SUMMARY
Dysfunctional bone marrow (BM) microenvironment is thought to contribute to the development of hematologic diseases. However, functional replacement of pathologic BM microenvironment through BM transplantation has not been possible. Furthermore, the study of hematopoietic inductive BM microenvironment is hampered by the lack of a functional non-hematopoietic reconstitution system. Here we show that deficiency of SH2-containing inositol-5′-phosphatase-1 (SHIP) in non-hematopoietic host microenvironment enables its functional reconstitution by wild-type donor cells. This microenvironment reconstitution normalizes hematopoiesis in peripheral blood and BM, also alleviates pathology of spleen and lung in the SHIP deficient recipients. SHIP deficient BM contains a significantly smaller population of multipotent stromal cells with distinct properties which may contribute to the reconstitution by wild-type cells. We further demonstrated that it is the non-hematopoietic donor cells that are responsible for the reconstitution. Thus, we have established a non-hematopoietic BM microenvironment reconstitution system to functionally study specific cell types in hematopoietic niches.
INTRODUCTION
The concept of hematopoietic stem cell (HSC) was first proven by Till and McCulloch following their pioneer studies of blood system regeneration in vivo (Till and McCulloch, 1961). A wealth of experimentation since then formed a solid foundation for stem cell research (Orkin and Zon, 2008). Non-hematopoietic cells within the bone marrow (BM) are collectively referred to as the BM microenvironment, which is essential for the maintenance and function of the HSCs (Bianco, 2011; Morrison and Spradling, 2008; Purton and Scadden, 2008; Schofield, 1978; Taichman, 2005). Non-hematopoietic cell types including mesenchymal-derived osteoblasts, endothelial cells and subendothelial reticular cells (pericytes) have been detected in the BM microenvironment, and have demonstrated concerted roles in regulating hematopoiesis (Calvi et al., 2003; Ding et al., 2012; Kiel et al., 2005; Omatsu et al., 2010; Sacchetti et al., 2007; Sugiyama et al., 2006; Zhang et al., 2003). Currently evolving concepts hypothesize that quiescent, long-term repopulating cells are in contact with the osteoblasts whereas more committed and proliferating hematopoietic stem and progenitors are in contact with the vasculature. However, in vivo imaging also suggested that the dichotomy between the endosteal and vascular niche is anatomically impossible (Lo Celso et al., 2009; Xie et al., 2009). Thus, the nature of the BM microenvironment and relative contribution of each niche component to hematopoiesis remain elusive.
The study of HSC has flourished after the establishment of a functional reconstitution assay in which the donor HSCs home to the marrow niches, engraft, proliferate, and eventually reconstitute the whole hematological and immunological repertoire of the lethally or sublethally irradiated recipient mice (Spangrude et al., 1988). In comparison, the study of BM microenvironment is lagging far behind, partially due to the lack of a model system to functionally study individual cell types involved in the BM microenvironment through non-hematopoietic reconstitution. Search for strategies to reconstitute BM microenvironment is warranted.
Dysfunctional BM microenvironment is now believed to contribute to the development of hematologic diseases such as myeloproliferative syndrome, myelodysplasia, leukemia and multiple myeloma (Lane et al., 2009). In hematologic malignancies, there are dynamic interactions between leukemic cells and cells of the BM microenvironment. Recent studies indicate that leukemogenic events in the hematopoietic system may create a tumor microenvironment (Colmone et al., 2008), or transform the niche into a milieu with dominant signals promoting and sustaining abnormal cell growth (Jones and Wagers, 2008; Li and Neaves, 2006). On the other hand, experiments in which selective gene targeting within BM microenvironment leading to deletion of the retinoic acid receptor γ (Walkley et al., 2007a), retinoblastoma (Walkley et al., 2007b) or Dicer1 specifically in osteoprogenitors (Raaijmakers et al., 2010) have provided critical evidence for genetic changes in the microenvironment contributing to or required for leukemogenesis. Furthermore, donor-cell derived hematopoietic disease is a well-recognized occurrence in allogeneic BM transplant recipients (Flynn and Kaufman, 2007), which provides clinical evidence implicating the BM microenvironment in leukemia development. In addition, multiple myeloma is an incurable blood disease characterized by clonal proliferation of malignant plasma cells, where BM microenvironment has a pivotal role in tumor pathogenesis (Raab et al., 2009). A complex interplay is involved in these hematologic diseases and provides a rationale for targeting the BM microenvironment. However, functional reconstitution of pathologic BM microenvironment through BM transplantation has not been possible. In standard irradiation and chemical ablation, the BM stroma remained functionally intact, where damaged sinusoidal endothelial cells could be replaced by host endothelial cells (Bierkens et al., 1991; Li et al., 2008). Thus, novel strategies to replace diseased BM microenvironment are needed.
SH2-containing inositol-5′-phosphatase-1 (SHIP) hydrolyzes the phosphatidylinositol-3-kinase (PI3K) product phosphatidylinositol-3,4,5-triphosphate (PI(3,4,5)P3) to PI(3,4)P2. SHIP blunts PI3K initiated signaling and prevents membrane recruitment and activation of pleckstrin homology domain-containing kinases (e.g. AKT and PDK) that serve as effectors of PI3K signaling (Rohrschneider et al., 2000). Within the SHIP deficient mice, a chronic progressive hyperplasia of myeloid cells was observed, perhaps at the expense of B-cell production. SHIP KO myeloid progenitors had enhanced responsiveness to cytokines and were less susceptible to apoptotic stimuli in vitro. Increased proliferation and decreased apoptosis may also contribute to the expanded HSC compartment in SHIP KO mice, though these HSCs have compromised homing and repopulating ability (Desponts et al., 2006; Helgason et al., 2003; Helgason et al., 1998; Liu et al., 1999). SHIP was initially thought to be restricted to hematopoietic cells, but it is now apparent that most cells of the BM and blood express SHIP. Hazen et al. demonstrated SHIP expression and participation in the function of non-hematopoietic cells of the BM, and SHIP KO mice had elevated levels of a number of hematopoietic cytokines in the serum (Hazen et al., 2009). In current study, we discovered that deficiency of SHIP in non-hematopoietic host microenvironment could efficiently enable its functional reconstitution by wild-type non-hematopoietic donor cells. Thus, we have established a model reconstitution system to functionally study specific cell types in the hematopoietic niches.
