Abstract
Bipolar disorder is a terrible and debilitating disease with limited treatment options. Circadian rhythm disruptions are prominent in bipolar subjects, and studies have shown that rhythm stabilization through psychosocial interventions can improve their symptoms. Furthermore, mice with a mutation in one of the central circadian proteins, CLOCK, have severely disrupted rhythms along with a behavioral profile that closely resembles human mania. A compound has been developed (CK01, similar to PF-670462) that inhibits the activity of casein kinase 1 (CK1), a critical protein involved in the timing of the molecular clock. Previous studies have shown that PF-670462 and other similar compounds are capable of entraining and stabilizing rhythms in arrhythmic animals. Here we show that chronic administration of CK01 leads to a reversal of the anxiety-related behavior, and partial reversal of the depression-related phenotypes of the Clock mutant mouse. This drug had no significant effects on the behavior of wild-type mice at the doses tested. These results suggest that CK1ε/δ inhibitors could be viable drugs for the treatment of bipolar disorder.
Keywords: anxiety, bipolar disorder, circadian rhythms, depression, mouse
Introduction
Bipolar disorder is a devastating disease that is characterized by periods of depression and mania. Although medications like lithium are effective in a number of individuals, the current mood stabilizers cause a number of significant side-effects and do not alleviate symptoms in individuals with this disorder. Thus, there is a need to develop better treatments. Several recent studies point towards disruptions in the circadian system as a strong contributing factor to the development of bipolar disorder (McClung, 2007). Psychosocial therapies such as interpersonal and social rhythm therapy have demonstrated that regulation of the circadian cycle leads to improved patient outcomes in a shorter time than traditional psychotherapy (Frank et al., 2000). Recently, several drugs have been designed to inhibit casein kinase 1 ε/δ (CK1ε/δ), critical proteins involved in the regulation of molecular rhythms. Circadian rhythms are controlled by a transcriptional/translational feedback loop that cycles over the course of 24 h (Ko and Takahashi, 2006). The CLOCK and BMAL1 proteins are the central transcriptional activators that regulate the expression of the Period (Per) and Cryptochrome (Cry) genes. These proteins then feed back and inhibit the activity of CLOCK/BMAL1. CK1ε/δ phosphorylates the PER proteins, leading to the recruitment of F-box protein β-TRCP, which then targets the PER proteins for degradation (Eide et al., 2005; Shirogane et al., 2005; Shanware et al., 2011). This action helps regulate the timing of the molecular rhythms. A specific CK1ε/δ inhibitor, PF-670462, which inhibits CK1ε/δ with a potency in the nanomolar range, has been developed previously (Badura et al., 2007). Studies have shown that PF-670462 inhibits PER3 nuclear translocation, and also leads to a dose-dependent phase delay in rhythms when administered chronically to wild-type (WT) rats in a light/dark cycle (Badura et al., 2007; Sprouse et al., 2010). These results were reproduced in diurnal cynomolgus monkeys, suggesting that they would have the same effect on humans (Sprouse et al., 2009). Recently, Meng et al. (2010) reported that the administration of PF-670462 could entrain mice with disrupted rhythms caused either by constant light or a mutation in the Vipr2 gene (Meng et al., 2010). These results suggest that PF-670462 or other CK1ε/δ inhibitors could be used as therapeutic tools to entrain the rhythms of individuals with rhythm disruptions.
Our lab previously reported that mice with a mutation in the Clock gene (ClockΔ19 mice, described in King et al., 1997) have a behavioral profile that is strikingly similar to human mania (King et al., 1997; Roybal et al., 2007). This includes hyperactivity, decreased anxiety (i.e. increased exploratory drive), decreased depression-like behavior, and a hyperhedonic response to rewarding stimuli (McClung et al., 2005; Roybal et al., 2007). Lithium treatment is able to restore the majority of these abnormal behavioral phenotypes to WT levels (Roybal et al., 2007), suggesting that this mouse can be used to screen additional compounds that might be effective mood-stabilizing drugs. We aimed to determine whether a CK1ε/δ inhibitor (CK01), a compound with a highly similar structure and function to PF-670462, would be able to restore normal behavior to the ClockΔ19 mouse. A positive result suggests that CK1ε/δ inhibitors may be effective compounds for rhythm and mood stabilization in bipolar subjects.
