, p65 and NFATc2 are bound followed later by Smad and CREB; ultimately, however, c-Rel and pCREB dissociate from the site. Finally, they showed that concomitant with the appearance of Smad to the promoter enhanceosome, there was decreased binding to the upstream enhancer. These studies thus show that NF-κB activation plays an important role in Foxp3 expression and that transcription factors initially binding to intronic enhancer sites can later participate in transcriptional activation of the Foxp3 promoter.
Turning our attention now to the role of enhancer sites in Foxp3 transcription it is best to start with a consideration of the major Foxp3 enhancer site in intron 2 extensively studied by Tone et al., the investigators already mentioned above in relation to their work on Foxp3 promoter function. Additional enhancer sites will be discussed subsequently in relation to epigenetic processes involved in Foxp3 gene regulation. Focusing on the possible regulatory role of conserved sequences in the intronic region (between exon -2a and -1, i.e., intron 2), Tone et al found that the 5′ intronic sequence, a 0.75kb segment located at +1988 to +2738, was associated with acetylated histone H4 in CD4+Foxp3+ primary T cells and was thus accessible to transcription factor binding. In addition, they showed with segment-driven luciferase assays, that this segment contained two regulatory elements one at +2137 to +2158 which bound NFAT and the other at +2177 to +2198 which bound Smad3 (presumably pSmad3) in anti-CD3/CD28 and TGF-β stimulated cells. Interestingly, the Smad3 bound not to the conventional Smad regulatory element in this region, but rather to an adjacent inverted repeat. These data indicated that the conserved sequence in intron 2 was indeed a bone fide enhancer element.
In further studies to determine the contributions of this enhancer to Foxp3 expression Tone et al. performed deletional analysis of the enhancer sequence using enhancer-driven luciferase assays and showed that both the NFAT and Smad binding sites contributed to enhancer activity. However, while deletion of the NFAT site led to loss of all activity, deletion of the Smad site led to residual activity induced by anti-CD3/CD28; thus, the latter stimulus can induce Foxp3 through NFAT in the absence of TGF-β. Finally, the importance of NFAT and Smad3 in Foxp3 expression was shown by the fact that addition of inhibitors of either Smad3 (SIS3) or NFAT (cyclosporin A) inhibited enhancer-driven luciferase activity.
The overall implication of the studies of Tone et al. is that the mechanism underlying TGF-β-driven of Foxp3 expression, at least in mice, involves the induction of activated Smad3 (pSmad3) which acts as a powerful transcription factor for the Foxp3 gene. As we have seen, induced Smad3 initially binds to an enhancer site in intron 2 and then interacts with NF-κB components, NFATc2 and CREB to form a complex (an enhanceosome) binding to the Foxp3 promoter. It should be noted, however, that there are no Smad3 binding sites in the promoter and therefore Smad3 probably interacts with the promoter indirectly via binding to other components of the enhanceosome. A second way of characterizing TGF-β induction of Foxp3 expression is based on a fact not yet discussed, namely that TGF-β induces increased H4 histone acetylation in the region of the NFAT/Smad binding. This implies that TGF-β is also involved in other epigenetic changes that, as discussed below, are a general feature of Foxp3 gene activation, including activation occurring in the thymus. In this scenario, TGF-β induction of activated Smad3 and its subsequent binding to the intron 2 enhancer also causes distant increases in the accessibility of the promoter to various transcription factors such as NFAT and c-Rel and in this way facilitates Foxp3 promoter activity. In this way one can explain the observation that Foxp3 expression is also regulated by Itch, a TGF-β-induced E3 ubiquitin ligase that ubiquitinates TIEG1, the latter a factor that binds to the SP-1 site in the Foxp3 promoter and induces Foxp3 transcription18.
