Abstract
Rationale
Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been implicated as a maladaptive mediator of cardiac ischemic injury. We hypothesized that the inflammatory response associated with in vivo ischemia/reperfusion (I/R) is initiated through CaMKII signaling.
Objective
To assess the contribution of CaMKIIδ to the development of inflammation, infarct and ventricular dysfunction following in vivo I/R and define early cardiomyocyte-autonomous events regulated by CaMKIIδ using cardiac-specific knockout (KO) mice.
Methods and Results
Wild-type (WT) and CaMKIIδ KO mice were subjected to in vivo I/R by occlusion of the left anterior descending (LAD) artery for 1-hr followed by reperfusion for various times. CaMKIIδ deletion protected the heart against I/R damage as evidenced by decreased infarct size, attenuated apoptosis and improved functional recovery. CaMKIIδ deletion also attenuated I/R induced inflammation and upregulation of NF-κB target genes. Further studies demonstrated that I/R rapidly increases CaMKII activity, leading to NF-κB activation within minutes of reperfusion. Experiments using cyclosporine A and cardiac-specific CaMKIIδ knockout mice indicate that NF-κB activation is initiated independent of necrosis and within cardiomyocytes. Expression of activated CaMKII in cardiomyocytes lead to I kappa B kinase (IKK) phosphorylation and concomitant increases in nuclear p65. Experiments using an IKK inhibitor support the conclusion that this is a proximal site of CaMKII-mediated NF-κB activation.
Conclusions
This is the first study demonstrating that CaMKIIδ mediates NF-κB activation in cardiomyocytes following in vivo I/R and suggests that CaMKIIδ serves to trigger, as well as to sustain subsequent changes in inflammatory gene expression that contribute to myocardial I/R damage.
Keywords: Ischemic heart disease, myocardial inflammation, reperfusion injury, nuclear factor kappa B, Ca2+/calmodulin-dependent protein kinase II
INTRODUCTION
Cardiac reperfusion following an acute myocardial infarction (MI) contributes to myocyte damage, generating what is referred to as myocardial ischemia/reperfusion (I/R) injury. Cardiomyocyte cell death in response to I/R results from increases in Ca2+ and reactive oxygen species (ROS) and is mediated through opening of the mitochondrial permeability transition (PT) pore1, 2. Subsequent to necrotic cardiomyocyte cell death, an inflammatory cascade which perpetuates further damage to cardiac tissue is activated3–5. One of the central players in inflammatory signaling is the transcription factor nuclear factor kappa B (NF-κB). Numerous reports have shown that NF-κB is activated following myocardial I/R6–9. Activated NF-κB translocates to the nucleus following release from its inhibitory subunit IκBα leading to transcriptional regulation of the expression of interleukins and cytokines. The NF-κB-mediated inflammatory cascade can be perpetuated through the ability of NF-κB targets such as TNFα and interleukins to further activate NF-κB signaling, as well as by the infiltration of neutrophils and other mononuclear inflammatory cells at sites of injury. The observation that cardiac specific overexpression of a dominant-negative IκBα10 or inhibition of IκBα degradation11 block NF-κB activation, reduce infarct size and preserve heart function in response to ischemia/reperfusion indicate that the activation of NF-κB within the cardiomyocyte is critical to this series of events. Pharmacological inhibitors of NF-κB activation also provide protection against cardiac I/R injury, attenuate the release of TNFα and IL-6, inhibit inflammation and apoptosis, reduce infarct size and improve functional recovery12–14. Thus inflammation mediated through NF-κB plays a critical role in infarct development following I/R.
While a maladaptive role of NF-κB in I/R injury is well documented, the initiating signals and cellular sites at which this response is triggered have not been fully elucidated. Ca2+/calmodulin-dependent kinase II (CaMKII), a multifunctional heteromeric serine/threonine protein kinase, is activated by both Ca2+ and oxidative stress15. CaMKII has been demonstrated to mediate ex vivo I/R injury and cardiac remodeling after myocardial infarction based on experiments using pharmacological inhibitors or transgenic mice overexpressing a CaMKII inhibitory peptide16–19. CaM kinase has also been demonstrated to play a role in inflammatory gene expression in macrophages20–22. Our laboratory generated conventional knockout (KO) mice23 and more recently cardiac specific knockout mice (CKO) in which the predominant cardiac CaMKII isoform, CaMKII delta (δ)24–27 is deleted. The studies presented here use these CaMKIIδ KO mouse lines to test the hypothesis that CaMKII is required for the initiation of I/R induced NF-κB activation in cardiomyocytes and plays a critical role in the subsequent inflammatory responses that contribute to myocardial I/R damage.
METHODS
Please see detailed Methods in the Online Supplement which provide expanded details of in vivo I/R and hemodynamic measurements, heart tissue preparation and nuclear fractionation, TUNEL, DNA laddering, CaMKII and NF-κB activity assays, immunofluorescence staining and affymetrix gene array analysis. Generation of global CaMKIIδ KO mice was described previously23. Cardiac specific CaMKIIδ KO mice were generated by crossing heterozygous floxed CaMKIIδ mice23 with MLC 2v-Cre mice28 as detailed in the Online Supplement. Myocardial I/R was induced by left anterior descending (LAD) coronary artery occlusion for 1 hr followed by reperfusion for various times up to 24 hrs. All animal studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of UCSD.
Statistical analysis
Data are presented as mean ± SEM as indicated and were analyzed by 2-tailed Student’s t test between 2 groups or by ANOVA when 3 or more groups were compared. P values of less than 0.05 were considered statistically significant.
