Abstract
Background
Plerixafor (Mozobil, AMD3100) with granulocyte-colony stimulating factor (G-CSF) mobilizes more CD34+ cells/kg compared to G-CSF alone. Given that plerixafor enhances mobilization of multiple WBC lineages, we determined if more storage space is required for products collected from patients mobilized with plerixafor.
Methods
A review of the medical records of 15 patients mobilized with chemotherapy and G-CSF (control) and 14 patients mobilized with plerixafor plus G-CSF (plerixafor) was performed. Data on demographics, baseline characteristics, CD34+ cells/kg, total nucleated cells, total mononuclear cells, total apheresis sessions, and total bags for storage were collected. Mean values were determined and compared using Student’s t-test.
Results
We found that the proportion of CD34+ cells among total nucleated cells was less in the plerixafor group compared to the control group (P=0.0427). More nucleated cells (10.7 × 1010 vs 7.1 × 1010, P=0.0452) and mononuclear cells (9.7 × 1010 vs 5.9 × 1010, P=0.0059) were mobilized with plerixafor plus G-CSF. However, there was no significant difference in CD34+ cells/kg, total CD34+ cells or the proportion of mononuclear cells among total nucleated cells between the two groups. More storage bags were required for the plerixafor group compared to the control group (15 vs 9, P=0.0299).
Conclusion
Mobilization with plerixafor plus G-CSF resulted in more total nucleated cells relative to CD34+ cells collected and thus a greater number of storage bags. An increase in the number of bags required for stem cell storage may be logistically problematic and will also lead to increased costs for storage of stem cells.
Keywords: AMD3100, stem cells, chemotherapy, bags
INTRODUCTION
Plerixafor (Mozobil, AMD3100) is a reversible inhibitor of stromal cell-derived factor-1 (SDF-1) binding to its chemokine receptor CXC chemokine receptor 4 (CXCR4) [1]. Early studies in HIV infected individuals and healthy volunteers treated with plerixafor showed an enhancement of circulating white blood cells (WBCs) and peripheral blood stem cells (PBSCs) [2–4]. Numerous studies have now shown that plerixafor plus G-CSF is superior to G-CSF alone at mobilizing hematopoietic stem cells in patients with non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma for autologous peripheral blood stem cell (PBSC) transplantation. More CD34+ cells/kg were collected with plerixafor plus G-CSF in fewer apheresis days compared to G-CSF alone [5–11]. Furthermore, it is possible to mobilize some patients with plerixafor who failed initial mobilization [5,9,11]. There was timely engraftment of PBSCs mobilized after plerixafor plus G-CSF [1]. Additionally, we have demonstrated at our institution that plerixafor plus G-CSF and cyclophosphamide plus G-CSF result in similar and adequate stem cell harvests for two or more autologous stem cell transplants for patients with multiple myeloma [12]. Both approaches were superior to G-CSF alone.
We set out in this analysis to determine if other factors pertaining to stem cell collection and storage may help inform the choice of mobilization regimen prior to high-dose chemotherapy and hematopoietic stem cell transplantation. We considered this possible since plerixafor has been shown to substantially increase the number of nucleated cells of many lineages in the periphery [4,13]. At our institution, each storage bag contains 1.4 – 2.6 × 108 cells/ml resuspended in 70ml dimethyl sulfoxide (DMSO), Normosol-R and autologous plasma mixture. The bags are then stored in tanks in the vapor phase of liquid nitrogen. Minimizing the number of bags per patient is highly desired because of limited space as well as to contain costs.
We retrospectively reviewed the medical records of 15 patients who received chemotherapy and G-CSF (control group) and 14 patients who received plerixafor plus G-CSF (plerixafor group). These patients were undergoing autologous PBSC transplantation for multiple myeloma, non-Hodgkin’s lymphoma, amyloidosis and acute promyelocytic leukemia. We compared the proportion of CD34+ cells among total nucleated cells, CD34+ cells/kg, total CD34+ cells, apheresis parameters, peripheral blood cell counts and the number of storage bags in both groups.
METHODS
This is a retrospective study conducted to compare the storage bag requirements after mobilization with plerixafor plus G-CSF or chemotherapy plus G-CSF. All of the patients that were included in this study had no prior mobilizations or stem cell collections. Medical records from 15 patients mobilized with chemotherapy and G-CSF and 14 patients mobilized with plerixafor plus G-CSF from March to December 2009 were analyzed. Patients with a diagnosis of multiple myeloma, non-Hodgkin’s lymphoma, amyloidosis and acute promyelocytic leukemia were included in the study.