Methods
Subjects and housing
ClockΔ19 mutant mice were created by N-ethyl-N-nitrosourea mutagenesis and produce a dominant-negative CLOCK protein defective in transcriptional activation activity as described (King et al., 1997). For all experiments using ClockΔ19 mutants, 8–10-weeks-old adult male mutant (Clock/Clock; Mut) and WT (+/+) littermate controls on a mixed BALBc; C57BL/6 background were used. Mice were group housed in sets of two to four per cage on a 12:12 h light/dark cycle (lights on at 06:00 h, lights off at 18:00 h) with food and water freely available.
Lithium administration
Lithium-treated mice received 600 mg/l of LiCl in drinking water for 10 days before behavioral testing, and throughout the course of the testing. This administration results in a stable serum concentration of lithium in the low therapeutic range for humans (0.41±0.06 mmol/l), with little to no adverse health consequence (Roybal et al., 2007).
CK01 preparation and administration
CK01 was dissolved in 20% sulfobutyl ether β-cyclodextrin in sterile water at three doses (5.6, 17.8, 32.0 mg/kg). Approximately 4.8 microliters of 1N HCl was added per milligram of CK01 used to the vehicle solution. The CK01 solution was injected daily subcutaneously for 10 days before behavioral testing, and daily injections continued throughout testing. The compound was administered at 06:30–07:00 h (Zeitgeber time 1) each day. The 5.6 mg/kg dose led to no changes in behavior throughout the study (data not shown) and thus results are presented for the higher doses only.
Behavioral assays
Locomotor response to novelty
Mice were placed into individual automated locomotor activity chambers that were equipped with infrared photobeams (San Diego Instruments, San Diego, California, USA). Activity measurements commenced upon the first beam break and were measured continuously, with data collected in 5-min blocks over a period of 2 h.
Elevated plus maze
The plus maze apparatus consisted of closed and open arms (all arms are 30×5 cm, with 25cm tall walls on the closed arms). Mice were placed in the center of an elevated plus maze and the time spent in the open arms, closed arms, and center of the maze, along with the number of entries into the open and closed arms of the maze were determined using Ethovision 3.0 video tracking software (Noldus, Leesburg, Virginia, USA). The time spent on the open arms and the percent of entries into the open arms were used to determine the anxiety-related behavior. The plus maze apparatus was sanitized and allowed to dry before each mouse was tested.
Dark/light test
The dark/light apparatus is a two-chambered box (25×26 cm for each side; Med Associates, St Albans, Vermont, USA), one side of which was kept dark and the other side was brightly lit by a fluorescent bulb at the top of the chamber. Mice were allowed to habituate to the dark side of the box for 2 min. Following the habituation period, the door between the compartments was opened and they were allowed to explore freely on both sides of the apparatus for 10 min. Anxiety-like behavior was measured as the percent of time spent in the light side.
Forced swim test
Mice were placed in 4-liter Pyrex glass beakers filled with 3 liters of water at 21–25°C for 6min. All test sessions were recorded from the side of the beakers by a video camera. Water was changed between subjects. The video was analyzed and scored by an observer blind to the genotypes and treatment groups. After a 2-min habituation period, latency to immobility was determined as the first cessation of movement. Total immobility was measured during the last 4 min of the test and was measured as the time spent without movement except for a single limb paddling to maintain flotation.
Statistics
All data were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc tests, using GraphPad Prism 5 statistical software for Windows (GraphPad Software Inc., La Jolla, California, USA). All data are presented as means±SEM with P value less than 0.05 considered statistically significant.