RESULTS
Infarct size and heart function
To test the role of CaMKIIδ in myocardial tolerance to I/R injury, CaMKIIδ KO and WT mice were subjected to left anterior descending coronary artery ligation for 1 hr followed by reperfusion for various times. Using a direct kinase activity assay, we demonstrate that CaMKII is activated (Ca2+ independent “autonomous” activity is increased) in WT mice at 3-min reperfusion following 1-hr in vivo ischemia (Figure 1A). As a further readout of CaMKII activation we measured phosphorylation of two substrates, the type two ryanodine receptor (RyR2) and phospholamban (PLN), at their CaMKII-specific phosphorylation sites. Marked time-dependent increases in RyR2 Ser2814 and PLN Thr17 phosphorylation were observed following reperfusion in WT but not in CaMKIIδ KO mice (Figure 1B).
Figure 1. CaMKII is activated by in vivo myocardial ischemia/reperfusion and CaMKIIδ deletion reduces infarct size and enhances functional recovery.
Animals were subjected to 1-hr ischemia followed by various times of reperfusion. (A) Increases in autonomous CaMKII activity were measured at 3-min reperfusion. Data are mean ± SEM of values from 3 mice. *p<0.05 versus Sham. (B) Phosphorylation of RyR2 and PLN at the CaMKII site was assessed in WT and CaMKIIδ KO mice at various times of reperfusion. Data are mean ± SEM of values from 3 determinations. *p<0.05 vs WT Sham. (C) Area at risk (AAR) and the ratio of infarct size to AAR were determined in WT and KO mice at 24-hr reperfusion. Data are mean ± SEM of values from 6 mice. (D) The maximum rates of rise and decline of left ventricular pressure (dP/dtmax and dP/dtmin) assessed at 24-hr reperfusion. Data are mean ± SEM of values from 8 mice. *p<0.05 versus WT. The dP/dtmax and dP/dtmin values in sham-operated mice (~8500 and ~−6500 mmHg/sec respectively) were not significantly different for WT and KO.
Susceptibility to and recovery from I/R injury was then assessed. The area at risk (AAR) was not different in CaMKIIδ KO and WT littermates but infarct size following I/R was significantly reduced in the KO hearts (Figure 1C; infarct size/AAR: 46.5±7.5% in WT vs. 32.8 ± 4.3% in KO, p<0.05). The decreased infarct was accompanied by improved functional recovery. Maximal left ventricular pressure (LVPmax), dP/dtmax and dP/dtmin were not different in WT or KO mice at baseline23 or under sham conditions (Online Table I and Figure 1 legend). Values for dP/dtmax and dP/dtmin measured at 24-hr reperfusion were, however, both significantly greater (p<0.05) in KO than in WT mice (Figure 1D). The difference in dP/dtmax was likely not due to altered preload since LVEDP was normal and did not differ between the two groups (Online Table I). The improved dP/dtmin observed following I/R in the KO mice may have reflected the higher LVPmax in this group since tau, a preferable measure of global LV relaxation, did not differ between WT and KO mice.
Apoptosis and inflammation
It is well accepted that cardiomyocyte cell death contributes to I/R injury. We examined the extent of apoptosis at 24-hr reperfusion following 1-hr ischemia. Apoptosis assessed by TUNEL staining (Figure 2A), DNA laddering (Figure 2B) and the level of cleaved caspase-3 (Figure 2C) were significantly greater in WT than in KO mice. Inflammation, a recognized consequence of myocardial ischemic insult, was evidenced by enhanced mononuclear cell infiltration as assessed by CD68 staining. The increase in CD68 staining observed in WT hearts at 24-hr reperfusion was significantly diminished by CaMKIIδ deletion (Figure 2D). The expression of monocyte chemotactic protein-1 (MCP-1/CCR2) was also significantly increased in hearts of WT but not in KO mice following I/R (Figure 2E).
Figure 2. CaMKIIδ deletion reduces apoptosis and inflammation following in vivo ischemia/reperfusion.
Endpoints indicative of apoptosis and inflammation were assessed at 24-hr reperfusion following 1-hr ischemia. (A) TUNEL staining; apoptotic nuclei were stained (green) and cardiomyocytes were detected by wheat germ agglutinin staining (red). Original magnification is 20×. Data are mean ± SEM of values from 3 hearts per group, with at least 5,000 nuclei examined per heart. *P < 0.05 versus WT I/R. (B) DNA laddering. (C) Cleaved caspase-3 as detected by Western blotting. Data are mean ± SEM of values from 3–4 determinations. *p<0.05 vs Sham; #p<0.05 vs WT I/R. (D) Inflammatory cells were identified by CD68 staining. Original magnification is 20×. (E) MCP-1 expression levels measured by Western blotting. Data are mean ± SEM of values from 3–4 determinations. *p<0.05 vs Sham; #p<0.05 vs WT.