Conserved Non-Coding Sequences and Epigenetic Control of Foxp3 Expression
Epigenetic control of gene expression encompasses changes in histones and DNA that affect the accessibility of promoter and enhancer regions to transcription factors, including conserved non-coding sequences that serve as enhancers. Quiescent genes are characterized by CpG sequences in which the 5′ cytosines are methylated since methylation retards transcription factor binding to DNA, either directly or by interacting with transcriptional repressors such as methyl binding proteins and methyl transferases. In addition, methyl binding proteins prevent histone acetylation (a characteristic of open chromatin) by recruiting histone deacetylases. Active genes, in contrast, are characterized by demethylated CpG sequences and acetylated histones in both promoter and enhancer regions. In general, stable and perhaps irreversible activation of particular genes during cell differentiation is characterized by the above epigenetic changes in these genes; however, partial or incomplete epigenetic changes can occur in genes being turned on and off.
As indicated above, Mantel et al. discovered several conserved non-coding sequences (CNS) in the human FOXP3 gene, including the two in intron 2, already mentioned, as well as one in the promoter and perhaps several others in downstream areas. In studies of the murine Foxp3 gene Kim and Leonard19 corroborated this finding although in this case the intronic CNS regions had somewhat different locations. In addition, they showed that the most downstream (or 3′) of these regions located at +4301 to +4500 had very considerable promoter activity in luciferase reporter assays conducted in stimulated Jurkat cells and was thus an enhancer region. Further analysis revealed that this enhancer contained a cyclic AMP response element binding protein(CREB)/activating factor (ATF) motif which in fact bound CREB in EMSA. In addition, mutation of this site or a dominant-negative CREB greatly inhibited the enhancer acitivity of this CNS in a luciferase reporter assay.
Kim and Leonard also examined the methylation status of CpG sequences in the Foxp3 gene of naïve and regulatory cells not expressing or expressing Foxp3 respectively recognizing, as discussed above, that demethylation of CpG correlates with gene expression. They found that the promoter and intron 2 CNS enhancer regions of the Foxp3 gene in regulatory cells contained virtually unmethylated DNA whereas corresponding regions in naïve cells exhibited a high degree of DNA methylation. This correlation was particularly striking with respect to the 3′ intron 2 CNS enhancer which contained only a single CpG island. Finally, along similar lines, they found that naïve CD4+ T cells exposed to TGF-β to induce expression of Foxp3 also displayed a lack of methylation, especially in this 3′ intron 2 CNS enhancer. Overall, these studies were compatible with the view that T cell activation and/or TGF-β signaling results in epigenetic modifications of the Foxp3 gene at least in part mediated by CREB binding to the 5′ enhancer in the Foxp3 gene intron 2. These modifications involve demethylation of CpG motifs both in the promoter and the 3′ intron 2 enhancer which significantly increases accessibility of gene regulatory regions to transcription factors.
Further studies focused on the role of epigenetic factors in maintaining stable Foxp3 expression. Floess et al.20 found that a conserved region in intron 2 corresponding to the more 3′ CNS referred to above as well as a conserved region in the intron between exons 7 and 8 contained mainly demethylated CpG sequences and exhibited histone acetylation in Foxp3+CD25+CD4+ cells from spleen and lymph nodes whereas the same sequences in Foxp3−CD25−CD4+ cells contained methylated CpGs and no histone acetylation. In addition, TGF-β-induced Foxp3+CD4+ cells manifest only partially demethylated CpG sequences in the intron 2 conserved region and no demethylation in a downstream intron between exons 7 and 8 (intron 10). This relative lack of demethylation in TGF-β-induced cells correlated with the fact that these cells lose Foxp3 expression upon restimulation in the absence of TGF-β, leading Floess et al. to conclude that the demethylation was necessary to maintain a stable Treg phenotype.