Patients were mobilized with 10 µg/kg/day G-CSF for four days and 240 µg/kg/day plerixafor one day prior to the start of collection. G-CSF and plerixafor were given for an additional three days after the start of collection. Stem cell collections were initiated when the peripheral blood CD34+ cell count was at least 6/µL in the chemotherapy plus GCSF control group. We did not have a minimum peripheral CD34+ cell count for the Plerixafor group. The peripheral CD34+ counts one day prior to the start of collection (prior to the first dose of Plerixafor) were not always ≥6/ul; however when we measured peripheral CD34+ counts on the day of collection, prior to the start of collection, for a subset of patients in the Plerixafor group (7/14), we found the peripheral CD34+ count to be >6/ul.
Most of the PBSC collections were performed with a COBE Spectra cell separator (COBE Spectra, Caridian BTC, Lakewood, CO) using the manual mononuclear cell collection protocol. The color of the collect line was 2% hematocrit. Two instruments used operating software version 4.7, one instrument used version 6.1 and another instrument used version 7.0. Three collections were performed with the Fenwal CS 3000 Plus cell separator (Baxter, Deerfield, IL) using the automatic method. The CS3000 Plus software was used. The interface detector offset was 100. Two collections were performed with the Fenwal Amicus cell separator (Baxter, Deerfield, IL) using the automatic method. Software version 2.5 was used. The following changes were made according to manufacturer recommendations to optimize stem cell collections: RBC offset was decreased from 2.3 to 1.5 and the RBC collect volume was decreased from 6.8 to 6.3. The Amicus and CS3000 collections occurred in the Plerixafor group. Each procedure took approximately 3 hours and the volume of blood processed was 6–15 L. The anticoagulant ACD-A (Baxter Healthcare Corp., Deerfield, IL) to whole blood volume ratio was started at 1:12 for all three machines and was ramped up to 1:15 for the COBE Spectra cell separator. The citrate was infused at a rate of 0.8 mL/min. The standard blood draw flow rate during leukapheresis was 85 mL/min for all three machines. The collect replace pump was set at 1.0mL/min for the COBE Spectra cell separator. If the procedure was stopped due to an adverse reaction, a slower blood flow rate of 50 mL/min was used upon resumption of the procedure which was gradually increased to 85 mL/min, as tolerated. PBSC collections were performed using this protocol regardless of the patient’s underlying hematologic malignancy. The CD34+ stem cell collection goal for patients with multiple myeloma was 6.0 ×106/kg while the goal for patients with other malignancies was 4.0 ×106/kg.
PBSCs were stored in 250ml Cryocyte freezing bags (Baxter, Deerfield, IL). Each storage bag contained 1.4 – 2.6 × 108 cells/ml resuspended in 70ml of freezing medium (10% dimethyl sulfoxide (DMSO) and 45% Normosol-R in autologous plasma). These bags were stored in tanks in the vapor phase of liquid nitrogen. Mononuclear cell content of products collected with the COBE Spectra was approximately 70–90%, CS3000 was approximately 90% and Amicus was approximately 50–80%. RBC content of products collected with the COBE Spectra was approximately 2–4%, CS3000 was approximately 16–20% and Amicus was approximately 5%. Platelet content of products ranged from 100s to 1000s. The Small Volume Collection Container (SVCC) used for the CS3000+.
Total nucleated cells in the stem cell product and peripheral blood were measured using the Coulter LH780 (Beckman Coulter, Fullerton, CA). Mononuclear cells in the stem cell product and peripheral blood were determined by multiplying the total nucleated cells by the estimated mononuclear cell percentage which was determined by manual differential. CD34+ cells were measured by flow cytometry (FACS Calibur, Becton-Dickinson, Franklin Lakes, NJ).
Data were plotted and statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA). Mean values were compared between the two groups using a 2-tailed Student’s t-test. P<0.05 was considered statistically significant.
The chemotherapy mobilization group received conventional regimens including cyclophosphamide and G-CSF (n=10); etoposide, methylprednisolone, cytarabine and cisplatin (ESHAP) (n=2); ifosphamide, carboplatin, and etoposide (ICE) (n=1); Rituximab, ifosfamide, carboplatin, etoposide (RICE) (n=1); and cyclophosphamide, vincristine, adriamycin and dexamethasone (CVAD/Hyper-CVAD) (n=1).