NF-κB activation
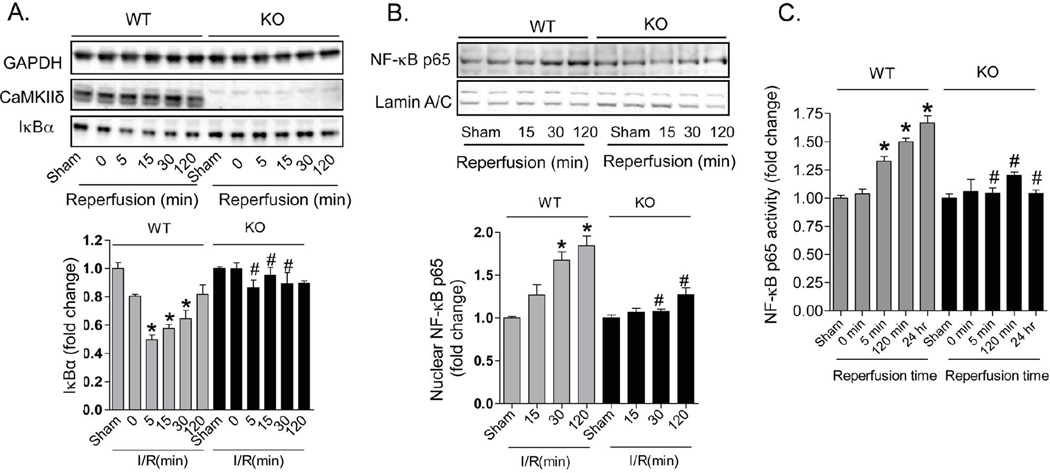
The NF-κB family of transcription factors plays a central role in regulating the expression of inflammatory and cell death genes linked to cardiovascular pathology. We tested the hypothesis that NF-κB activation in response to I/R is regulated through CaMKIIδ. An early event in NF-κB activation is the degradation of IκBα. In WT mice, reperfusion following 1-hr ischemia was accompanied by a rapid and transient decrease in IκBα expression; this response was seen as early as 5 minutes after reperfusion and prevented by CaMKIIδ deletion (Figure 3A). Subcellular fractionation of hearts isolated at various times after I/R demonstrated increases in the p65 NF-κB subunit in nuclear fractions from WT but not KO mice (Figure 3B). Finally we assessed NF-κB “activity” in ventricular lysates through binding to its consensus sequence (TransAM NF-κB oligonucleotide). NF-κB binding was not changed during ischemia, but quickly increased in WT hearts at 5 minutes and at 2 hrs of reperfusion; this increase was sustained throughout 24 hrs of reperfusion. (Figure 3C). In contrast, activation of NF-κB was not significantly increased in KO hearts at any time following reperfusion (Figure 3C).
Figure 3. CaMKIIδ deletion inhibits IκBα degradation, NF-κB nuclear localization and NF-κB activity in response to in vivo I/R.
Animals were subjected to 1-hr ischemia followed by various times of reperfusion. (A) Degradation of IκBα was detected by Western blotting in hearts exposed to in vivo ischemia followed by various times of reperfusion. Data are mean ± SEM of values from 3–6 determinations. *p<0.05 vs Sham; #p<0.05 vs WT I/R. (B) Translocation of NF-κB p65 to the nucleus was detected by Western blotting following nuclear fractionation. Lamin A/C was used as a loading control. Data are mean ± SEM of values from 3–6 determinations. *p<0.05 vs Sham; #p<0.05 vs WT I/R. (C) NF-κB activity was measured by the TransAM oligonucleotide binding assay. Data are mean ± SEM of values from 3–6 samples. *p<0.05 vs Sham; #p<0.05 vs WT I/R.
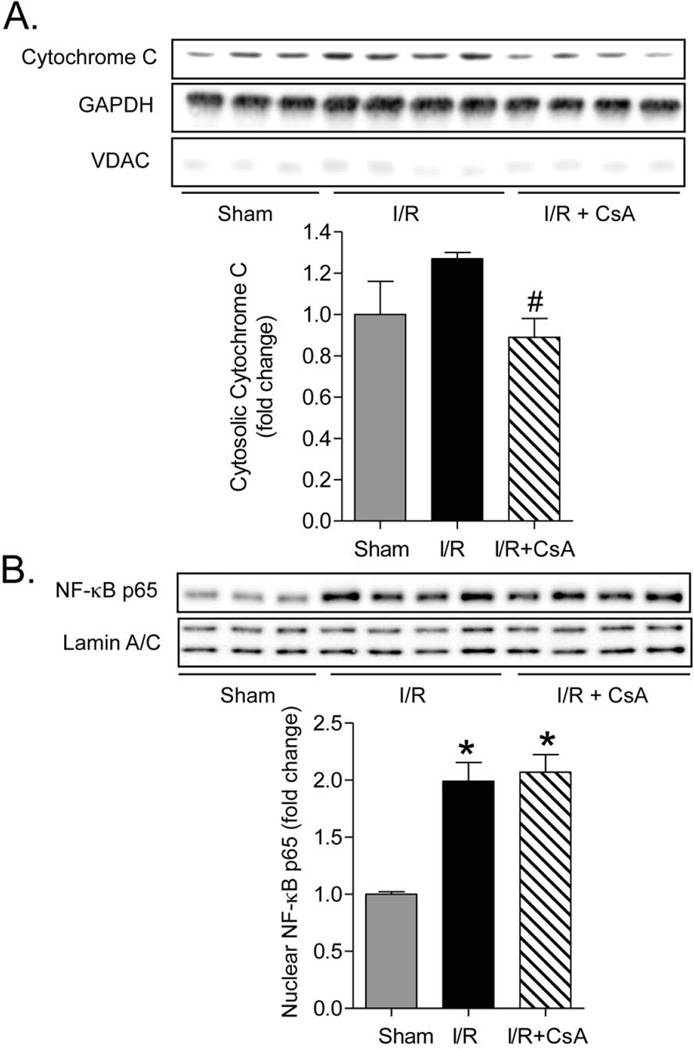
To explore the dependence of NF-κB activation on opening of the mitochondrial PT pore and development of necrosis, mice were treated with cyclosporin A (CsA 10mg/kg, administrated intravenously 10 min before reperfusion). CsA treatment reduced cytochrome C release into cytosolic fractions following I/R, indicating effective PT pore inhibition (Figure 4A). Nuclear p65 translocation in response to I/R was, however, unchanged by CsA treatment (Figure 4B). This observation suggests that I/R can induce NF-κB activation independent of necrotic damage to mitochondria, consistent with the very rapid onset of NF-κB activation.
Figure 4. Inhibition of necrosis by CsA does not reduce NF-κB activation in response to in vivo I/R.