RESULTS
SHIP-deficient niche leads to impaired hematopoiesis
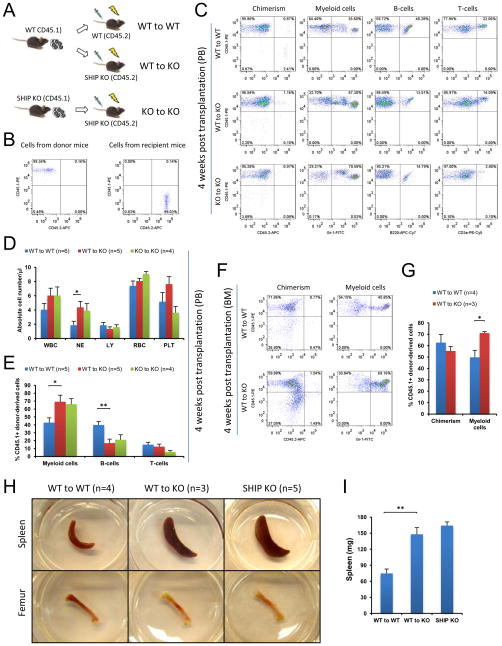
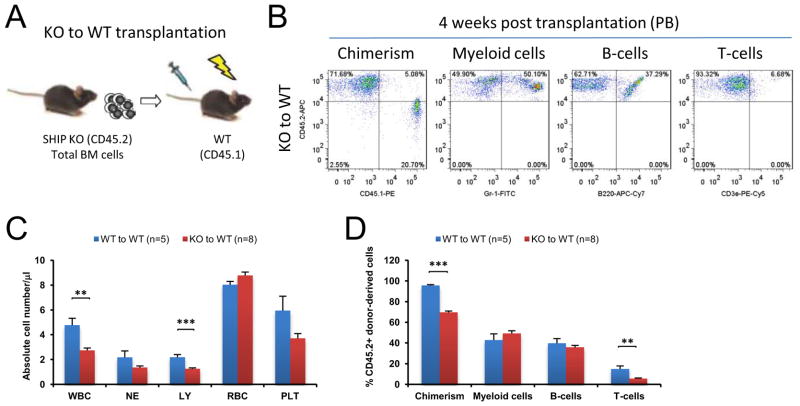
Consistent with the reported data, we observed that SHIP knock-out (KO) mice had significantly higher myeloid population and lower B-cell population in both peripheral blood (PB) and the BM compared to WT mice (data not shown). To provide direct evidence that SHIP disruption in HSC niche is responsible for the observed hematopoiesis defect, we performed reverse transplantation experiments where whole BM cells from CD45.1+ WT mice were intravenously transplanted into lethally irradiated CD45.2+ WT or SHIP KO mice. As a control, whole BM cells from CD45.1+ SHIP KO mice were transplanted into lethally irradiated CD45.2+ SHIP KO mice (Figures 1A and1B). Four weeks after transplantation, all three experimental groups (WT-to-WT, WT-to-KO and KO-to-KO) achieved equally high chimerism in peripheral blood (CD45.1-PE+ and CD45.2-APC− population, Figure 1C). Both complete blood cell count and lineage analysis indicated that WT-to-KO and KO-to-KO recipients exhibited SHIP KO hematopoietic phenotype, where CD45.1+ donor-derived hematopoietic cells produced significantly higher myeloid population and significantly lower B-cell population in PB compared to WT-to-WT recipients (Figures 1D and 1E). Similar to what was observed in PB, despite of a comparable donor-cell chimerism in the BM (CD45.1-PE+ and CD45.2-APC− population), donor-derived myeloid cell population in WT-to-KO recipients was significantly higher than that of the WT-to-WT recipients (Figures 1F and 1G). Earlier studies (Helgason et al., 1998; Liu et al., 1999) as well as our own observation indicated that SHIP KO mice suffer from profound splenomegaly and have less red blood cells in the BM than WT mice. Large spleen and pale femurs were phenotypical characteristics for SHIP KO mice. The spleen size and femoral appearance between WT-to-WT and WT-to-KO recipients remained readily distinguishable, with the latter nearly identical to those of the SHIP KO mice (Figures 1H and 1I). In addition, we transplanted whole BM cells from CD45.2+ SHIP KO mice into lethally irradiated CD45.1+ WT recipients. The results at 4w after transplantation indicated that SHIP KO cells had diminished ability to form chimerism in WT recipients. However, the levels of SHIP KO donor-derived myeloid cells and B-cells were comparable to those of the WT-to-WT recipient mice (Figure 2). Taken together, these results suggested that SHIP deficiency in the BM microenvironment was responsible for the defective hematopoietic phenotype observed in the SHIP KO mice. Interestingly, we also observed a reduced donor derived T-cell production in the KO-to-WT recipient, indicating that SHIP may also play a role in T cell recovery after irradiation.
Figure 1. SHIP deficiency in the BM microenvironment determined defective hematopoiesis at 4 weeks post transplantation.
(A) BM cells of CD45.1 WT mice were transplanted into lethally irradiated CD45.2 WT mice or littermate SHIP KO mice. As a control, BM cells of CD45.1 SHIP KO mice were also transplanted into lethally irradiated CD45.2 SHIP KO mice. (B) Examples of genotyping by FACS analysis of CD45.1+ SHIP KO mice (left, cells from donor mice) and CD45.2+ SHIP KO mice (right, cells from recipient mice). (C) Representative FACS profiles of hematopoietic cell lineage analysis in PB of transplant recipients. (D) Absolute blood cell number and (E) donor-derived blood cell composition in PB of transplant recipients. (F–G) Transplanted WT BM cells acquired SHIP KO hematopoietic phenotype in the BM of SHIP KO recipient mice 4 weeks after reverse transplantation. Shown are donor chimerism (similar for both WT-to-WT and WT-to-KO) and donor-derived myeloid cell population (higher in WT-to-KO than in WT-to-WT). (H–I) Transplantation of WT BM cells to the SHIP KO mice could not rescue the altered spleen size and the gross appearance of femur at 4 weeks post transplantation. (H) Apparent difference of spleen and femur between WT and SHIP KO recipients and representative pictures of spleen and femur of a SHIP KO mouse at comparable age are also shown. (I) Quantification of the spleen weight difference. WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). Data in (D), (E), (G) and (I) are mean ± SEM.*p<0.05, **p<0.01, two-tailed t-Test. See also Figure S1.
Figure 2. SHIP disruption in hematopoietic lineages led to impaired homing and engraftment of these cells, but was not responsible for the hematopoiesis defects observed in the SHIP KO mice.
(A) Experimental design for transplant assays. (B) Representative FACS lineage analysis. (C) Absolute cell number in PB of WT transplant recipient mice. Total white blood cell count and lymphocyte count were lower in KO-to-WT transplant recipients. (D) Donor-derived blood cell composition in PB of WT transplant recipient mice. Total donor chimerism and donor-derived T cell percentage were lower in KO-to-WT transplant recipients. WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). Data in (C) and (D) are mean ± SEM. **p<0.01, ***p<0.001 by two-tailed t-Test.