RESULTS
Patients were chosen consecutively over a period of approximately a year that were mobilized with standard regimens of chemotherapy and G-CSF or plerixafor and G-CSF. There were more males than females (control, 53%; plerixafor 64%). The mean age of patients in both groups was similar (58 years old). Most patients carried a diagnosis of multiple myeloma (control, n=10, 67%; plerixafor, n=9, 64%). Five (33%) control patients and three (21%) plerixafor patients had non-Hodgkin’s lymphoma. One (7%) patient with amyloidosis and one (7%) with acute promyelocytic leukemia were also included in the plerixafor group (Table 1).
TABLE I.
Patient demographic and baseline characteristics
| Characteristics | Control (n=15) |
Plerixafor (n=14) |
|---|---|---|
| Gender | ||
| Male | 8 (53%) | 9 (64%) |
| Female | 7 (47%) | 5 (36%) |
| Age (mean, years) | 58 | 58 |
| Diagnosis | ||
| Multiple myeloma | 10 (67%) | 9 (64%) |
| Non-hodgkin’s Lymphoma | 5 (33%) | 3 (21%) |
| Amyloidosis | None | 1 (7%) |
| Acute promyelocytic leukemia | None | 1 (7%) |
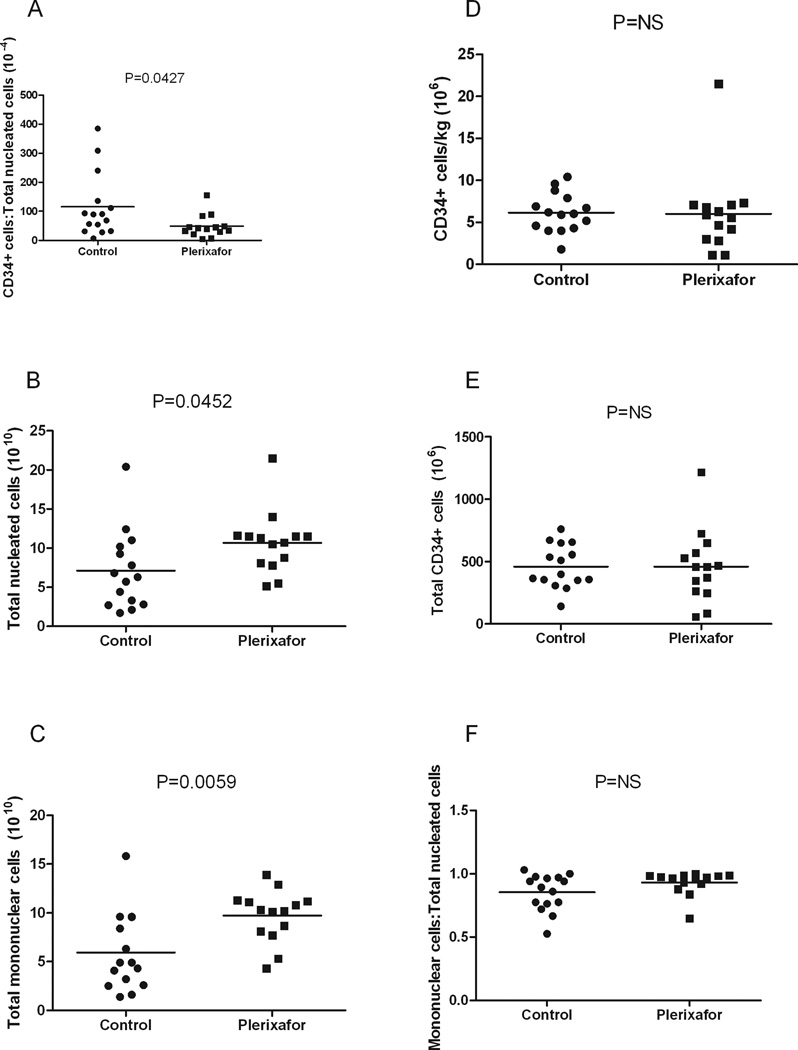
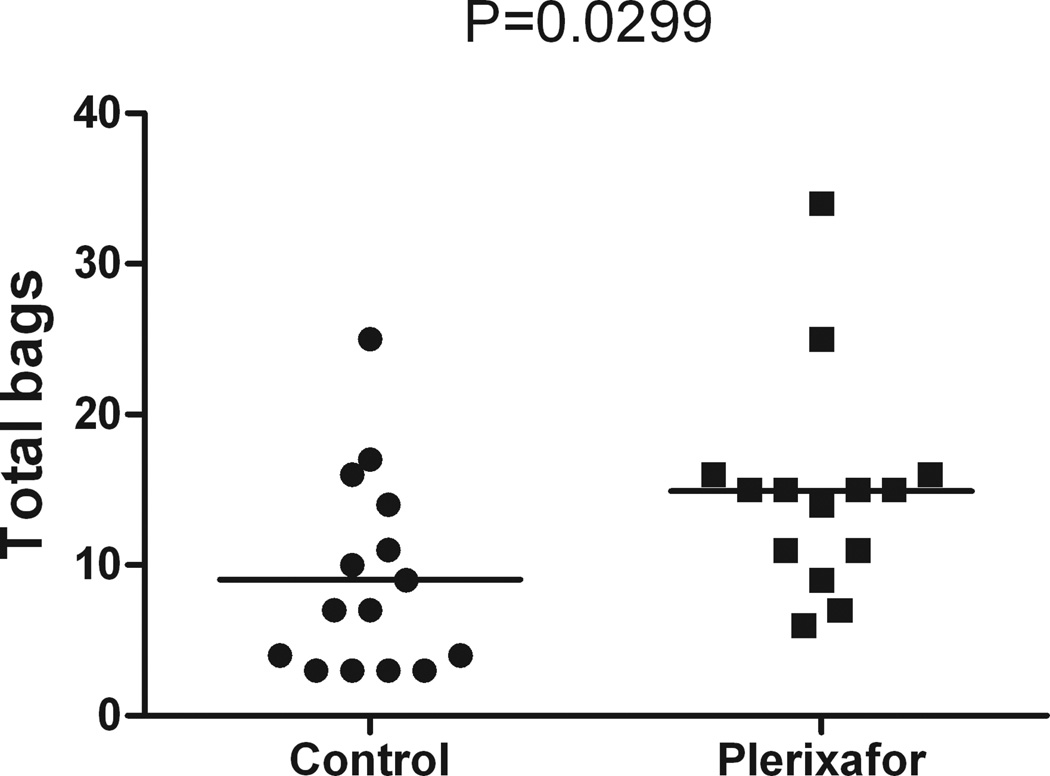
A smaller proportion of CD34+ cells among total nucleated cells was found in the plerixafor group compared to the control group (mean, plerixafor: 49.2 × 10−4, control: 115.6 × 10−4, P=0.0427) (Figure 1A). More nucleated cells and mononuclear cells were mobilized with plerixafor plus G-CSF (mean, nucleated cells: 10.7 × 1010, mononuclear cells: 9.7 × 1010) compared to chemotherapy plus G-CSF (mean, nucleated cells: 7.1 × 1010, mononuclear cells: 5.9 ×1010) (P=0.0452 and P=0.0059, respectively) (Figures 