Similar findings were reported by Lal et al.21 who in this case focused on a far upstream CNS some 5–6000 bp 5′ to the transcription start site that also contained a CpG island. This region was heavily methylated in non-Treg cells and even in TGF-β-induced Tregs but not in thymus-derived Tregs. In addition, this region was characterized by bound methyl-binding proteins and methyl transferases. Not unexpectantly, exposure of cells with this methylated DNA region to a DNA methylase inhibitor 5-aza-2′-deoxycytidine resulted in the induction of histone acetylation as well as binding of transcription factor to down-stream promoter sites such as TIEG1 and Sp-1 binding. In addition, it resulted in stable Foxp3 expression and acquisition of suppressor activity upon TGF-β stimulation. On the other hand, an inhibitor of Foxp3 expression, IL-6, led to methylation of this region22. These studies thus raise the question of whether methyl transferase inhibitors can regulate suppressor T cell generation and function.
That latter idea was further examined by Josefowicz et al.23 who showed that mice with methyl transferase I deficiency exhibit greatly increased induction of Foxp3+ thymocytes and peripheral T cells by TCR stimulation alone and such induction is not further increased by TGF-β. Methyl transferase recruitment is a cell cycle dependent event and thus the authors proposed that the function of TGF-β in Foxp3 expression is, in part, repression of cell-cycle dependent recruitment of methyl transferase either directly or via negative regulation of cell proliferation caused by p27 activation. This theory, however, may be too sweeping since the main enhancer affecting Foxp3 expression, the 5′ enhancer in intron 2 investigated by Tone et al. is not a region subject to methylation. A theory somewhat more compatible with the data presently at hand is that demethylation of the Foxp3 gene in some or all of the conserved non-coding sequences discussed above relates mainly to the effects of TCR (and possibly IL-2) stimulation occuring first in the thymus with respect to “natural” Tregs and second in the periphery with respect TGF-β-induced “adaptive” Tregs. Furthermore, these epigenetic changes, while initiated by cell stimulation, are mediated by the induction of factors such as CREB/ATF or, as indicated below, by Foxp3-Runx1 complexes.
Most recently, a sweeping advance in our knowledge of how conserved regions in and around the Foxp3 gene operate has come from a recent study by Zheng et al.24 defining the function of these regions with mice in which the various conserved regions were deleted. These investigators identified a conserved region in intron 3 as a “pioneer” CNS whose deletion results in a greatly reduced numbers of Tregs; this region therefore determines the overall size of both the thymic and peripheral Treg population. This region binds c-Rel (as does a promoter site described above) which is presumably generated by TCR stimulation. A second CNS region, one corresponding to the region identified above as the 3′ enhancer in intron 2, is necessary for induction of Tregs in the periphery by TGF-β, consistent with the prior studies of Tone et. al. Deletion of this CNS leads to reduced numbers of Tregs in the mucosal tissues in correlation with the fact that these are the tissues most associated with dendritic cell mediated TGF-β- and retinoic acid-induction of Tregs. A third and final CNS region corresponding to the 3′ enhancer in intron 2, was not involved in either thymic generation of Tregs nor in peripheral induction of Tregs; it was, however, necessary for the maintenance of Foxp3 expression in dividing cells and thus deletion of this region was associated with age-associated decreases in Tregs. Interestingly, this region was shown to bind Foxp3 itself when in a demethylated (i.e., active) state; thus, production of Foxp3 may itself be necessary for the maintenance of a stable Treg population. Finally, previous studies had shown that Runx1 (bound to Cbf-β) binds to various CNS regions and has effects on Foxp3 expression25. This observation was expanded upon by Zheng et al.24 who showed that Foxp3 binding to the intron 2 enhancer actually involves the Foxp3 complexed to Runx1 and occurs at Runx1 binding sequences in the enhancer.
Overall, the Zheng et al. studies of the enhancer activity of conserved non-coding sequences as well as previous studies of the role of epigenetic effects on Foxp3 gene expression greatly expand our knowledge of the molecular regulation of the Foxp3. Nevertheless, they still leave unanswered many questions concerning the way these distal regions physically initiate/facilitate Foxp3 transcription. Some inkling of the way this might occur comes from the recent studies of Ruan et al.16 in that these studies provide evidence of molecular interactions between enhancer and promoter regions, but additional studies are necessary to fully elucidate this question.