SHIP deficiency in the niche enables its reconstitution
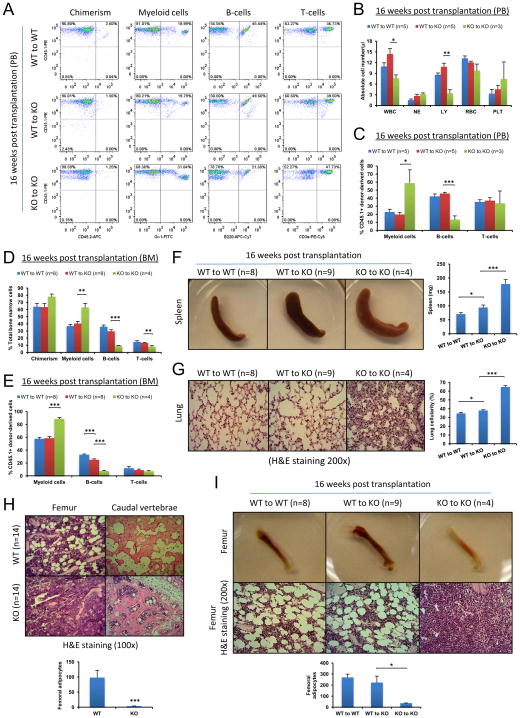
Many genes, such as retinoic acid receptor γ(Walkley et al., 2007a) have been implicated in niche function. Disruption of these genes also leads to hematopoiesis defects. These defects remained infinitely even after the WT-to-KO reverse transplantation. Intriguingly, in the WT-to-SHIP KO recipients, the levels of both myeloid and B-cell populations in PB shifted towards those of the WT-to-WT recipient mice at 8w, 12w (Figure S1), and 16w after reverse transplantation (Figures 3A–3C, Figure S2G). The similar shift was also observed in the BM (Figures 3D and 3E, Figures S2A and S2B), although it was somewhat weaker compared to the shift in the PB (Figure 3E). The spleen size of the WT-to-KO recipients at 16w was still larger than that of the WT-to-WT recipients, but it was significantly smaller compared to that of the KO-to-KO recipients (Figure 3F). Another prominent characteristic of the SHIP KO mouse is the elevated cellularity in the lung due to myeloid cell infiltration (Helgason et al., 1998). This phenotype was also significantly reversed to a much lesser extent in the WT-to-KO recipients (Figure 3G, Figure S2C). All these results indicate that transplantation of WT whole BM cells may gradually reconstitute the BM microenvironment in the SHIP deficient mice.
Figure 3. Transplantation of WT whole BM cells reversed the hematopoietic phenotype and alleviated pathology in the SHIP deficient mice.
(A–E) Transplantation of WT whole BM cells reversed the hematopoietic phenotype. (A) Representative FACS profiles of hematopoietic cell lineage analysis of PB from transplant recipients at 16 weeks post transplantation. (B) Absolute blood cell number and (C) donor-derived blood cell composition in PB of transplant recipients at 16 weeks post transplantation. Analysis of BM hematopoietic cell composition both in total BM cells (D) and in donor-derived cells (E). WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). (F–I) Transplantation of WT whole BM cells alleviated pathological phenotype and restored adipogenesis in the BM of SHIP deficient mice. (F) Gross appearance of spleen and quantification of spleen weight of WT-to-WT, WT-to-KO and KO-to-KO transplant recipients. (G) H&E staining of lung histological sections (200x) and quantification of lung cellularity of WT-to-WT, WT-to-KO and KO-to-KO transplant recipients. (H) Representative femur and caudal vertebrae sections after H&E staining and quantification of femoral adipocytes in WT and SHIP KO mice. (I) Gross appearance of femur, H&E staining of femur sections and quantification of femoral adipocytes of WT-to-WT, WT-to-KO and KO-to-KO transplant recipients. Data in (B), (C), (D), (E), (F), (G), (H) and (I) are mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, two-tailed t-Test. See also Figure S2.
We next directly investigated the structure of the BM. Hematoxylin and Eosin (H&E) staining as well as immunohistochemical staining (IHC) using an antibody against adipocyte-specific perilipin of bone sections revealed that SHIP KO mice had no discernible adipocytes in the trabecular region of the femur, and had significantly fewer adipocytes in the caudal vertebrae compared to WT mice (Figure 3H, Figures S2D and S2E). Subsequent histological analysis showed that the trabecular region of femurs of the WT-to-KO recipients contained numerous adipocytes similar to the WT-to-WT control mice, but in stark contrast to the KO-to-KO recipient mice (Figure 3I, Figure S2F). As illustrated in Figure 1H, pale femurs were phenotypical characteristics for SHIP KO mice (Figure 3I).
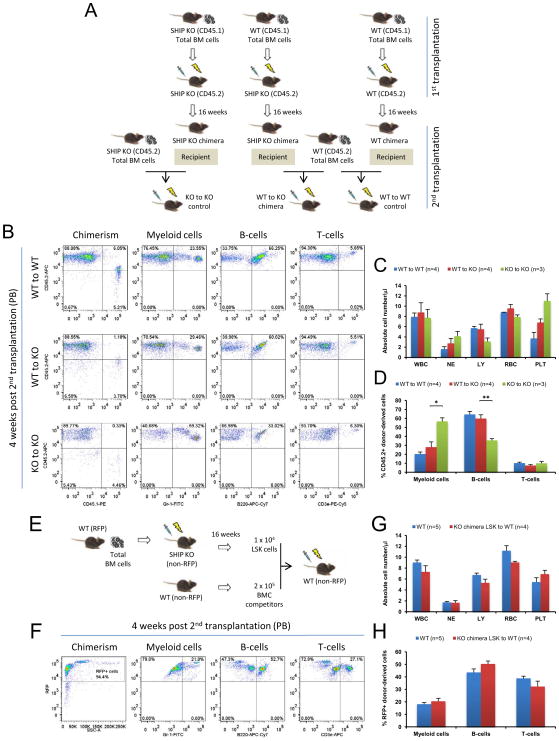
To definitively prove that transplantation of WT whole BM cells had reconstituted the BM microenvironment in the SHIP deficient mice, a second reverse transplantation was performed in the CD45.1 chimera mice at 16 weeks after the first reverse transplantation (Figure 4A). Four weeks after the second reverse transplantation, similar to WT-to-WT recipients, WT-to-KO recipients exhibited WT hematopoietic phenotype, in contrast to KO-to-KO recipients (Figures 4B–4D), confirming that SHIP KO chimera mice already possessed a WT BM microenvironment. To further demonstrate that a WT hematopoiesis has been established in the SHIP KO chimera mice, lineage- (CD3e−, CD4−, CD8a−, B220−, CD19−, Gr-1−, and CD14−), Sca-1+, c-kit+ (LSK) hematopoietic stem and progenitor cells (Morrison and Weissman, 1994) were isolated from the WT-to-KO recipients and transplanted into lethally irradiated WT mice (Figure 4E). As expected, all recipient mice exhibited WT hematopoietic phenotype (Figures 4F–4H).