1B and 1C, Tables II and III). There was no difference in CD34+ cells/kg, total CD34+ cells and proportion of mononuclear cells to total nucleated cells between the two groups (Figures 1D–F, Tables II and III). More storage bags were required per patient for the plerixafor group compared to the control group (mean, plerixafor: 15, control: 9, P=0.0299) (Figure 2, Tables II and III).
Figure 1. Plerixafor mobilization leads to a smaller proportion of CD34+ cells among total nucleated cells.
A. Mean ratio of CD34+ cells to total nucleated cells in the control group (115.6 × 10−4) per patient was greater compared to the plerixafor group (49.2 × 10−4), (P=0.0427).
B. Mean total nucleated cells in the control group (7.1 × 1010) was less compared to the plerixafor group per patient (10.7 × 1010), (P=0.0452). Total nucleated cells were determined using the Coulter LH780.
C. Mean total mononuclear cells in the control group (5.9 × 1010) was less compared to the plerixafor group (9.7 × 1010), (P=0.0059). Mononuclear cells were determined by manual differential.
D. Mean CD34+ cells/kg in the control group (6.2 × 106) was similar to the plerixafor group (6 × 106), (P=NS). The number of CD34+ cells was determined using flow cytometry.
E. Mean total CD34+ cells per patient in the control group (460.1 × 106) was similar to the plerixafor group (460.8 × 106), (P=NS). Total CD34+ cells were determined by multiplying CD34+ cell/kg by the patients’ weight.
F. Mean ratio of mononuclear cells to total nucleated cells per patient in the control group (0.85) was similar to the plerixafor group (0.93), (P=NS).
TABLE II.