Hearts were collected from mice treated ±CsA at 15-min reperfusion following 1-hr ischemia (A) Cytochrome C expression detected by Western blotting in mouse heart cytosolic fractions. GAPDH and VDAC were used as cytosolic and mitochondrial loading controls to assess purity of cytosolic fractions. Data are mean ± SEM of values from 3–4 determinations. #p<0.05 vs I/R (B) Translocation of NF-κB p65 to the nucleus was detected by Western blotting in nuclear fractions from the same hearts. Lamin A/C was used as a loading control. Data are mean ± SEM of values from 3–4 mice. *p<0.05 versus Sham.
Transcriptome analysis of I/R injury in WT and CaMKIIδ hearts
We used Affymetrix Gene arrays to compare changes in mRNA levels at 24-hrs reperfusion following 1-hr ischemia in WT and KO mouse hearts. There were 3129 differentially expressed transcripts in WT versus CaMKIIδ KO after I/R (interaction P<0.05 and minimum fold between groups > 20%) as shown in Figure 5A. The 5kb promoters of these differentially expressed genes were scanned for enrichment of evolutionarily conserved (human to mouse) transcription factor binding sites using the Whole Genome RVista tool29. Transcription factor binding sites enriched in the differentially regulated genes included those for the known CaMKIIδ target MEF2c (−log10, P=12.3)30 and those for NF-κB (−log10 P=10.3). There were 313 predicted NF-κB target genes upregulated in WT hearts which were significantly suppressed or downregulated in CaMKIIδ KO hearts (Online Table II). Many of these genes clustered by Gene Ontology (GO) analysis into categories related to apoptosis, cytokine signaling and regulation of NF-κB signaling. A diagrammatic representation including some of the I/R regulated, CaMKII dependent NF-κB target genes is shown in Figure 5B.
Figure 5. Clustering and functional annotation of transcripts differentially regulated by I/R in WT and CaMKIIδ hearts.
(A) We identified 3129 transcripts that had a significant interaction (P<0.05) by genotype and I/R and clustered them with the HOPACH algorithm to identify major patterns of regulation. Rows represent probesets, columns are arrays; red indicates increase in expression and green indicates decreases in expression compared to baseline (sham) for each genotype (B) Network representation of selected gene ontology processes for the NF-κB target genes that are upregulated by I/R in WT versus CaMKIIδ KO hearts (i.e. putative NF-κB target genes from clusters 2, 3 and 9). Grey nodes represent gene ontology terms. Red and green nodes respectively represent genes upregulated and downregulated by I/R, with intensity correlated with the magnitude of the response. The central core color shows fold changes in WT hearts and the surrounding ring shows fold changes in CaMKIIδ KO hearts.
CaMKII signaling to NF-κB
To directly demonstrate and determine the mechanism for CaMKII effects on NF-κB activation we expressed constitutively active CaMKIIδ in neonatal rat cardiomyocytes. The amount of nuclear p65 was increased in cells expressing CaMKIIδ (Figure 6A). In addition we determined that I kappa B kinase (IKK), a proximal kinase in the NF-κB signaling cascade, was phosphorylated when CaMKII activity was increased (Figure 6B). Pharmacological inhibiton of IKK with BMS-345541 blocked the ability of CaMKII to increase nuclear p65 (Figure 6A). These data suggest that CaMKII activates IKK to initiate NF-κB signaling. To test the importance of IKK activation and NF-κB signaling using the in vivo I/R injury model, BMS-345541 was administrated to mice intravenously at 2mg/kg 10 min before reperfusion. The efficacy of IKK inhibition was evidenced by the ability of this drug to significantly decrease I/R induced NF-κB activation in WT mice (Figure 6C, third vs. fourth bars). Treatment of WT mice with BMS-345541 lead to a 31% decrease in infarct size (Figure 6D). In contrast the inhibitor did not further reduce I/R induced NF-κB activation or infarct size in KO mice (Figure 6C,D). The lack of additive protection by CaMKIIδ deletion plus NF-κB inhibition is consistent with there being a common mechanism underlying the benefit of deleting CaMKIIδ and of inhibiting NF-κB signaling. The finding that there is no additional benefit to CaMKII deletion when IKK is inhibited supports the cell based studies showing that CaMKII regulates NF-κB signaling in cardiomyocytes through IKK, rather than by regulating a more distal process in the NF-κB activation cascade.
Figure 6. CaMKII induces I kappa B kinase (IKK) phosphorylation and inhibition of IKK reduces NF-κB activation and infarct development in response to in vivo I/R.
For A and B, neonatal rat ventricular myocytes (NRVMs) were infected with adenovirus expressing GFP or constitutively active CaMKIIδ at 40 M.O.I. and harvested 24-hr after infection. (A) Translocation of NF-κB to the nucleus was detected by Western blotting following nuclear fractionation of cells treated ±15µM BMS-345541. Lamin A/C was used as a loading control. Data are mean ± SEM of values from 3 samples. *p<0.05 versus AdCMV; #p<0.05 vs CaMKIIδ. (B) Phosphorylated IKK was detected by Western blot. Data are mean ± SEM of values from 3 samples. *p<0.05 versus AdCMV. For C and D, hearts were collected at 24-hr reperfusion following 1-hr ischemia. (C) NF-κB activation by in vivo I/R measured by the TransAM oligonucleotide binding assay in WT and KO mice pretreated for 10 min with 2mg/kg BMS-345541 or vehicle. Data are mean ± SEM of values from 3 mice. *p<0.05 vs WT without BMS treatment. (D) Area at risk (AAR) and Infarct size to AAR ratio (IS/AAR) following in vivo I/R ±BMS treatment. Data are mean ± SEM of values from 4–6 determinations. *p<0.05 vs WT without BMS treatment.