The Regulation of TGF-β-Induced Foxp3 Expression by Cytokines
Numerous studies have established beyond doubt that cytokines regulate Foxp3 expression both positively and negatively (Figure 2). This is not unexpected given the fact that there is an absolute need for the immune response to regulate the level of Treg activity in any given immune response and the most salient way of accomplishing this is through the secretion of various cytokines. Such regulation at once correlates effector cell activity in host defense and inflammation on one hand with the need to control over-exuberant responses on the other.
Figure 2.
Possible mechanisms of the effect of retinoic acid on Foxp3 gene transcription. As indicated in the Figure, retinoic acid may have direct effects by acting via RAR/RXR or may have indirect effects on various cytokines that positively and negative influence Foxp3 expression. See text for specific details.
A. Positive Cytokine Regulation
Regulation of TGF-β-induced Foxp3 by IL-2
Regulation of TGF-β-induced Foxp3 by IL-2 needs to be discussed first, given the known importance of this cytokine in Treg development in the thymus (reviewed elswhere26,27). As alluded to above, this relationship first came to light with the observation that IL-2 deficiency is associated with the autoimmunity manifesting as colitis and early studies showing that such autoimmunity is in fact due to reduced secretion of regulatory cytokines28–30. Later, it was shown that IL-2 was also necessary for TGF-β-induced Foxp3 expression and Treg development31. Recently, the importance of IL-2 to Treg development has been studied at the molecular level. In initial studies by Burchill et al.32 it was found that T cell-specific deletion of Stat5 (the main Stat induced by IL-2) and reconstitution of IL-2Rβ-deficient mice with bone marrow cells expressing a mutant IL-2Rβ that only activates Stat5 restores the ability of the mice to develop Tregs. That Stat5 was acting to induce Foxp3 expression was suggested by concomitant studies showing that Stat5 bound to the Foxp3 promoter. Later studies by Zorn et al.33 showed that IL-2 increased Foxp3 expression in human CD25+ T cells (but not CD25− T cells) and that a luciferase reporter gene driven by human Foxp3 enhancer (the more 3′ intron 2 CNS) gave a positive signal if co-transfected with either a Stat5- or Stat3-expressing vector. In addition, these authors showed that treatment of patients that have undergone bone marrow treatment with IL-2 leads to substantial increases in Foxp3 expression in CD25+ cells. Perhaps the most definitive studies of the relation of Stat5 to Foxp3 expression, however, have come from O’Shea and his associates34,35 who showed that Stat5-deficient mice as well as Jak3- and Il2rg-deficient mice with defective IL-2 signaling, lacked cells in the thymus or the periphery that expressed Foxp3. Moreover, stem cell transplantation studies and studies of tissue specific Cre mice mated to mice with floxed Stat5 genes strongly suggested that the T cell-specific Stat5 deficiency also results in lack of Foxp3 expression. On the other hand, mice with conditional Stat3-deficiency had normal numbers of cells expressing Foxp3. Thus, they could distinguish between the effects of Stat5 and Stat3 in the positive regulation of Foxp3. Finally, this group of authors assessed the importance of IL-2 to TGF-β-induced Tregs and found that, here again, induction was greatly impaired by defective IL-2 signaling.
Related to the above results is the fact that two Stat5 binding sites were found in the promoter region of Foxp3 gene as well as one in the intron 2 region corresponding to the more 3′ CNS which also is a CREB/ATF binding region as discussed above. ChIP studies provided evidence that Stat5 binds to these sites in CD25+ T cells obtained from the thymus and spleen and then cultured in the presence of IL-234. Thus, IL-2 signaling through Stat5 appears to be an important positive stimulus for Foxp3 expression.