Mean apheresis yields in the control group per patient
| Patient | Mobilization | Peripheral WBC (103/ul) |
CD34+ cells/kg (106) |
Total CD34+ cells (106) |
Total nucleated cells (1010) |
Total mononuclear cells (1010) |
No of bags |
|---|---|---|---|---|---|---|---|
| 1 | Cy/G-CSF | 19.2 | 7.9 | 510.34 | 5.7 | 4.9 | 7 |
| 2 | Cy/G-CSF | 64.5 | 9.6 | 648.96 | 2.1 | 1.4 | 3 |
| 3 | Hyper CVAD/G-CSF | 30.3 | 1.8 | 141.3 | 20.4 | 15.8 | 25 |
| 4 | Cy/G-CSF | 31.5 | 4.6 | 286.58 | 9.3 | 9.6 | 14 |
| 5 | ESHAP/G-CSF | 10 | 6.2 | 365.8 | 2.7 | 2.6 | 4 |
| 6 | Cy/G-CSF | 56.6 | 8.8 | 672.32 | 2.8 | 2.5 | 3 |
| 7 | Cy/G-CSF | 54.7 | 6.9 | 536.13 | 7.8 | 4.1 | 11 |
| 8 | Cy/G-CSF | 50.3 | 6 | 760.8 | 6.8 | 4.9 | 10 |
| 9 | Cy/G-CSF | 17.6 | 6.7 | 355.77 | 11 | 8.4 | 3 |
| 10 | ICE/G-CSF | 14.5 | 5.2 | 397.8 | 4.4 | 4.3 | 7 |
| 11 | ESHAP/G-CSF | 14.4 | 4 | 356.8 | 6.3 | 6.3 | 9 |
| 12 | RICE/G-CSF | 21.7 | 4.3 | 307.45 | 3.3 | 3.2 | 4 |
| 13 | Cy/G-CSF | 20.5 | 4 | 350.8 | 12.4 | 9.6 | 17 |
| 14 | Cy/G-CSF | 68.4 | 10.4 | 655.2 | 1.7 | 1.6 | 3 |
| 15 | Cy/G-CSF | 23.5 | 5.9 | 555.78 | 10.2 | 9.6 | 16 |
Abbreviations: Cy: Cyclophosphamide; G-CSF: granulocyte-colony stimulating factor; CVAD: Cyclophosphamide, vincristine, adriamycin, dexamethasone; ESHAP: Etoposide, methylprednisolone, cytarabine, cisplatin; RICE: Rituximab, ifosfamide, carboplatin, etoposide; ICE: ifosfamide, carboplatin, etoposide
TABLE III.
Mean apheresis yields in the plerixafor group per patient
| Patient | Peripheral WBC (103/ul) |
CD34+ cells/kg (106) |
Total CD34+ cells (106) |
Total nucleated cells (1010) |
Total mononuclear cells (1010) |
No of bags |
|---|---|---|---|---|---|---|
| 1 | 77.4 | 7.1 | 528.95 | 11.6 | 10.8 | 16 |
| 2 | 58.3 | 21.5 | 1216.9 | 7.8 | 7.7 | 9 |
| 3 | 14.6 | 5.6 | 459.2 | 11.5 | 11.2 | 15 |
| 4 | 25.3 | 7.3 | 649.7 | 14 | 12.9 | 16 |
| 5 | 50.7 | 4.7 | 372.24 | 10.7 | 10.3 | 14 |
| 6 | 28.8 | 1.1 | 57.86 | 10.5 | 10.2 | 15 |
| 7 | 32.3 | 3 | 247.8 | 11.5 | 10.1 | 15 |
| 8 | 23.3 | 5.9 | 469.64 | 5.5 | 5.3 | 6 |
| 9 | 29.7 | 6.3 | 724.5 | 21.5 | 13.9 | 25 |
| 10 | 56.6 | 2.8 | 263.76 | 8.8 | 8.7 | 11 |
| 11 | 24.5 | 6.8 | 569.16 | 11.5 | 11.3 | 34 |
| 12 | 28.5 | 4.2 | 348.18 | 8.1 | 8.1 | 11 |
| 13 | 54.8 | 7.1 | 459.37 | 5.14 | 4.31 | 7 |
| 14 | 32.5 | 1.1 | 84.26 | 11.3 | 11.1 | 15 |
Figure 2. Plerixafor mobilization leads to increased storage bag requirements.
Mean total storage bags per patient required in the control group (9 bags) was less compared to the plerixafor group (15 bags), (P=0.0299).