Figure 4. Conversion to normal hematopoietic niche and establishment of normal hematopoiesis in the SHIP KO chimera.
(A–D) Conversion to normal hematopoietic niche in the SHIP KO chimera was confirmed by a second reverse transplantation. (A) BM cells of CD45.2 WT mice were transplanted into lethally irradiated WT CD45.1 chimeric mice or littermate SHIP KO CD45.1 chimeric mice. As a control, BM cells of CD45.2 SHIP KO mice were transplanted into lethally irradiated SHIP KO CD45.1 chimeric mice. (B) Representative FACS profiles of hematopoietic cell lineage analysis in PB of second reverse transplant recipients at 4 weeks post-transplantation. (C) Absolute blood cell number and (D) donor-derived blood cell composition in PB of second reverse transplant recipients at 4 weeks post-transplantation. (E–H) Normal hematopoiesis established in the SHIP KO chimera was confirmed by a secondary transplantation. (E) BM cells of red fluorescent protein (RFP) WT mice were transplanted into lethally irradiated SHIP KO mice. After 16 weeks, LSK hematopoietic stem and progenitor cells were isolated and transplanted into lethally irradiated WT mice. (F) Representative FACS profiles of hematopoietic cell lineage analysis in PB of secondary transplant recipients. (G) Absolute blood cell number and (H) donor-derived blood cell composition in PB of secondary transplant recipients (red bars) compared with WT mice (blue bars). WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). Data in (C), (D), (G) and (H) are mean ± SEM. *p<0.05, **p<0.01, two-tailed t-Test.
Non-hematopoietic donor cells accomplish niche reconstitution
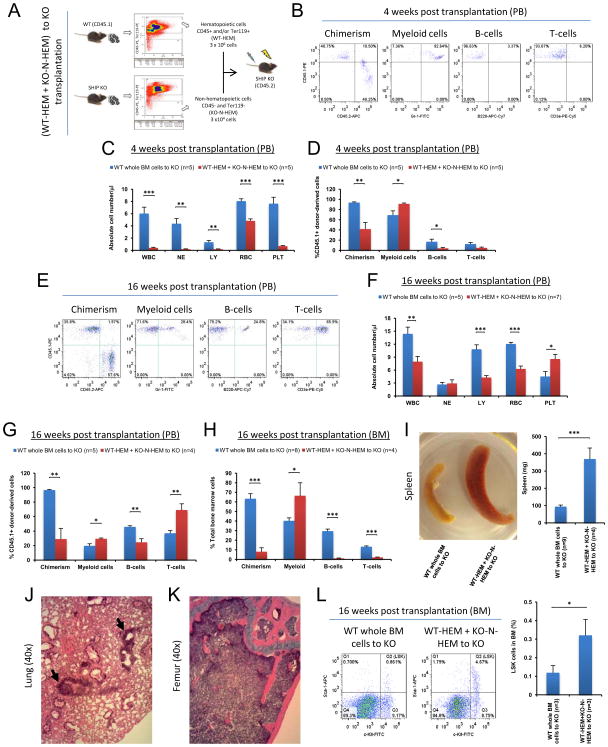
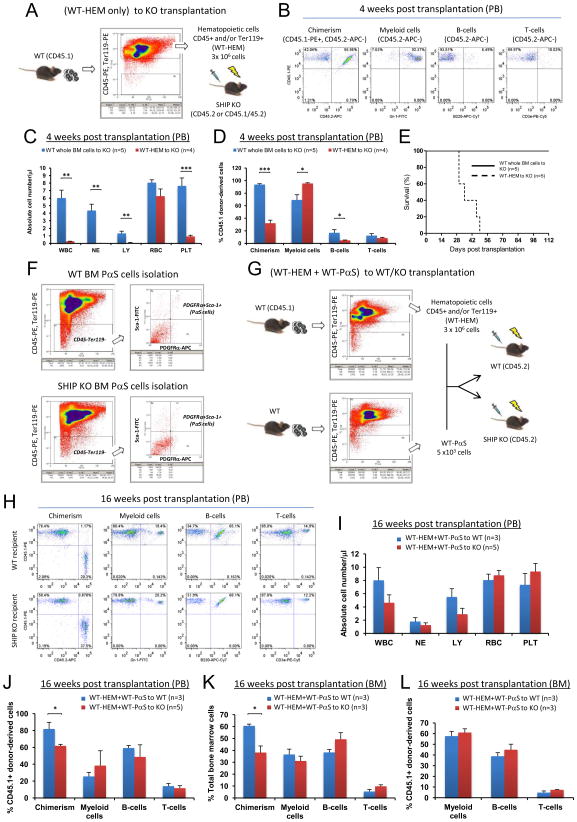
Transplantation of WT whole BM cells to SHIP KO mice converted BM microenvironment from a SHIP KO phenotype to a WT phenotype. To determine which population of the transplanted WT BM cells contributed to the non-hematopoietic reconstitution in the SHIP KO recipient mice, we combined WT hematopoietic cells (CD45+ and/or Ter119+) (WT-HEM) and SHIP deficient non-hematopoietic cells (CD45− and Ter119−) (KO-N-HEM) in a ratio of 100:1, which is comparable to the composition found in normal BM (non-hematopoietic cells were approximately 0.5–1% of the BM cells), and then transplanted them into lethally irradiated SHIP KO mice (Figure 5A). The resulting donor chimerism after transplantation was significantly lower than that of the WT (whole BM)-to-KO transplantation. Although the absolute numbers of donor-derived white blood cells were low at 4 weeks post transplantation, they consisted primarily of myeloid cells with very few lymphocytes (Figures 5B–5D). This SHIP KO hematopoietic phenotype persisted at both 4 weeks and 16 weeks after transplantation (Figures 5E–5H), suggesting that WT hematopoietic donor cells were not sufficient to reconstitute the SHIP deficient microenvironment, and WT non-hematopoietic cells were critical for successful niche reconstitution. Consistently, the pathological phenotype associated with SHIP deficiency also remained when SHIP KO non-hematopoietic cells were used as donor cells (Figures 5I–5K). We also analyzed the number of LSK cells in the BM of WT-HEM+KO-N-HEM recipients, which appeared similar to that of SHIP KO mice (Figure 5L, Figure S3A). In a similar experiment, we transplanted WT hematopoietic cells alone (WT-HEM) into lethally irradiated SHIP KO mice (Figure 6A). Without WT non-hematopoietic cells, the resulting donor chimerism after transplantation was significantly lower than that of the WT (whole BM)-to-KO transplantation. Similarly, despite minuscule amount of donor-derived white blood cells, they consisted primarily of myeloid cells with very few lymphocytes, a phenotype of the SHIP KO mice (Figures 6B–6D). The recipient mice eventually died between 4w and 7w post transplantation (Figure 6E). We then adopted a technique reported by Morikawa et al. (Morikawa et al., 2009) to isolate CD45−, Ter119−, PDGFRα+, and Sca-1+ (PαS) multipotent stromal cells from mouse BM (Figure 6F). We combined WT hematopoietic cells and WT PαS cells to transplant into lethally irradiated WT or SHIP KO mice (Figure 6G). After 16 weeks, SHIP KO recipients had significantly lower chimerism than that of the WT recipients. However, the SHIP KO recipients exhibited comparable hematopoietic lineage composition in PB and BM as the WT recipients (Figures 6H–6L). Collectively, these results demonstrate that WT hematopoietic cells alone were not sufficient to sustain hematopoiesis in lethally irradiated SHIP KO mice, and WT non-hematopoietic cells, including the PαS cells are required for the BM reconstitution in SHIP KO recipients.