Cardiac specific CaMKIIδ deletion and NF-κB signaling
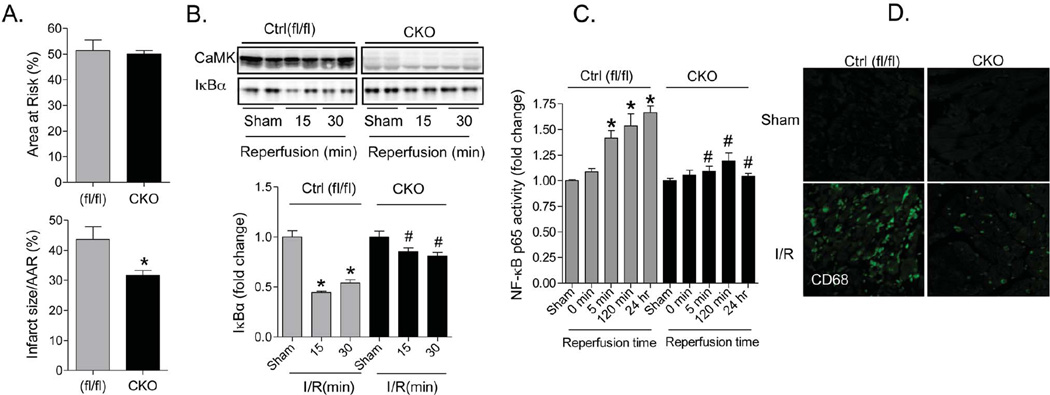
The rapid activation of CaMKII and NF-κB suggests that this pathway is directly regulated by increases in Ca2+ and ROS signaling in cardiomyocytes. Nonetheless, it is possible that CaMKII regulates NF-κB signaling in inflammatory cells that are activated in or recruited to the heart during reperfusion. To directly demonstrate that the I/R protection afforded by CaMKIIδ deletion results from effects in the cardiomyocyte compartment we used cardiac specific CaMKIIδ KO (CKO) mice generated by crossing CaMKIIδfl/fl with MLC2v-Cre mice28. Consistent with our reported findings using global KO hearts23, and indicative of selective loss of CaMKII activity in the CKO mice, the basal phosphorylation of RyR2 and PLN at their CaMKII, but not at their PKA, phosphorylation sites was significantly reduced (Online Figure IIA). Cardiac specific deletion of CaMKIIδ did not, however, alter ventricular chamber dimension, wall thickness or cardiac function as assessed by echocardiography in mice at 2 months of age (Online Figure IIB). Cardiac specific CaMKIIδ KO mice subject to in vivo I/R were protected to an extent similar to that observed in the global CaMKIIδ KO mice. Infarct size/AAR was reduced to 31.7±1.6% in CKO vs. 43.7±4.2% in Ctrl (fl/fl) mice (Figure 7A). The degradation of IκBα and activation of NF-κB observed at early times (5 to 30 min) after reperfusion was also significantly inhibited by cardiac specific CaMKIIδ deletion (Figure 7 B–C). In addition, the subsequent development of inflammation, assessed by CD68 staining at 24-hrs, was markedly attenuated in the cardiac specific CaMKIIδ KO mouse hearts (Figure 7D). Thus CaMKII signaling within the cardiomyocyte compartment initiates NF-κB activation and thereby contributes to I/R induced inflammation and infarct development.
Figure 7. Cardiac specific deletion of CaMKIIδ protects against in vivo I/R injury.
Animals were subjected to 1-hr ischemia followed by 24-hr reperfusion. (A) Area at risk (AAR) and Infarct size to AAR ratio (IS/AAR) in control and CKO hearts following I/R. Data are mean ± SEM of values from 4–5 determinations. *p<0.05 vs Ctrl I/R. (B) Degradation of IκBα detected by Western Blotting in control and CKO mouse hearts following I/R. Data are mean ± SEM of values from 3 determinations. *p<0.05 vs control Sham; #p<0.05 vs CKO Sham. (C) NF-κB activity was determined at the end of 1hr ischemia and after various times of reperfusion in control and KO hearts by TransAM oligonucleotide binding assay. Data are mean ± SEM of values from 3–4 determinations. *p<0.05 vs Sham; #p<0.05 vs Ctrl I/R. (D) CaMKIIδ deletion reduces inflammation following I/R. Inflammatory cells were identified by CD68 staining. Original magnification is 20×.
DISCUSSION
The role of CaMKII in ischemic heart diseases
The current study provides evidence that CaMKIIδ is a mediator of the effects of I/R on IκBα degradation, NF-κB activation and upregulaton of putative NF-κB target genes and that its actions are mediated through IKK phosphorylation. This is the first report to utilize the cardiac specific CaMKIIδ KO mice generated in our laboratory. Studies using these mice provide evidence that deletion of CaMKIIδ within the cardiomyocyte results in no overt basal phenotype but markedly limits the ability of I/R to increase NF-κB activation. Concomitantly, CaMKIIδ gene deletion attenuates I/R induced cardiac inflammation, cardiomyocyte death, infarct development and loss of contractile function.
A series of studies from the Mattiazi group used the ex vivo isolated perfused heart to examine the involvement of CaMKII in global I/R injury16, 17. These studies concluded that the salutary effects of CaMKII inhibition result from preventing the disordered cardiomyocyte Ca2+ homeostasis that contributes to cell death and mechanical dysfunction following I/R. A primary target of CaMKII action was suggested by these studies to be the phosphorylation of phospholamban (PLN). We demonstrate that in vivo I/R increases phosphorylation of PLN and ryanodine receptor (RyR2) at CaMKII sites in WT but not in CaMKIIδ KO mice (Figure 1B). Thus I/R induced activation of CaMKII and phosphorylation of cytosolic Ca2+ handling proteins could contribute to mitochondrial dysfunction and subsequent necrotic cell death. Importantly, however, our data demonstrate that there is also a rapid CaMKII-mediated activation of NF-κB which is not secondary to necrosis and, while it may initially be protective31, sets into motion a subsequent series of proinflammatory processes. Furthermore whereas the ex vivo model used in the studies cited above can only assess short term responses, in vivo I/R brings into play a more physiologically complex and prolonged sequence of events. Reperfusion following ischemia in vivo is associated with influx of leukocytes and activation of endogenous inflammatory cells that contribute to development of apoptosis and cardiac inflammation. The studies presented here indicate that averting the early and proximal cardiomyocyte autonomous activation of NF-κB by CaMKIIδ deletion also significantly attenuates these more chronic inflammatory responses as well as the ventricular dysfunction observed 24 hrs after reperfusion.