Finally, it should be noted that human cells are somewhat different from mouse cells with respect to IL-2 and Stat5, as already suggested above in studies of the human and mouse Foxp3 promoter function. Thus, as shown by Passerini et al.36 IL-2 alone is sufficient to induce Foxp3 in TCR-stimulated human CD4+ T cells, as are (to a lesser extent) other Stat5 inducing cytokines such as IL-15 and IL-7 but not with cytokines not inducing Stat5 such as IL-4 and TGF-β. In the latter regard, IL-2 mediated induction was not augmented by TGF-β. These results correlated with molecular studies which showed that transfection of cells with a vector expressing Stat5 was sufficient to induce Foxp3 in activated cells. Thus, IL-2 and Stat5 in the absence of TGF-β are more capable of inducing Foxp3 in human cells than in mouse cells. However, it should be noted that the induced cells are not anergic and do not exhibit suppressor function13; thus, it is not clear that IL-2 alone is capable of inducing functional Tregs even in humans.
B. Negative Cytokine Regulation
Regulation of TGF-β-Induced Foxp3 Expression by IL-6
Negative regulation of Foxp3 and suppressor cell development is provided by IL-6 was initially shown by Pasare and Medzhitov37 and later by other investigators38,39,40. Strong evidence that such regulation occurs via Stat3 was provided by Yao et al.34 who showed that IL-6 fails to regulate TGF-β-induced Foxp3 expression in mice that are Stat3 deficient due to a conditional T cell-specific Stat3 gene deletion. In addition, Yao et al. showed with ChIP studies that Stat3 binds to the same conserved region in intron 2 of the Foxp3 gene as does Stat5, probably to the same target site since the Stat3 and the Stat5 target sites are very similar. Stat3 binding to this site, however, was much weaker than Stat5 binding, raising the possibility that Stat5 binding to this site inhibits Stat3 binding except under conditions in which the cell is exposed to IL-6 and expresses high amounts of activated Stat3. Finally, it should be noted that while Stat3 appears to be implicated in IL-6-mediated down-regulation of TGF-β-induced Foxp3 expression it also appears to have a positive effect on TGF-β-induced Foxp3 expression or on the maintenance of natural Treg phenotype and function41,42,43,44,45. Thus, Stat3 may exert both positive and negative regulatory effects on Foxp3 expression depending on dose of activated Stat3 or on the presence of as yet undefined regulatory co-factors.
Two other cytokines that induce Stat3, IL-2146 and IL-2747,48 (see discussion below), also has inhibitory effects on TGF-β-induced Foxp3 expression; on this basis it is curious and unexplained that IL-10, another Stat3 inducing cytokine has little effect in vitro and one study showed that IL-10 can maintain Foxp3 expression of regulatory T cells in a colitis model49. This may relate to the relative capacity of these various cytokines to induce SOC3, a factor that inhibits Stat3 activation.
Regulation of TGF-β-Induced Foxp3 Expression by IL-27
Negative regulation of Foxp3 and suppressor function of Tregs was shown first by Neufert et al.47 These investigators showed that the effect was a direct activity of IL-27 rather than one exerted through another cytokine and, in particular, it was independent on the positive effect of IL-2. In addition, they showed that the inhibitory effect was also observed in Stat1-deficient mice and was thus independent of Stat1. This was in contrast to the inhibitory effect of IL-27 on Th17 development, which was Stat1-dependent, a result also found by Huber et al.48 Interestingly, such independence of TGF-β-induced Treg development appears to contrast with the dependence of natural Treg development on Stat150. These results documenting the lack of Stat1 dependence of the negative IL-27 effect correlate with studies showing that knock-down of Stat3 by specific Stat3 siRNA substantially neutralized the down-regulatory effect of IL-27 on TGF-β-induced Foxp3 expression. However, such knock-down had a greater effect on the down-regulatory effects of IL-6 than those of IL-27, suggesting that IL-27 inhibition is mediated by other factors as well.