Figure 5. Transplanted WT non-hematopoietic cells were responsible for the reconstitution of BM microenvironment in the SHIP KO recipients.
(A) Strategy to combine WT hematopoietic cells (WT-HEM) and SHIP KO non-hematopoietic cells (KO-N-HEM) in the transplantation. (B) Representative FACS profiles of donor-cell chimerism and hematopoietic cell lineage analysis of PB of WT-HEM + KO-N-HEM to KO transplant recipients at 4 weeks post transplantation. (C) Absolute blood cell number and (D) donor-derived blood cell composition in PB of WT-HEM + KO-N-HEM to KO transplant recipients at 4 weeks post transplantation. (E–L) At 16 weeks post transplantation, SHIP KO hematopoietic phenotype and pathological phenotype associated with SHIP deficiency persisted when WT non-hematopoietic donor cells were replaced with SHIP deficient non-hematopoietic donor cells. (E) Representative FACS profiles of donor-cell chimerism and hematopoietic cell lineage analysis of PB of WT-HEM + KO-N-HEM to KO transplant recipients at 16 weeks post transplantation. (F) Absolute blood cell number and (G) the percentage among donor-derived cell population in the PB of transplant recipients at 16 weeks post transplantation. (H) Total hematopoietic cell composition (percentage) in the BM of transplant recipients at 16 weeks post transplantation. (I) Apparent and quantitative difference of spleen between WT whole BM recipients and WT-HEM + KO-N-HEM recipients at 16 weeks after reverse transplantation. (J) Elevated cellularity and myeloid infiltration (black arrows) remained visible in the lungs of WT-HEM + KO-N-HEM transplant recipients (n=4). (K) No discernible adipocytes in the femurs of WT-HEM + KO-N-HEM transplant recipients (n=4). (L) Representative FACS analysis (left) and quantification (right) of total BM LSK cells of WT whole BM recipients and WT-HEM + KO-N-HEM recipients at 16 weeks after reverse transplantation. WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). Data in (C), (D), (F), (G), (H), (I) and (L) are mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, two-tailed t-Test. See also Figure S3.
Figure 6. WT non-hematopoietic BM cells were required for the reconstitution of BM microenvironment in the SHIP KO recipients.
(A–E) WT hematopoietic cells alone were not sufficient to sustain hematopoiesis in lethally irradiated SHIP KO mice. (A) Strategy of experiments in which WT hematopoietic cells (CD45+ and/or Ter119+, WT-HEM) alone were transplanted into lethally irradiated SHIP KO mice. (B) Representative FACS analysis profile, (C) absolute blood cell number, and (D) donor-derived cell population in the PB of WT-HEM to KO recipients at 4 weeks post transplantation. (E) Kaplan-Meier survival analysis of the recipients. (F–L) WT PαS cells contribute to the formation of a hematopoietic microenvironment in the SHIP KO recipients. (F) Isolation of PαS cells by FACS sorting. Shown are representative FACS profiles of WT and SHIP KO PαS cells. (G) Strategy of experiments in which WT hematopoietic cells (CD45+ and/or Ter119+, WT-HEM) were combined with isolated WT-PαS cells to transplant into either WT or SHIP KO mice. (H) Representative FACS analysis profile. (I) Absolute blood cell number and (J) donor-derived cell population in the PB at 16 weeks post transplantation. Analysis of BM hematopoietic cell composition both in total BM cells (K) and in donor-derived cells (L). WBC: white blood cells (x 103); NE: neutrophils (x 103); LY: lymphocytes (x 103); RBC: red blood cells (x 106) and PLT: platelets (x 105). Data in (C), (D), (I), (J), (K) and (L) are mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, two-tailed t-Test.