Results
CK01 has no effect on general locomotor activity
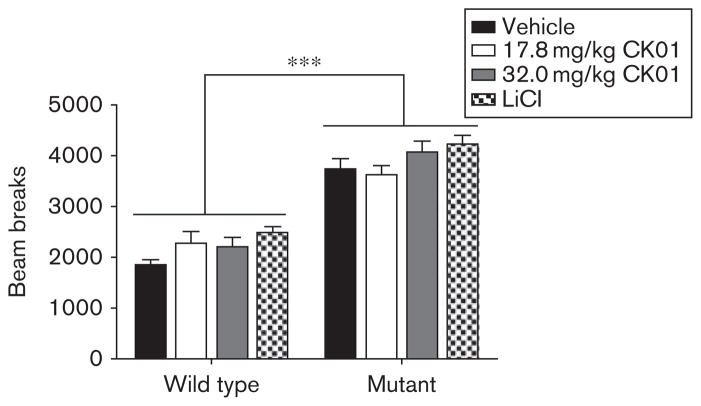
To determine the effects of CK01 administration on manic-like behaviors, ClockΔ19 mice and WT littermates received 10 days of systemic CK01 at three doses (5.6, 17.8, and 32.0 mg/kg) or sulfobutyl ether-β-cyclodextrin, and were compared with ClockΔ19 and WT mice receiving lithium treatment, following a battery of behavioral tests. As the 5.6mg/kg dose led to no discernable behavioral effects on any measure, results are shown only for the two higher doses. Mice were first assessed for their locomotor response to novelty. As reported previously (Roybal et al., 2007), ClockΔ19 mice are hyperactive when compared with WT animals (Fig. 1), and CK01 administration had no significant effect on locomotor activity, similar to mice receiving lithium treatment (Fig. 1). Because treatment had no effect on locomotor activity, effects on the other behavioral tests are interpreted as changes in anxiety-related and depression-related behavior.
Fig. 1.
CK01 administration has no effect on the locomotor response to novelty. Locomotor activity was measured in the ClockΔ19 mice and the wild-type littermates for 2 h following 10 days of CK01 or lithium administration. Analysis by two-way analysis of variance revealed a significant main effect of genotype [F(1,90) =145.94, P<0.001]. Bonferroni’s post-hoc tests revealed that there was no significant effect of any treatment on locomotor response to novelty (n=12–14/group). LiCl, lithium chloride. ***P<0.001.
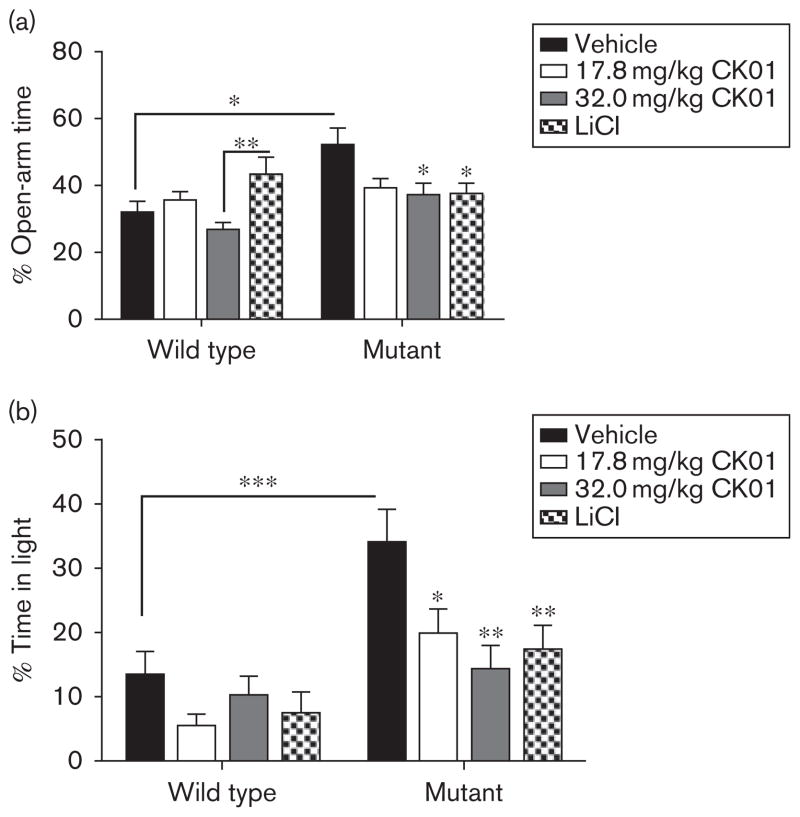
CK01 normalizes the anxiolytic behavior of the ClockΔ19 mice
To examine the effects of CK01 administration on anxiety-related behavior, mice were subjected to two different measures: the elevated plus maze and the dark/light test. In the elevated plus maze, the excess exploratory behavior of ClockΔ19 mice was rescued by the 32.0 mg/kg dose of CK01, as seen by a reduction in the amount of time spent in the open arms of the maze, similar to lithium treatment (Fig. 2a). The 17.8 mg/kg dose also caused a near-significant decrease in open-arm time (Fig. 2a). In the dark/light test, CK01 also had anxiogenic effects similar to lithium treatment (Fig. 