A dissenting note regarding the role of IL-27 (and Stat1) in Foxp3 expression has come from Ouaked et al.51 who found that, in human T cells, both IL-27 and IFN-γ (but not IL-12) substantially enhance TGF-β-induced Foxp3 expression and cells so induced exhibited suppressor function. In addition, they showed that the FOXP3 promoter had two Stat binding sites in the proximal FOXP3 promoter which in fact bound Stat1 generated in cells stimulated in the presence of IL-27 in ChIP assays. Finally, these investigators showed with luciferase reporter studies conducted in primary human T cells that promoter constructs with intact Stat1 sites induced positive signals in cells stimulated by TGF-β, IL-27 and IL-2. These data obtained with studies of human cells are difficult to reconcile with studies of mouse T cells where, as indicated above, IL-27 has a down-regulatory effects on TGF-β-induced Foxp3 expression and such effects were dependent on Stat3. They also differ from the studies of Tone et al. discussed above in which Foxp3 promoter constructs did not drive appear, in itself to have activity in luciferase reporter assays15. Resolution of this discrepancy awaits additional studies of human cells.
Regulation of TGF-β-Induced Foxp3 Expression by IL-4
IL-4 is yet another cytokine with negative regulatory effects on TGF-β-induced Foxp3 expression. In initial studies on this point by Mantel et al.52, it was shown that TGF-β had little capacity to induce Foxp3 expression in human cells undergoing Th2 differentiation and that differentiated Th2 cells lack Foxp3. Then, in studies of the molecular basis of this IL-4-induced effect, it was shown that GATA-3 (a factor induced by IL-4) binds to a conserved site in the Foxp3 promoter about 300 bp upstream of the transcription start site and that over-expression of GATA-3 in cells expressing a Foxp3 promoter driven luciferase reporter is associated with reduced luciferase expression. In addition, mutation of the GATA-3 binding site in the Foxp3 promoter diminished the negative effect of GATA-3 in this system. On this basis it was concluded that GATA-3 is a repressor of Foxp3 transcription and, as such, negates the TGF-β inductive effect.
A somewhat different picture obtained in studies of IL-4 effects on TGF-β-induced Foxp3 expression in mouse cells53. In parallel with the studies of human cells, inclusion of IL-4 (but not IL-10) into activated CD4+ T cell cultures suppressed TGF-β induction of Foxp3 to an even greater extent than IL-6. However, in this case, the negative effect of IL-4 was not seen in Stat6-deficient cells and GATA-3 was not detectable in cells at early time points when the IL-4 suppression was already evident; thus, the suppressor effect of IL-4 appeared to be due to Stat6 rather than GATA-3. In subsequent studies of the mechanism of the IL-4 effect ChIP studies of virtually the entire promoter and intronic enhancer regions of the Foxp3 gene in primary T cells were performed in the presence and absence of IL-4 to determine if IL-4 affected histone acetylation and therefore chromatin accessibility. These studies revealed that TGF-β induced histone acetylation in an area between the 5′ and 3′ enhancers in intron 2 (at +2459 to +2866) but not in the promoter or either of the two enhancers themselves and that this effect was not blocked by IL-4. Furthermore, it was shown that Stat6 binds to this site in the presence but not in the absence of IL-4. Finally, luciferase reporter studies in which a large 10.2 kb fragment spanning the Foxp3 promoter and enhancer regions to drive the reporter were conducted to assess the effect of the Stat6 binding site on promoter activity. Whereas deletion of the NFAT-Smad3 binding element reduced the luciferase signal as in the Tone et al. studies, deletion of the Stat6 binding element had no effect on the signal. However, deletion of the latter did abolish the suppressor effect of IL-4. Thus, the authors concluded that an IL-4 induced Stat6-mediated silencer at this site accounts for the negative effect of IL-4 on TGF-β-induced Foxp3 expression.