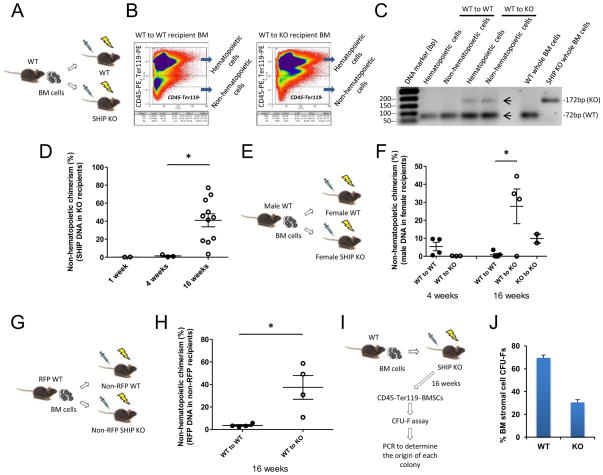
We applied several different approaches to quantify non-hematopoietic chimerism in the BM of SHIP KO recipients. First, under rigorous gating conditions, we isolated CD45– and Ter119- non-hematopoietic cells from the BM (Figures 7A and 7B). We then used conventional PCR (Figure 7C) to detect and quantitative real-time PCR (Figure 7D) to measure WT SHIP DNA in SHIP KO recipients. Next, we quantified Y-chromosome specific DNA in sex-mismatched recipients to demonstrate that while WT-to-KO transplantation resulted in chimerism, WT-to-WT transplantation did not (Figures 7E and 7F). Finally, we measured RFP DNA in donor-derived RFP gene positive cells in non-RFP recipients (Figures 7G and 7H). While there was no significant chimerism in WT-to-WT recipients, the non-hematopoietic chimerism estimated by all three Q-PCR methods was about 30–40% in the WT-to-KO recipients at 16w after the reverse transplantation (Figures 7D, 7F and 7H). In the above experiments, the targeted non-hematopoietic cell population is a mixture of multiple cell types, including multipotent BM stromal cells, fibroblasts (reticular connective tissue), and endothelial cells. In order to examine the reconstitution of multipotent stromal cells in the WT-to-KO recipients, we performed a colony forming assay (CFU-Fs) (Friedenstein et al., 1974; Kuznetsov et al., 2009) with the CD45−Ter119− BM cells from WT-to-KO recipients (Figure 7I). Total genomic DNA was isolated from individual colonies, and genotyping was performed to determine whether the cells were of WT donor or of SHIP KO recipient origin. The results indicated that up to 70% of the multipotent BM stromal cells of the KO recipients came from WT donor (Figure 7J). Taken together, these results directly show that cells originated from transplanted non-hematopoietic cells were indeed incorporated into the BM microenvironment in the SHIP KO recipient mice, and suggest that there is a selective advantage to allow engraftment of WT non-hematopoietic cells in the SHIP KO BM microenvironment. It is noteworthy that in the third approach we used whole BM cells from RFP (or GFP) transgenic mice for transplantation. We initially intended to use FACS to quantify non-hematopoietic chimerism in SHIP KO recipient mice. Interestingly, we discovered that BM cells in the non-hematopoietic compartment of the RFP or GFP mice were not fluorescent (Figure S4). It has been a problem in the BM stromal field that often times a reporter gene is silenced in these cells for reasons that are not clear. In both mice, the expression of RFP or GFP is under the control of CMV early enhancer/chicken β actin (CAG) promoter. Despite the ability of the CAG promoter to drive gene expression in almost all tissues, its activity appears to be developmentally regulated in specific cell lineages (Baup et al., 2009). Accordingly, we decided to quantify the RFP DNA in the SHIP KO recipients using PCR instead (Figure 7H).
Figure 7. Quantification of non-hematopoietic chimerism in the SHIP KO mouse BM microenvironment.
(A) Strategy to detect WT SHIP DNA in SHIP KO recipients. (B) Representative FACS profile of isolation of hematopoietic cells (CD45+ and/or Ter119+) and non-hematopoietic cells (CD45- and Ter119-) from WT and SHIP KO mouse recipient BM is shown. (C) Conventional PCR genotyping of hematopoietic and non-hematopoietic cells from the BM of transplant recipients. SHIP WT band (72bp) and SHIP KO band (172bp) are indicated. (D) Quantitative Real-Time PCR determination of WT SHIP DNA in SHIP KO transplant recipients. (E) Strategy to detect male DNA in female recipients. (F) Quantitative Real-Time PCR determination of male DNA in female transplant recipients. (G) Strategy to detect red fluorescent protein (RFP) reporter gene in non-RFP SHIP KO recipients. (H) Quantitative Real-Time PCR determination of RFP reporter gene in non-RFP SHIP KO recipients. Non-hematopoietic cells from RFP BM were isolated by using APC-labeled anti-mouse CD45 and Ter119 antibodies. (I) Strategy of colony forming assay (CFU-Fs) with the CD45-Ter119- BM stromal cells from WT-to-KO recipients. Four SHIP KO recipients were used in this experiment; approximately twenty colonies from each mouse were selected for genomic DNA isolation. (J) Conventional PCR was performed to determine the origin of individual colonies. Data in (J) are mean ± SEM. *p<0.05, two-tailed t-Test. See also Figure S4.
SHIP-deficient BM microenvironment
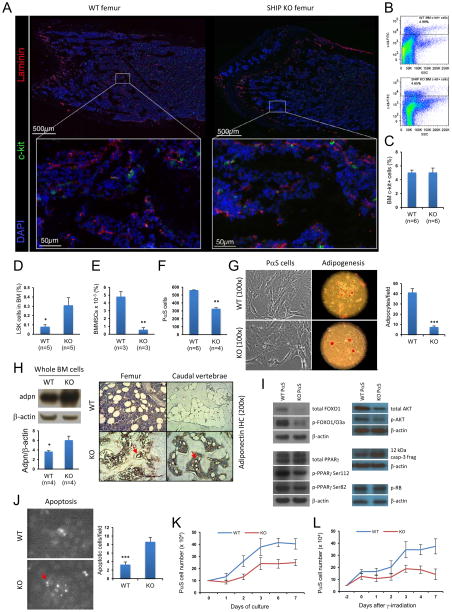
We next explore the mechanism by which the SHIP KO BM microenvironment affects normal hematopoiesis. Using laser scanning cytometry imaging technique, we found that SHIP KO mouse BM had a similar number and distribution of c-kit+ progenitor cells in the femur as WT mice despite the absence of adipocytes (Figures 8A–8C). Both WT and SHIP KO mouse BM contained comparable total number of cells. As previously reported, SHIP KO mouse BM contained 5-fold more LSK hematopoietic stem and progenitor cells (Figure 8D, Figure S3A). We also measured the amount of BM multipotent stromal cells (BMMSCs), which can give rise to multiple mesenchymal cell lineages, including chondrocytes, osteocytes, and adipocytes. Intriguingly, SHIP KO mouse BM contained about 10-fold less CD45−, CD11b−, CD29+, CD44+, CD105+, CD106+, and Sca-1+ multipotent stromal cells (Zhu et al., 2010) (Figure 8E, Figure S3B), and about 1-fold less CD45−, Ter119−, PDGFRα+, and Sca-1+ (PαS) multipotent stromal cells (Morikawa et al., 2009) (Figure 8F). Initial isolation of BM stromal cells based on their plastic-adherence properties (Peister et al., 2004) indicated that significantly more cells, presumably macrophages and monocytes, from the SHIP KO mouse BM adhered to the plastic tissue culture dish (Figure S5A). In separate cell adhesion assay under controlled shear flow, we could confirm that myeloid cells from SHIP KO mouse BM exhibited higher degree of adhesion to plastic surface than those from WT mouse BM (data not shown). We then performed a colony forming assay (CFU-Fs) (Friedenstein et al., 1974; Kuznetsov et al., 2009) using CD45−−Ter119− BM stromal cells to exclude possible contamination from BM macrophages. The results indicated that both WT and SHIP KO BM stromal cells had similar abilities to form fibroblast-like colonies (Figure S5B).
Figure 8. Characterization of SHIP-deficient BM microenvironment.