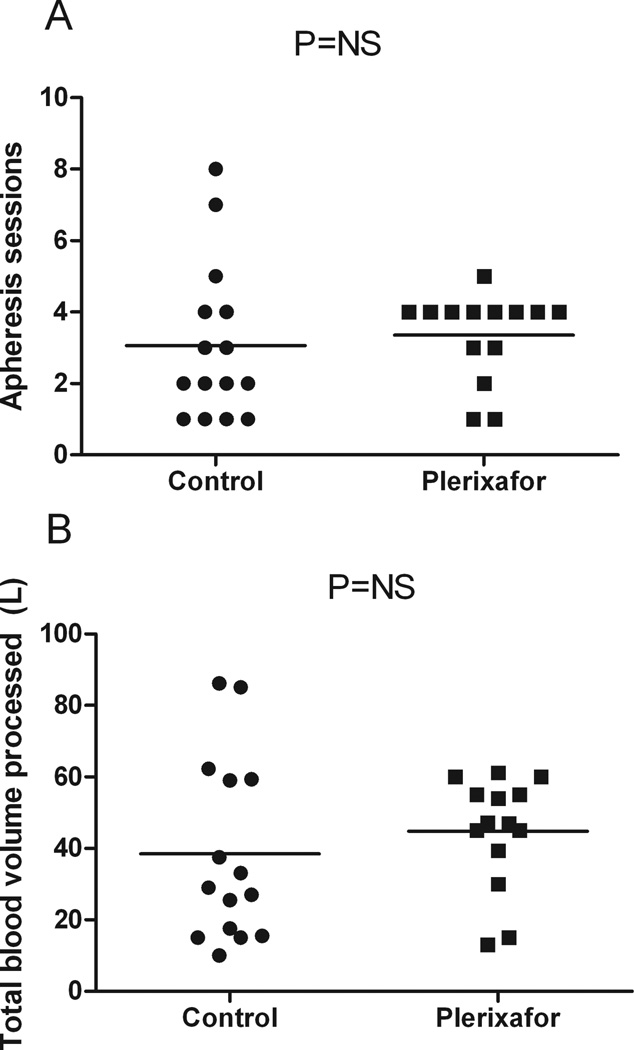
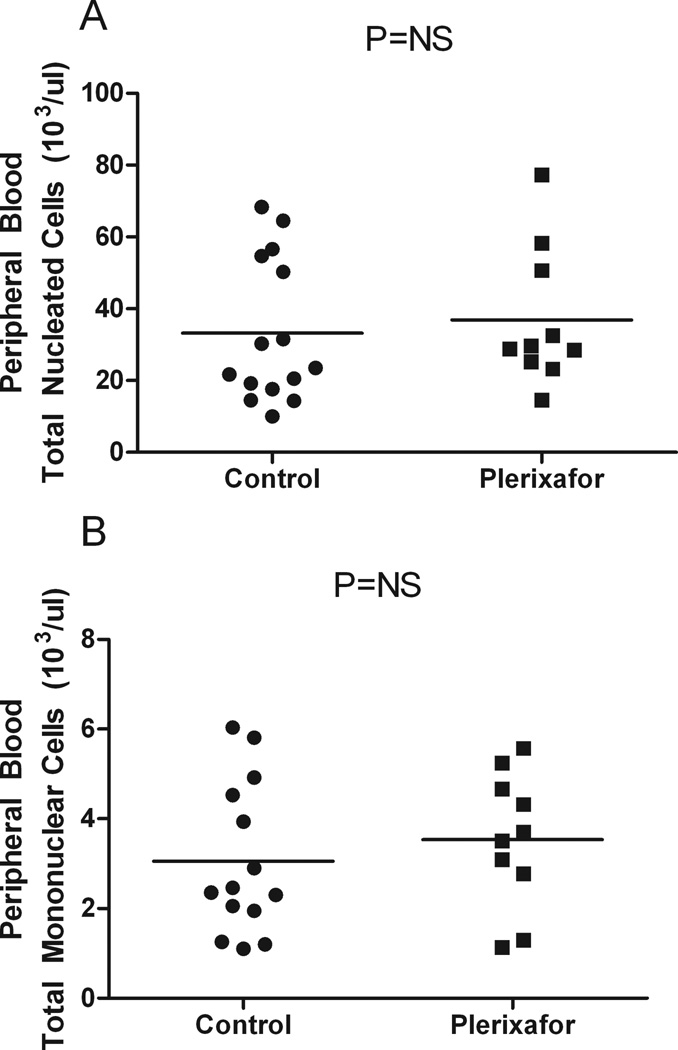
The number of apheresis sessions to reach the goal was similar between the two groups (mean of 3) (Figure 3A). Total blood volume processed per patient was also similar between the two groups (mean, control: 38.5L, plerixafor: 44.8L) (Figure 3B). No statistically significant differences were found in the peripheral blood total nucleated cells (mean, control: 33.2 × 103/ul, plerixafor: 38.4 × 103/ul) or mononuclear cells (mean, control: 3.1 × 103/ul, plerixafor: 4.2 × 103/ul) in both groups the day prior to the start of collection and plerixafor administration (Figure 4, Tables II and III).