In studies exploring the role of CaMKII in myocardial infarction, Singh et al linked CaMKII to inflammation, specifically focusing on complement factor B (CFB) gene expression and the deleterious effects of LPS and TNFα19. CaMKII activation was shown to be required for CFB expression in response to LPS and TNFα in isolated cardiomyocytes. Further studies demonstrated that LPS-mediated activation of toll-like receptors, which contributes to cardiac NF-κB activation5, 32, 33, leads to CaMKII oxidation and activation in response to myocardial infarction34. The notion that increases in TLR and MyD88 activation lead to sustained CaMKII oxidation, contributing to NF-κB-mediated inflammatory gene expression following myocardial infarction, is wholly compatible with our data showing CaMKII-mediated increases in proinflammatory gene expression following in vivo I/R. What is most compelling, however, is that our studies also suggest that CaMKII is an initiator of inflammation through its direct role in NF-κB activation in the cardiomyocyte. Thus CaMKII serves as a trigger as well as an intermediate player in a sustained myocardial inflammatory cycle.
The role of CaMKII in NF-κB activation
As indicated in the introduction, NF-κB has been extensively implicated in myocardial I/R injury. There is abundant evidence that blocking NF-κB signaling limits infarct size, reduces neutrophil invasion and decreases inflammatory gene expression10–14, 35. Activation of NF-κB proceeds through a number of distinct pathways that converge on the phosphorylation and subsequent proteasomal degradation of the IκB inhibitor protein. Degradation of this protein facilitates the translocation of NF-κB dimers to the nucleus where they bind target genes and activate transcription. In our studies comparing WT and CaMKIIδ KO mice we report, consistent with published studies12, that I/R leads to a rapid decrease in IκBα levels in WT mice. We demonstrate that IκBα degradation is attenuated by CaMKIIδ deletion suggesting that CaMKII either affects steps upstream of its phosphorylation or alters its proteasomal processing. We further show that both NF-κB nuclear translocation and it transcriptional activity are regulated through CaMKIIδ following I/R.
Our studies using BMS-345541, an inhibitor of IKK, the kinase responsible for IkB phosphorylation and its subsequent degradation, confirm the important role of NF-κB activation in infarct development following I/R. The data showing that pharmacological blockade of IκB kinase has no additional ameliorative effect when CaMKIIδ is deleted further imply that a common mechanism underlies the beneficial effects of blocking CaMKII and NF-κB signaling and suggests that NF-κB activation is directly downstream of CaMKII. Previous studies using neonatal rat cardiomyocytes and macrophages provided evidence that CaMKII can regulate NF-κB signaling19, 36, 37. Specifically, in neonatal rat cardiomyocytes CaMKIIδ overexpression was shown to increase IκBα degradation and NF-κB-dependent promoter/luciferase activity36. We show here that a more proximal event, the activation of IKK, is regulated through CaMKIIδ in these cells. Whether IKK is a direct target of CaMKII-mediated phosphorylation remains to be determined. Nonetheless the finding that inhibiting IKK blocks the ability of CaMKII to elicit NF-κB activation in NRVMs and prevents further effects of CaMKII deletion on infarct development in the in vivo I/R model support the conclusion that IKK is the proximal site of CaMKII action.
NF-κB targets in CaMKII mediated I/R injury
We identified a significant number of genes with NF-κB binding sites in their 5’ upstream promoters that are upregulated by I/R in WT but not in CaMKIIδ KO mice. Which of these genes contribute to I/R-induced and CaMKII-mediated cardiomyocyte cell death, infarct development and inflammation is not yet known. Anderson’s group focused on complement factor B (CFB) as a gene that responds to lipopolysaccharides (LPS) in a CaMKII dependent manner both in vitro and in vivo19. This is, however, only one of many genes detected in their gene arrays as CaMKII dependent19 and only one of the many genes identified in our arrays as being both CaMKII dependent and potentially NF-κB regulated. We specifically examined changes in the NF-κB regulated chemokine MCP-1 as a marker of inflammation, and demonstrated that the increases in MCP-1 protein expression seen 24 hrs after I/R are prevented by CaMKII deletion. Interestingly MCP-1 not only participates in chemoattraction but also regulates cell death pathways38, thus MCP-1 is a potential mediator through which NF-κB signals contribute to cardiomyocyte death and infarct expansion. Undoubtedly a multitude of proinflammatory gene products conspire to induce cardiomyocyte injury and contractile dysfunction following I/R. The profound ameliorative effect of CaMKIIδ deletion on the expression of myriad NF-κB target genes categorized as regulators of cytokine signaling and apoptosis implicates CaMKII as a nodal regulator of this process.
In summary, we demonstrate for the first time that CaMKIIδ mediates rapid NF-κB activation and the development of apoptosis and inflammation in response to I/R in the in vivo mouse heart. Our studies using cardiac specific CaMKIIδ KO mice reveal that the maladaptive effects of CaMKII signaling elicited by I/R are early events that occur in the cardiomyocyte compartment. The activation of NF-κB signaling is independent of necrosis and results in regulation of multiple NF-κB target genes. We suggest that oxidative stress or Ca2+ release during reperfusion initiate rapid CaMKIIδ activation which leads to cardiomyocyte autonomous IKK phosphorylation and NF-κB activation. The ensuing changes in cardiac gene expression and recruitment of infiltrating and resident inflammatory cells fuel the subsequent inflammatory cascade that contributes to further cardiomyocyte injury. Inhibiting CaMKII during reperfusion could provide significant therapeutic advantage by targeting an early causal event in this cascade.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Calcium/calmodulin dependent kinase (CaMKII) is activated by calcium and reactive oxygen species (ROS).