2b). A significant decrease in time spent in the light side of the light/dark box was observed following administration of 17.8 mg/kg CK01 and a more robust decrease was observed following 32.0 mg/kg CK01 treatment. CK01 treatment had no detectable effect on WTanimals in any measure of anxiety-related behavior (Fig. 2a and b); however, lithium treatment had a slight anxiolytic effect on open-arm time in the elevated plus maze when compared with the 32.0 mg/kg dose of CK01, but did not differ significantly from either vehicle or the 17.8 mg/kg dose of CK01.
Fig. 2.
CK01 administration normalized anxiety-related behavior in the ClockΔ19 mice. (a, b) The ClockΔ19 mice and the wild-type (WT) littermates were assessed for anxiety-related behavior following CK01 and lithium administration in both the elevated plus maze (EPM) (a) and dark/light test (b). Analysis by two-way analysis of variance revealed that ClockΔ19 mice display previously reported decreased anxiety-related behavior by spending more time in the open arms of the EPM [main effect of genotype; F(1,83) =6.39, P<0.02] and light side of the dark/light box [main effect of genotype; F(1,85)=21.93, P<0.001]. Bonferroni’s post-tests revealed that 17.8 mg/kg CK01 treatment caused a reduction in open arm time that did not reach significance in the EPM (a) and a significant reduction in time spent in the light side of the dark/light test (b, t =2.553, P<0.05). 32.0 mg/kg CK01 treatment in ClockΔ19 mice caused a significant decrease in EPM open arm time (a, t=2.55, P<0.05) and time spent on the light side of the dark/light test (b, t=3.62, P<0.01). As described previously, lithium had antimanic effects on the ClockΔ19 mice by restoring open-arm time in the EPM (a, t=2.75, P<0.05) and time in the light side of the dark/light test (b, t=3.25, P<0.05) to WT levels. CK01 treatment had no significant effect on the anxiety-related behavior when compared with the vehicle-treated WT mice; however, Bonferroni’s post-hoc tests revealed that lithium treatment caused an anxiolytic effect that differed significantly from mice receiving 32.0 mg/kg CK01 (a, t =3.04, P<0.01), but not vehicle or 17.8 mg/kg CK01 treated mice. LiCl, lithium chloride. *P<0.05, **P<0.01, ***P<0.001.