Retinoic Acid Enhancement of TGF-β-Induced Foxp3 Expression
As mentioned above, a major recent finding regarding TGF-β-induced Foxp3 expression is that all-trans-retinoic acid (RA) produced by mucosal CD103+ dendritic cells can greatly enhance the TGF-β effect9,10,54 but, as initially shown by Hill et al.55, has no effect on Foxp3 expression on it own. The question therefore arises: what is the molecular mechanism of this enhancement?
One analysis addressing this issue was conducted by Hill et al. who first presented evidence that RA failed to enhance TGF-β induced T cell Foxp3 expression in cells from mice with deletion of RARα, one of the three main nuclear receptors for RA (and which mediate RA transcriptional activity). However, RARα-deficient mice had normal numbers of Tregs. Further studies revealed that RA did not change the molecular “signature” of the Tregs in that they continued to lack molecular components associated with natural Tregs. This suggested that RA acts indirectly as an enhancer of Foxp3 expression, perhaps by abolishing the inhibitory effects of other cells on such expression. In support of this idea Hill et al. provided evidence that mature (CD44hi) CD4+ T cells secrete various cytokines (IL-4, IFN-γ and IL-21) that suppress TGF-β induced Foxp3 expression, especially when present together, and that RA suppresses the secretion of these cytokines. These findings were consistent with previous studies discussed above showing that particular cytokines (e.g., IL-4) have potent effects on TGF-β induction.
This conclusion has been challenged, however, by Mucida et al.56 who provided data showing that highly purified populations of naïve T cells devoid of mature cells undergo unfettered induction of Foxp3 in the presence of TGF-β. Thus, while it is possible that RA does in fact inhibit the secretion of inhibitory cytokines and may have an indirect effect on TGF-β induction in the tissue milieu, the main effect is a direct effect on the target naïve cell. This view is supported by Takaki et al.53 who showed that IL-4, the most suppressive cytokine identified in the Hill et al. study, does not prevent the enhancing effect of RA. In addition, these authors showed that RA opposed the effect of IL-4 on histone deacetylation and thus prevented Stat6 binding to its intronic site. Finally, these authors identified an RARα/RXRα binding site between +2114 and +2350 and then showed that disruption of this site impaired the ability of a construct containing the site to drive a luciferase reporter. These data, as well as recent data from Nolting et al.57 showing that while CD44+ T cells do indeed produce the cytokines previously identified by Hill et al. as inhibitors of the induction of Foxp3 in naïve cells, RA overrides these inhibitory effects. Thus, the weight of evidence favors the idea that RA directly enhances Foxp3 expression.
In another line of investigation, Xiao et al.58 provided data showing that RA enhances the phosphorylation of Smad3 and, the same time, inhibits the expression of IL-6Rα, IRF-4 and IL-23R factors that would favor Th17 differentiation and thus divert the cell from becoming a Treg under TGF-β stimulation. That the latter actually occurs in vivo was shown by the fact that RA administration inhibits Th17-mediated inflammation (EAE). These data also favor a direct effect of RA in that they support the notion that RA enhances Smad3 activation and signaling, presumably via the mechanism suggested by Tone et al above. It should be noted, however, that while as already mentioned Nolting et al. provided evidence that the enhancing effect of RA is not due to indirect effects on inhibiting cytokines, neither is it due to effects on Smad3 since they produced data showing that while RA does enhance Smad3 activation, Smad3 cannot be involved in RA enhancing effects because the latter are slightly diminished in Smad3-deficient mice.
Finally, Elias et al.59 have examined the role of Stat3 and Stat5 in RA enhancement of TGF-β induction of Foxp3. They found that T cells from Stat3-or Stat5- deficient mice exhibited robust RA enhancement and thus RA does not act by reversing the inhibiting effect of Stat3-inducing cytokines such as IL-6 and IL-27 nor by does it involve augmentation of the positive effect of IL-2. The latter fact was corroborated by studies showing that RA enhancing effects occur in the presence of anti-IL-2.