Reperfusion of the ischemic heart is associated with increased myocardial calcium and ROS generation, which lead to cardiac dysfunction.
NF-κB, a master regulator of inflammation, contributes to tissue injury induced by cardiac ischemia/ reperfusion (I/R.)
What New Information Does This Article Contribute?
CaMKII is activated within minutes of reperfusion and is required for NF-κB activation following in vivo I/R.
CaMKII activates NF-κB directly by phosphorylating its upstream kinase - IKK.
Activation of NF-κB by CaMKII is an early, cardiomyoctyte autonomous response that is critical for long- term inflammation, myocardial tissue injury and contractile dysfunction.
The mechanisms responsible for NF-κB activation in response to cardiac ischemia/reperfusion are unknown. I/R associated increases in Ca2+ or ROS should activate CaMKII, but direct evidence is lacking. We found that CaMKII activity is rapidly increased in response to reperfusion and that CaMKII is required for subsequent activation of NF-κB. We demonstrate that these responses occur in a cardiomyocyte in a necrosis-independent manner and that CaMKII increases NF-κB activity through phosphorylation of its upstream kinase IKK. Deletion of CaMKII in cardiomyocytes prevents I/R mediated increases in NF-κB target gene expression, leads to a decrease in infarct size, and improved ventricular function at 24 h after I/R. These findings suggest that CaMKII-mediated NF-κB activation is an initiating signal that occurs in cardiomyocytes and is responsible for subsequent I/R injury. Blocking CaMKII could ameliorate excessive NF-κB mediated responses associated with various inflammatory diseases and in particular prove to be a viable therapeutic target for preventing the deleterious effects of myocardial reperfusion.
ACKNOWLEDGMENTS
We thank Melisssa Barlow for animal care and breeding and Dr. Shigeki Miyamoto for his advice and critical review of the manuscript.
SOURCES OF FUNDING
This work was supported by NIH grant HL080101 to J.H.B. H. Ling was supported by an AHA Postdoctoral Fellowship. CBBG was supported by an NIH Predoctoral Training Grant (T32 GM007752) and is a member of the UCSD Biomedical Science Graduate Program. ACZ is supported by grants 1U54HK08460-01, 8UL1TR000100-03, P01HL098053 and an AHA Scientist Development Grant.
Nonstandard Abbreviations
- I/R
ischemia/reperfusion
- CaMKII
Ca2+/calmodulin-dependent kinase II
- KO
knockout
- CKO
cardiac specific knockout
- NF-κB
nuclear factor kappa B
- TNFα
tumor necrosis factor alpha
- IL- 6
Interleukin-6
- LAD
left anterior descending
- IS
infarction size
- AAR
area at risk
- LV
left ventricle
- CsA
cyclosporin A
- IKK
I kappa B kinase
- RyR2
type 2 ryanodine receptor
- PLN
phospholamban
- PT pore
mitochondrial permeability transition pore
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiological reviews. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circulation research. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 3.Coggins M, Rosenzweig A. The fire within: Cardiac inflammatory signaling in health and disease. Circulation research. 2012;110:116–125. doi: 10.1161/CIRCRESAHA.111.243196. [DOI] [PubMed] [Google Scholar]
- 4.Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, Smith CW. Inflammation in the course of early myocardial ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5:2529–2537. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 5.Marchant DJ, Boyd JH, Lin DC, Granville DJ, Garmaroudi FS, McManus BM. Inflammation in myocardial diseases. Circulation research. 2012;110:126–144. doi: 10.1161/CIRCRESAHA.111.243170. [DOI] [PubMed] [Google Scholar]
- 6.Li C, Browder W, Kao RL. Early activation of transcription factor nf-kappab during ischemia in perfused rat heart. The American journal of physiology. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-kappab and induces neutrophil infiltration via lipopolysaccharide-induced cxc chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 8.Izumi T, Saito Y, Kishimoto I, Harada M, Kuwahara K, Hamanaka I, Takahashi N, Kawakami R, Li Y, Takemura G, Fujiwara H, Garbers DL, Mochizuki S, Nakao K. Blockade of the natriuretic peptide receptor guanylyl cyclase-a inhibits nf-kappab activation and alleviates myocardial ischemia/reperfusion injury. The Journal of clinical investigation. 2001;108:203–213. doi: 10.1172/JCI12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kis A, Yellon DM, Baxter GF. Role of nuclear factor-kappa b activation in acute ischaemia-reperfusion injury in myocardium. British journal of pharmacology. 2003;138:894–900. doi: 10.1038/sj.bjp.0705108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK. Cardiac-specific blockade of nf-kappab in cardiac pathophysiology: Differences between acute and chronic stimuli in vivo. American journal of physiology. Heart and circulatory physiology. 2005;289:H466–H476. doi: 10.1152/ajpheart.00170.2004. [DOI] [PubMed] [Google Scholar]
- 11.Pye J, Ardeshirpour F, McCain A, Bellinger DA, Merricks E, Adams J, Elliott PJ, Pien C, Fischer TH, Baldwin AS, Jr, Nichols TC. Proteasome inhibition ablates activation of nf-kappa b in myocardial reperfusion and reduces reperfusion injury. American journal of physiology. Heart and circulatory physiology. 2003;284:H919–H926. doi: 10.1152/ajpheart.00851.2002. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Kim JS, Kwon JS, Jeong MH, Cho JG, Park JC, Kang JC, Ahn Y. Bay 11-7082, a nuclear factor-kappab inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. International heart journal. 2010;51:348–353. doi: 10.1536/ihj.51.348. [DOI] [PubMed] [Google Scholar]