(A–C) SHIP KO mouse BM had a similar number and distribution of c-kit+ progenitor cells in the femur as WT mice. (A) Laser scanning cytometry imaging of the trabecular region of femur from WT and SHIP KO mice. Blood vessels (red) and c-kit+ cells (green) were stained using specific antibodies against laminin and c-kit, respectively. Slides were counterstained with DAPI (blue). Images in the upper panel were composite of areas shown in the lower panel. Three mice were used in each group. (B–C) Analysis of c-kit+ cell population showing similar numbers in WT and SHIP KO mouse BM. (D–L) Disruption of SHIP led to reduced BM multipotent stromal cell (BMMSC) population, impaired adipogenic differentiation, and enhanced apoptosis of these cells. Comparison of LSK (D) and BMMSC (CD45-, CD11b-, CD29+, CD44+, CD105+, CD106+, and Sca-1+) (E) populations between WT and SHIP KO mouse BM. (F) Comparison of PαS cell (CD45-, Ter119-, PDGFRα+, and Sca-1+) populations between WT and SHIP KO mouse BM. Shown are average PαS cell numbers obtained from two pairs of femur and tibia. (G) SHIP KO PαS cells formed fewer adipocytes compared to WT PαS cells. The adipogenic potential was assessed by Oil red O staining (100x) at 14 days after adipogenic induction of WT and SHIP KO PαS cells. (H) SHIP KO adipocytes expressed much higher level of adiponectin in both femur and caudal vertebrae compared to WT mice. Western blots of whole BM cells (left) and IHC staining (right) were conducted using specific antibodies against adiponectin. Red arrows in IHC indicate adipocytes in SHIP KO mouse BM. (I) Western blots of key regulators of adipogenic process, cell-cycle progression, and markers of apoptotic process. (J) SHIP KO PαS cells were more prone to apoptosis in vitro. Apoptosis was assessed by TUNEL staining (100x). (K) Representative growth curves of WT and SHIP KO PαS cells in regular culture. (L) Representative growth curves of WT and SHIP KO PαS cells after γ-irradiation. Data in (C), (D), (E), (F), (G), (H), (J), (K) and (L) are mean ± SEM.*p<0.05, **p<0.01, ***p<0.001, two-tailed t-Test. See also Figure S5.
In order to further study BM multipotent stromal cells, we isolated PαS multipotent stromal cells from WT and SHIP KO mouse BM (Figure 6F). SHIP KO PαS cells formed fewer adipocytes compared to WT PαS cells (Figure 8G). Interestingly, although SHIP KO PαS cells formed fewer adipocytes, these cells appeared to have produced more lipid droplets than WT adipocytes as judged by Oil Red O staining (Figure 8G). Western blots of whole BM cells and immunohistochemical staining (IHC) showed that SHIP KO BM adipocytes expressed much higher level of adiponectin in both femur and caudal vertebrae compared to WT mice (Figure 8H, Figure S5C). Thus, the capability of fat cell formation in vitro closely reflected aforementioned in vivo observations of SHIP KO mouse BM. SHIP KO PαS cells also appeared to have impaired ability to form osteoblastic nodule upon osteogenic induction (Figures S5D and S5E). It is noteworthy that a previous study by Takeshita et al. showed that SHIP does not modulate bone formation in vivo (Takeshita et al., 2002) (please also see Discussion).
It was recently reported that tumor suppressor retinoblastoma protein (RB) can regulate interactions between HSCs and BM microenvironment (Walkley et al., 2007b) as well as cell fate choice and lineage commitment in vivo (Calo et al., 2010). However, the level of phospho- RB was similar between the WT and SHIP KO PαS cells (Figure 8I). Since progression of a cell through G1 and S phase requires inactivation of RB by phosphorylation (Harbour and Dean, 2000), similar level of phospho-RB indicates normal cell cycle progression of SHIP KO PαS cells. Further investigation revealed that SHIP KO PαS cells expressed less FOXO1 and diminished levels of phospho-PPARγ but equivalent level of total PPARγ compared with WTPαS cells (Figure 8I). Both FOXO1 and phosphorylation of PPARγ are known to inhibit PPARγ activities in adipogenesis (Hu et al., 1996; Nakae et al., 2003); and depletion of FOXO1 prevents osteoblast differentiation (Siqueira et al., 2011). SHIP KO PαS cells also expressed less AKT and phospho-AKT, and produced larger amount of the 12 kDa cleaved caspase-3 fragment (Figure 8I). Consistently, SHIP KO PαS cells were more prone to apoptosis in vitro under normal culture conditions when compared with WT PαS cells (Figure 8J). A recent study also showed that SHIP inhibition can cause apoptosis of hematopoietic cancer cells (Brooks et al., 2010). Starting with the same number of BM PαS cells, the total number of cells derived from SHIP KO PαS cells was about 40% less than that from the WT PαS cells in normal culture over 7 days (Figure 8K). We also observed that SHIP deficient CD45−Ter119− BM stromal cells lost viability in long term culture (Figures S5F and S5G). These results are consistent with earlier PαS cell isolation (Figure 8F) and apoptosis results (Figure 8J). While irradiated WT BM PαS cells recovered rapidly, the number of irradiated SHIP KO BM PαS cells did not increase over time (Figure 8L). Thus, the propensity of apoptosis and impaired growth after irradiation may contribute to the non-hematopoietic reconstitution by wild-type donor cells in the SHIP deficient recipient mice.
DISCUSSION
Hematopoiesis is the process by which all lineages of blood cells are produced in a hierarchical and stepwise manner in the BM and at the apex of this hierarchy are the HSCs (Orkin and Zon, 2008). Previous studies suggested that SHIP might play a role in HSC signaling and thus HSC homeostasis, homing and reconstituting potential (Desponts et al., 2006; Helgason et al., 2003). On further investigation, our data support the notion that SHIP KO mice have a distinct state of hematopoiesis including defective hematopoietic cellular composition skewing towards myeloid cells on the cost of lymphopoiesis both in the PB and BM, significantly more HSCs despite of comparable total BM cellularity. A recent study by Hazen et al. suggested that the defective hematopoiesis in SHIP KO mice was not HSC cell autonomous (Hazen et al., 2009). In the present study, we provided direct evidence by using reverse transplantation to demonstrate that SHIP deficient BM microenvironment indeed can determine defective hematopoiesis at 4 weeks post transplantation. However, this is the prelude of what we observed at later time points, when hematopoiesis in SHIP KO recipients was surprisingly normalized. We then investigated what might have caused such normalization, which revealed that a significant non-hematopoietic chimerism took place in the BM of SHIP KO mice. Thus, a significant non-hematopoietic chimerism resulting in a normal hematopoiesis has been demonstrated. Mechanistically, we studied PαS cells from the BM of SHIP KO mice and discovered that their distinct properties including propensity of apoptosis and impaired growth after irradiation may contribute to the non-hematopoietic chimerism. Furthermore, our results indicate that WT non-hematopoietic cells are required for the BM reconstitution in SHIP KO recipients.