Figure 3. Technical factors in apheresis do not account for differences in total nucleated and mononuclear cell counts.
A. Mean number of apheresis sessions for the control group (3) was similar to the plerixafor group (3), (P=NS).
B. Mean total blood volume processed per patient for the control group (38.5L) was similar to the plerixafor group (44.8L), (P=NS).
Figure 4. Pre-collection peripheral blood cell count does not account for differences in total nucleated and mononuclear cell counts.
A. Mean peripheral blood total nucleated cells for the control group (33.2 × 103/ul) is similar to the plerixafor group (38.4 × 103/ul), (P=NS).
B. Mean peripheral blood total mononuclear cells for the control group (3.1 × 103/ul) is similar to the plerixafor group (4.2 × 103/ul), (P=NS).
DISCUSSION
Here we report that plerixafor plus G-CSF mobilization leads to an unanticipated requirement of more storage bags. The increased requirement (Figure 2) is due to the greater number of mobilized mononuclear and nucleated cells relative to CD34+ cells. In other words, CD34+ cells constituted a smaller proportion of total nucleated cells (Figure 1A) in the plerixafor plus G-CSF group. This is the first study to our knowledge that evaluated the number of storage bags required for patients mobilized with plerixafor plus G-CSF compared to chemotherapy plus G-CSF.
Differences in pre-collection peripheral blood total nucleated cell and mononuclear cell counts as well as technical factors during apheresis could potentially account for the differences in total nucleated and mononuclear cell counts observed in the stem cell product. However, we show that the pre-collection peripheral blood total nucleated and mononuclear cell counts as well as the apheresis parameters such as the number of apheresis sessions and total blood volume processed were similar between the two groups (Figures 3 & 4) indicating that pre-collection peripheral blood counts and technical factors during apheresis did not account for the increased mononuclear and total nucleated cell counts observed.
We believe that this is the first report to show that the proportion of CD34+ cells among total nucleated cells in the product was decreased with plerixafor. Total nucleated cells and total mononuclear cells were statistically significantly increased in the plerixafor group compared to the control group. Since CD34+ cell yield is similar between the two groups (Figure 1D and 1E), then an increase in CD34− mononuclear cells such as B and T cells may account for the increased storage bags needed. Liles et al reported that plerixafor-mobilized leukapheresis products contained significantly greater numbers of T and B cells compared to G-CSF stimulated leukapheresis products [4]. More recently, Donahue et al reported that plerixafor and G-CSF mobilize different CD34+ cell populations [13]. By using global gene and microRNA expression microarrays, they show that plerixafor mobilized cells were enriched for B cells, T cells and mast cell genes while G-CSF mobilized cells were enriched for neutrophils and mononuclear phagocyte genes [13]. It is important to note that it is possible to store mobilized products at slightly higher concentrations than used in our study. A study by Rowley SD et al [22] showed that high concentration of cells (3.7 ± 1.9 × 108 nucleated cells per ml in 127 ± 45ml) could be stored per bag without a loss of engraftment potential or an increase in toxicity after infusion. This is twice as concentrated as used in our institution and would reduce the overall number of bags stored per patient. Nonetheless, it does not change the fact that more storage bags will still be required for patients mobilized with plerixafor plus G-CSF compared to chemotherapy plus G-CSF.
Having more bags to store increases cost and is therefore undesirable. It is a straightforward decision to give plerixafor when patients have failed prior mobilization. Given that certain conditions are associated with poorer mobilization [8,9,14,15] and higher CD34+ cell numbers are associated with better outcome [16–20], there are situations when upfront use of plerixafor and G-CSF are recommended. These include patients with a history of melphalan exposure, extensive prior therapy or prolonged disease duration and extensive radiotherapy [21]. Because “supermobilizers” may have better outcomes and difficult to mobilize patients have poorer outcomes in autologous stem cell transplant for lymphomas [16], it may be reasonable to consider using plerixafor as first line therapy in additional cases. Thus, it is important to keep in mind during the process of deciding which mobilization regimen to use that if plerixafor will be used upfront in the mobilization regimen, more bags may be needed leading to higher costs.
In summary, we have shown that the proportion of CD34+ cells among total nucleated cells is less in patients mobilized with plerixafor and G-CSF compared to chemotherapy and G-CSF. This led to a greater number of storage bags required in the plerixafor plus G-CSF group of patients which could lead to higher costs.