- 13.Onai Y, Suzuki J, Kakuta T, Maejima Y, Haraguchi G, Fukasawa H, Muto S, Itai A, Isobe M. Inhibition of ikappab phosphorylation in cardiomyocytes attenuates myocardial ischemia/reperfusion injury. Cardiovascular research. 2004;63:51–59. doi: 10.1016/j.cardiores.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. Ikkbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. American journal of physiology. Heart and circulatory physiology. 2007;293:H2248–H2253. doi: 10.1152/ajpheart.00776.2007. [DOI] [PubMed] [Google Scholar]
- 15.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of camkii by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundina-Weilenmann C, Mattiazzi A. Camkii inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovascular research. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Salas MA, Valverde CA, Sanchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of camkii-mediated apoptosis and necrosis in the ischemia/reperfusion injury. Journal of molecular and cellular cardiology. 2010;48:1298–1306. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase ii inhibition protects against structural heart disease. Nature medicine. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 19.Singh MV, Kapoun A, Higgins L, Kutschke W, Thurman JM, Zhang R, Singh M, Yang J, Guan X, Lowe JS, Weiss RM, Zimmermann K, Yull FE, Blackwell TS, Mohler PJ, Anderson ME. Ca2+/calmodulin-dependent kinase ii triggers cell membrane injury by inducing complement factor b gene expression in the mouse heart. The Journal of clinical investigation. 2009;119:986–996. doi: 10.1172/JCI35814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (camk) iv mediates nucleocytoplasmic shuttling and release of hmgb1 during lipopolysaccharide stimulation of macrophages. J Immunol. 2008;181:5015–5023. doi: 10.4049/jimmunol.181.7.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Guo L, Collage RD, Stripay JL, Tsung A, Lee JS, Rosengart MR. Calcium/calmodulin-dependent protein kinase (camk) ialpha mediates the macrophage inflammatory response to sepsis. Journal of leukocyte biology. 2011;90:249–261. doi: 10.1189/jlb.0510286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Ghisletti S, Perissi V, Rosenfeld MG, Glass CK. Transcriptional integration of tlr2 and tlr4 signaling at the ncor derepression checkpoint. Molecular cell. 2009;35:48–57. doi: 10.1016/j.molcel.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for ca2+/calmodulin-dependent kinase ii in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. The Journal of clinical investigation. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase ii mrnas. The Journal of biological chemistry. 1989;264:17907–17912. [PubMed] [Google Scholar]
- 25.Schworer CM, Rothblum LI, Thekkumkara TJ, Singer HA. Identification of novel isoforms of the delta subunit of ca2+/calmodulin-dependent protein kinase ii. Differential expression in rat brain and aorta. The Journal of biological chemistry. 1993;268:14443–14449. [PubMed] [Google Scholar]
- 26.Mayer P, Mohlig M, Idlibe D, Pfeiffer A. Novel and uncommon isoforms of the calcium sensing enzyme calcium/calmodulin dependent protein kinase ii in heart tissue. Basic research in cardiology. 1995;90:372–379. doi: 10.1007/BF00788498. [DOI] [PubMed] [Google Scholar]
- 27.Baltas LG, Karczewski P, Krause EG. The cardiac sarcoplasmic reticulum phospholamban kinase is a distinct delta-cam kinase isozyme. FEBS letters. 1995;373:71–75. doi: 10.1016/0014-5793(95)00981-e. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the rxralpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–1949. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
- 29.Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by camp and protein kinase a. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. Cam kinase signaling induces cardiac hypertrophy and activates the mef2 transcription factor in vivo. The Journal of clinical investigation. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of nf-kappab in the heart: To be or not to nf-kappab. Circulation research. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 32.Haudek SB, Spencer E, Bryant DD, White DJ, Maass D, Horton JW, Chen ZJ, Giroir BP. Overexpression of cardiac i-kappabalpha prevents endotoxin-induced myocardial dysfunction. American journal of physiology. Heart and circulatory physiology. 2001;280:H962–H968. doi: 10.1152/ajpheart.2001.280.3.H962. [DOI] [PubMed] [Google Scholar]
- 33.Chao W. Toll-like receptor signaling: A critical modulator of cell survival and ischemic injury in the heart. American journal of physiology. Heart and circulatory physiology. 2009;296:H1–H12. doi: 10.1152/ajpheart.00995.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME. Myd88 mediated inflammatory signaling leads to camkii oxidation, cardiac hypertrophy and death after myocardial infarction. Journal of molecular and cellular cardiology. 2012;52:1135–1144. doi: 10.1016/j.yjmcc.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiemermann C. Inhibition of the activation of nuclear factor kappa b to reduce myocardial reperfusion injury and infarct size. Cardiovascular research. 2004;63:8–10. doi: 10.1016/j.cardiores.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Kashiwase K, Higuchi Y, Hirotani S, Yamaguchi O, Hikoso S, Takeda T, Watanabe T, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Ueno H, Nishida K, Hori M, Otsu K. Camkii activates ask1 and nf-kappab to induce cardiomyocyte hypertrophy. Biochemical and biophysical research communications. 2005;327:136–142. doi: 10.1016/j.bbrc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. Camkii promotes tlr-triggered proinflammatory cytokine and type i interferon production by directly binding and activating tak1 and irf3 in macrophages. Blood. 2008;112:4961–4970. doi: 10.1182/blood-2008-03-144022. [DOI] [PubMed] [Google Scholar]
- 38.Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/ccr2 pathway. Circulation research. 2012;110:174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.