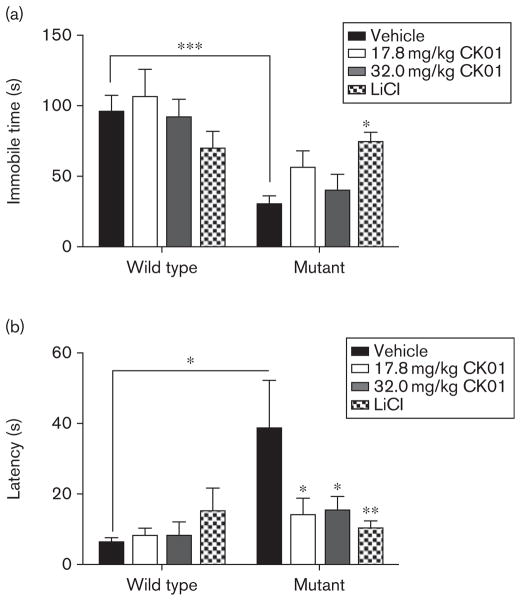
CK01 treatment partially normalizes the antidepressant effects of the ClockΔ19 mouse
In the forced swim test, ClockΔ19 mice displayed a significant decrease in depression-related behavior, as described previously (Fig. 3a; Roybal et al., 2007). Unlike lithium treatment, which normalizes the effects on depression-related behavior by causing an increase in total immobility time (Fig. 3a), CK01 treatment had no significant effect on immobility time. However, CK01 treatment did cause a significant decrease in latency to the first bout of immobility in ClockΔ19 mice at both doses, without affecting WT animals, suggesting a partial reversal of this phenotype (Fig. 3b).
Fig. 3.
CK01 administration has partial effects on ClockΔ19 depression-related behavior. (a) ClockΔ19 and wild-type (WT) mice were assessed for depression-related behavior using the forced swim test following CK01 and lithium treatment. Analysis by two-way analysis of variance (ANOVA) showed that the ClockΔ19 mice have decreased immobility time compared with the WT animals [main effect of genotype; F(1,88) =21.39, P<0.001]. Bonferroni’s post-hoc tests showed that lithium treatment had previously reported antimanic effects on ClockΔ19 depression-related behavior by increasing the total immobility time (t=2.52, P<0.05). CK01 treatment had no detectable effect on either ClockΔ19 or WT immobile time. (b) Latency to immobility was assessed following CK01 and lithium treatment in ClockΔ19 and WT animals. ClockΔ19 mice had a significantly longer latency to immobility than WT animals by two-way ANOVA [main effect of genotype; F(1,87) =6.30, P<0.02]. Bonferroni’s post-hoc tests revealed a significant effect of treatment with 17.8 mg/kg CK01 (t=2.82, P<0.05), 32.0 mg/kg CK01 (t =2.80, P<0.05), and lithium (t=3.40, P<0.01) treatment on latency to immobility in ClockΔ19 mice. WT animals were not significantly affected by treatment. LiCl, lithium chloride. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Our results show that CK01 treatment leads to a reversal of the abnormal anxiolytic behaviors of the ClockΔ19 mouse, which were more robust following the administration of a higher dose (32.0 mg/kg). Furthermore, there was a partial reversal of the antidepressant phenotype. Along with other abnormal circadian and reward-related phenotypes, these behaviors constitute a profile of abnormal behavioral responses in the ClockΔ19 mouse which together represent a manic-like phenotype reminiscent of human bipolar disorder. Interestingly, CK01 treatment does not reverse the hyperactivity in a novel environment that is prominent in the ClockΔ19 mouse. Lithium treatment also does not reverse this phenotype, and recent studies in our lab suggest that treatment with another mood-stabilizing agent, valproate, also has no effect on this particular behavior (unpublished observations). These results suggest that the hyperactivity in the ClockΔ19 mouse is controlled by a separate mechanism that is independent of the control of anxiety-related and mood-related behavior. This separation of mechanisms is particularly relevant as amphetamine-induced and other psychostimulant-induced locomotor activity is often used as a model of mania. Indeed, separate drugs may be needed to reverse specific endophenotypes of bipolar illness. Interestingly, a recent report found that PF-670462 does normalize amphetamine-induced hyperactivity likely through a regulation of Darpp-32-PP1-GlurR1 signaling in the nucleus accumbens (NAc) (Li et al., 2011). This suggests that CK1 inhibitors may be able to modulate certain behavioral abnormalities through circadian clock stabilization and others through effects on modulation of NAc output.