Taken together these various studies indicate that while RA can reduce the level of cytokines potentially able to inhibit RA enhancing effects on TGF-β-induction of Foxp3 (Figure 2), such reductions may be irrelevant because direct effects of RA on naïve T cells undergoing induction have been shown to negate negative cytokine activity. In addition, there is little or no evidence that RA enhancing effects occur via the modulation of either negative or positive cytokine regulation of Foxp3 expression. Finally, whether or not RA enhancing activity depends on Smad3 is quite unclear since evidence in favor of this view showing that RA enhances Smad3 expression is opposed by the finding that Smad3-deficient mice exhibit substantial enhancement57. At the moment, therefore, the mechanism of by which RA enhances TGF-β-induction of Foxp3 is poorly understood.
Conclusions
The body of work reviewed above relating to the molecular basis of TGF-β-induction of Foxp3 expression and thus the induction of iTregs has established the basic parameters governing such induction. It is clear that induction is dependent not only on promoter function, but also on enhancers embedded in conserved non-coding sequences located both in intron 2 and intron 3 as well as in other more distal conserved sequences. With respect to TGF-β-induced Tregs, the 5′ conserved non-coding sequence located in intron 2 appears to be of very great importance and recent work has shown that this enhancer has physical engagement with a promoter site binding NF-κB components such as c-Rel. This basic concept of Foxp3 regulation in induced Tregs receives strong support from the fact that Smad3, the essential transcription factor induced by TGF-β binds to the 5′ enhancer in intron 2 but not to a promoter site. Other conserved regions control the basic size of the Treg compartment and the maintenance of Treg stability.
Another basic feature of Foxp3 expression in both natural and induced Tregs is the importance of epigenetic processes, particularly as they relate to the role of demethylation as a means of enhancing promoter/enhancer accessibility. However, it is not clear how the binding of various factors (e.g., NFAT, Smad3, Foxp3 itself) to enhancer sites relate to these epigenetic processes: do they in fact initiate them or do they result from them. A similar question can be raised with respect to cytokine regulation: do cytokines influence Foxp3 gene expression by acting as transcription factors or by influencing gene accessibility.
Some of these questions bear on the mechanism of retinoic acid enhancement of TGF-β-induced Foxp3 expression. This mechanism is still shrouded in mystery as it is still unclear if enhancement involves regulation of Smad3 effects, direct activity of RA receptors as transcription factors or even regulation of epigenetic effects. Further studies will undoubtably address these issues.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 2.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 3.Chatila TA, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 7.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 13.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantel PY, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 15.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 16.Ruan Q, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Venuprasad K, et al. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–1451. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lal G, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182:259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 23.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010 doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klunker S, et al. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J Exp Med. 2009;206:2701–2715. doi: 10.1084/jem.20090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turka LA, Walsh PT. IL-2 signaling and CD4+ CD25+ Foxp3+ regulatory T cells. Front Biosci. 2008;13:1440–1446. doi: 10.2741/2773. [DOI] [PubMed] [Google Scholar]
- 27.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 28.Lúdvíksson BR, Ehrhardt RO, Strober W. TGF-beta production regulates the development of the 2,4,6-trinitrophenol-conjugated keyhole limpet hemocyanin-induced colonic inflammation in IL-2-deficient mice. J Immunol. 1997;159:3622–3628. [PubMed] [Google Scholar]
- 29.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 30.Schorle H, Holtschke T, Hünig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 31.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 32.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 33.Zorn E, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Z, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Passerini L, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 37.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 38.Dominitzki S, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 41.Doganci A, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallandre JR, et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 43.Kortylewski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong LY, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58:1023–1032. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neufert C, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 48.Huber M, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 49.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouaked N, et al. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 52.Mantel PY, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takaki H, et al. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 55.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mucida D, et al. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nolting J, et al. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao S, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elias KM, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]