REFERENCES
- 1.Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K, Dehner C, Gibney C, Bridger G, Calandra G. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010 Jan;45(1):39–47. doi: 10.1038/bmt.2009.119. Epub 2009 Jun 1. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ, Flexner CW, Bridger GJ, Badel K, MacFarland RT, Henson GW, Calandra G. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(2):1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 3.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 4.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, Christensen J, Wood B, Price TH, Dale DC. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45(3):295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 5.Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K, Grove B, Dye A, Bridger G. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41(4):331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 6.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27(28):4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 7.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 8.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K, Calandra G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K, Nelson V, Kovacsovics T, Leis J, Calandra G, Maziarz RT. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone Marrow Transplant. 2009;43(12):909–917. doi: 10.1038/bmt.2008.409. [DOI] [PubMed] [Google Scholar]
- 10.Fruehauf S, Veldwijk MR, Seeger T, Schubert M, Laufs S, Topaly J, Wuchter P, Dillmann F, Eckstein V, Wenz F, Goldschmidt H, Ho AD, Calandra G. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor AMD3100 (plerixafor) mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009:1–10. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 11.Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, Badel K, Calandra G. Treatment with plerixafor in non-Hodgkin's lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biol Blood Marrow Transplant. 2009;15(2):249–256. doi: 10.1016/j.bbmt.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Nazha A, Cook R, Vogl DT, Mangan PA, Hummel K, Cunningham K, Luger S, Porter D, Schuster SJ, O'Doherty U, Sell M, Siegel DL, Stadtmauer EA. Plerixafor and G-CSF Versus Cyclophosphamide and G-CSF for Stem Cell Mobilization in Patients with Multiple Myeloma. 2009 [Google Scholar]
- 13.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, Stroncek DF. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114(12):2530–2541. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhtar S, Weshi AE, Rahal M, Khafaga Y, Tbakhi A, Humaidan H, Maghfoor I. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma. 2008;49(4):769–778. doi: 10.1080/10428190701843213. [DOI] [PubMed] [Google Scholar]
- 15.Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. Prediction of mobilisation failure in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33(9):907–912. doi: 10.1038/sj.bmt.1704466. [DOI] [PubMed] [Google Scholar]
- 16.Bolwell BJ, Pohlman B, Rybicki L, Sobecks R, Dean R, Curtis J, Andresen S, Koo A, Mineff V, Kalaycio M, Sweetenham JW. Patients mobilizing large numbers of CD34+ cells ('super mobilizers') have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant. 2007;40(5):437–441. doi: 10.1038/sj.bmt.1705763. [DOI] [PubMed] [Google Scholar]
- 17.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–3969. [PubMed] [Google Scholar]
- 18.Sola C, Maroto P, Salazar R, Mesia R, Mendoza L, Brunet J, Lopez-Pousa A, Tabernero JM, Montesinos J, Pericay C, Martinez C, Cancelas JA, Lopez-Lopez JJ. Bone Marrow Transplantation: Prognostic Factors of Peripheral Blood Stem Cell Mobilization with Cyclophosphamide and Filgrastim (r-metHuG-CSF): The CD34+ Cell Dose Positively Affects the Time to Hematopoietic Recovery and Supportive Requirements after High-Dose Chemotherapy. Hematology. 1999;4(3):195–209. doi: 10.1080/10245332.1999.11746443. [DOI] [PubMed] [Google Scholar]
- 19.Limat S, Woronoff-Lemsi MC, Milpied N, Chartrin I, Ifrah N, Deconinck E, Gressin R, Colombat P, Cahn JY, Arveux P. Effect of cell determinant (CD)34+ cell dose on the cost and consequences of peripheral blood stem cell transplantation for non-Hodgkin's lymphoma patients in front-line therapy. Eur J Cancer. 2000;36(18):2360–2367. doi: 10.1016/s0959-8049(00)00327-0. [DOI] [PubMed] [Google Scholar]
- 20.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18(6):1360–1377. doi: 10.1200/JCO.2000.18.6.1360. [DOI] [PubMed] [Google Scholar]
- 21.Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Kumar S, Munshi NC, Dispenzieri A, Kyle R, Merlini G, San Miguel J, Ludwig H, Hajek R, Jagannath S, Blade J, Lonial S, Dimopoulos MA, Einsele H, Barlogie B, Anderson KC, Gertz M, Attal M, Tosi P, Sonneveld P, Boccadoro M, Morgan G, Sezer O, Mateos MV, Cavo M, Joshua D, Turesson I, Chen W, Shimizu K, Powles R, Richardson PG, Niesvizky R, Rajkumar SV, Durie BG. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23(10):1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 22.Rowley SD, Bensinger WI, Gooley TA, Buckner CD. Effect of cell concentration on bone marrow and peripheral blood stem cell cryopreservation. Blood. 1994;83(9):2731–2736. [PubMed] [Google Scholar]