Previous studies have found that CK01 treatment leads to phase delays and a lengthening of the period of WTanimals while it entrains the rhythms of animals that are arrhythmic (Meng et al., 2010). CK1δ inhibition leads to a daily enhancement of PER protein in the nucleus of the cell, which presumably results from decreased degradation of the PER proteins or enhanced nuclear translocation. In the ClockΔ19 mice, the PER protein levels are very low and rhythms in a light/dark cycle are sometimes weak (Vitaterna et al., 2006). Future studies will determine whether CK01 stabilizes the rhythms in these mice through increased PER protein concentrations in the suprachiasmatic nucleus. This rhythm stabilization could have therapeutic effects in the ClockΔ19 mice by affecting rhythms in the ventral tegmental area dopamine neurotransmission. Indeed, virtually all aspects of dopaminergic transmission have a circadian rhythm, and these rhythms are disrupted in the ClockΔ19 mice (McClung et al., 2005). It is possible that the administration of CK01 may normalize the rhythm of dopaminergic transmission and thus normalize the ClockΔ19 behavior. Future studies will determine whether CK01 does indeed alter rhythms in the ventral tegmental area dopaminergic transmission. CK01 may also normalize ClockΔ19 anxiety-related behavior by stabilizing rhythms in the amygdala, which is known to be an important regulator of these behaviors (Davis, 1992).
It is unclear why there was only a partial reversal of depression-related behavior in the ClockΔ19 mice in the forced swim test, in that the latency to immobility was significantly altered but the total time immobile was not. It is possible that a higher dose or more chronic treatment paradigm would be sufficient to alter both parameters of this measure. It is also possible that CK1 proteins have a more prominent role in the control of anxiety. Nevertheless, the results are promising and suggest that CK1ε/δ inhibitors might be able to normalize both anxiety-related and mood-related phenotypes in humans.
Acknowledgments
The authors thank Ariel Ketcherside for assistance with mouse husbandry and genotyping. The authors also thank Pat Seymour for useful discussions. This project was funded by a collaborative contract between Pfizer and UT Southwestern Medical Center. Dr McClung has also received research funding from GlaxoSmithKline on an unrelated project and honoraria from Pfizer, Servier, and GlaxoSmithKline.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Badura L, Swanson T, Adamowicz W, Adams J, Cianfrogna J, Fisher K, et al. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322:730–738. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Eide EJ, Kang H, Crapo S, Gallego M, Virshup DM. Casein kinase I in the mammalian circadian clock. Methods Enzymol. 2005;393:408–418. doi: 10.1016/S0076-6879(05)93019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biolog psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Li D, Herrera S, Bubula N, Nikitina E, Palmer AA, Hanck DA, et al. Casein kinase 1 enables nucleus accumbens amphetamine-induced locomotion by regulating AMPA receptor phosphorylation. J Neurochem. 2011;118:237–247. doi: 10.1111/j.1471-4159.2011.07308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QJ, Maywood ES, Bechtold DA, Lu WQ, Li J, Gibbs JE, et al. Entrainment of disrupted circadian behavior through inhibition of casein kinase 1 (CK1) enzymes. Proc Natl Acad Sci USA. 2010;107:15240–15245. doi: 10.1073/pnas.1005101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J Biol Chem. 2011;286:12766–12774. doi: 10.1074/jbc.M111.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirogane T, Jin J, Ang XL, Harper JW. SCFbeta-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per1) protein. J Biol Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Swanson TA, Engwall M. Inhibition of casein kinase I epsilon/delta produces phase shifts in the circadian rhythms of cynomolgus monkeys. Psychopharmacology (Berl) 2009;204:735–742. doi: 10.1007/s00213-009-1503-x. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Kleiman R, Tate B, Swanson TA, Pickard GE. Chronic treatment with a selective inhibitor of casein kinase I delta/epsilon yields cumulative phase delays in circadian rhythms. Psychopharmacology (Berl) 2010;210:569–576. doi: 10.1007/s00213-010-1860-5. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci USA. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]