Earlier studies by Hazen et al. also demonstrated that circulating levels of multiple cytokines were elevated in SHIP KO mice (Hazen et al., 2009). During the initial period following reverse transplantation of WT BM cells aberrant cytokine levels were likely to still persist in the BM microenvironment of SHIP KO recipients, which may contribute to the SHIP KO hematopoietic phenotype observed at 4w after transplantation. It is conceivable that once the WT non-hematopoietic cells have successfully engrafted and started to proliferate and differentiate, the reconstitution of BM microenvironment in SHIP KO mouse would take place, leading to normalization of hematopoiesis. On the other hand, it is also possible that the normal cytokine responsiveness of the WT hematopoietic progenitors contributed to the observed normalization of the hematopoiesis. To further dissect the potential mechanisms involved, we performed a series of experiments to validate our findings. First, whole BM cells from SHIP KO mice were transplanted into WT mice, and we found that overall levels of donor-derived myeloid cells and B-cells were comparable between WT and SHIP KO cell recipients at 4w after transplantation. At 16w after the transplantation, the SHIP KO cell recipients exhibited elevated myelopoiesis and reduced lymphopoiesis as reported previously (Helgason et al., 2003). In the second set of experiments, hematopoietic cells from WT mice combined with non-hematopoietic cells from SHIP KO mice were transplanted into lethally irradiated SHIP KO mice. In this setting, however, the donor chimerism was so poor that total white blood cell levels in the SHIP KO recipients were extremely low. Interestingly, those successfully engrafted donor-cells differentiated primarily into myeloid cells. In the third set of experiments, WT hematopoietic cells alone were transplanted into SHIP KO mice. They exhibited similar hematopoietic phenotype as WT-HEM + KO-N-HEM recipients. All the SHIP KO recipients in the latter experiment eventually died 4–6w post transplantation. In the next set of experiments, we combined WT hematopoietic cells and WT PαS cells to transplant into lethally irradiated SHIP KO mice, and these SHIP KO recipients exhibited WT hematopoietic lineage composition in PB and BM after transplantation. Collectively, these experiments indicated that the non-hematopoietic reconstitution observed in the BM of SHIP KO mice was not caused by the transplanted WT hematopoietic cells, and the WT non-hematopoietic cells, including the PαS cells were required to establish normal hematopoiesis in the recipients.
Although there was no comprehensive study on hematopoiesis after a double irradiation and transplantation, tandem hematopoietic stem cell transplantation has been a feasible clinical option in treating patients with severe blood malignancies, where total body irradiation was only given in one transplant procedure (Monjanel et al., 2010; Somlo et al., 2011). Nevertheless, such tandem transplantation inspired us to perform a second reverse transplantation to demonstrate that SHIP KO chimeras, due to non-hematopoietic chimerism, had a functional BM microenvironment capable of normal hematopoiesis. WT hematopoiesis established in the WT-to-KO transplant recipients was further confirmed by a secondary transplantation of the LSK hematopoietic stem and progenitor cells.
Despite the advance in understanding genetic events leading to pathogenesis of hematologic malignancies, significant improvements in therapeutic outcome based on these insights have not been forthcoming in many cases. Dysfunctional BM microenvironment is now also believed to contribute to the disease development. Thus, one potential therapeutic strategy is to replace the defective microenvironment. So far, functional replacement of diseased BM microenvironment through BM transplantation has not been possible. Despite the report by Rombouts and Ploemacher (Rombouts and Ploemacher, 2003), in most studies of murine experiments or in clinical transplantations where primary BM cells were infused, donor non-hematopoietic stromal cells could rarely be detected in the recipient BM (Laver et al., 1987; Morikawa et al., 2009; Simmons et al., 1987). Our results with WT recipients which had negligible non-hematopoietic chimerism in BM are consistent with these reports. Here, we show that lipid phosphatase SHIP deficiency may enable a functional reconstitution of BM microenvironment by wild-type cells. In this study, the total non-hematopoietic chimerism in SHIP KO BM at 16 weeks after transplantation was estimated about 30–40%, which was capable to produce a wild-type hematopoietic phenotype both in PB and BM. To examine the reconstitution of multipotent stromal cells in the WT-to-KO recipients, we performed a colony forming assay (CFU-Fs) with the CD45−Ter119− BM cells from WT-to-KO recipients and found that up to 70% of the BM multipotent stromal cells of the KO recipients came from WT donor. The numerous adipocytes seen in the WT-to-KO recipients strongly indicate that donor multipotent stromal cells were a major contributor in this reconstitution. The study by Morikawa et al. (Morikawa et al., 2009) showed that primary PαS cells can home to appropriate sites and differentiate in response to cues from the niche environment. However, significant engraftment is only possible when the recipient’s BM multipotent stromal cells were debilitated by standard conditioning in sufficient numbers to leave their niche available to the donor multipotent stromal cells. Indeed, the propensity of apoptosis of SHIP KO PαS cells and the lack of growth after irradiation could potentially facilitate their replacement by WT PαS cells. Although we showed that PαS cells were defective in the SHIP deficient mice, this cell type is not exclusively functioning as HSC niche. Normal hematopoiesis was restored only after the transplantation of WT PαS cells into the SHIP deficient recipients. However, this finding cannot completely rule out the possibility that the endogenous niche cells in the SHIP deficient mice are also critical for hematopoiesis. The SHIP deficient BM may still sustain different niche components such as osteoblastic cells and vascular cells. Additionally, considering the splenomegaly phenotype, it is possible that spleen may function as an alternative niche to rescue the defect in BM niche. Consistently, significant hematopoietic reconstitution was also observed in the niche-defective SHIP KO mice at 16 weeks post transplantation (WT-HEM + KO-N-HEM to KO, Figures 5F–5H). Sacchetti et al. have identified perivascular cells as multipotent stem cells within the BM stroma (Sacchetti et al., 2007). Later Mendez-Ferrer et al. reported a subset of perivascular multipotent stromal cells expressing nestin (Mendez-Ferrer et al., 2010), and Omatsu et al. provided new insight on the CXCL12 abundant reticular cells that support HSCs in BM (Omatsu et al., 2010). Besides osteogenic differentiation, these cells are also capable to differentiate into adipocytes, which are thought to exert an inhibitory role in hematopoiesis (Naveiras et al., 2009). Recently, Park et al. reported that BM-derived multipotent stromal cells may be a heterogeneous population with the newly identified Mx1+ population (Park et al., 2012). In addition, VCAM-1 was also reported to be a relevant marker for BM stromal cells (Chou et al., 2012). It is currently unclear whether or how much these cell populations overlap (Nombela-Arrieta et al., 2011).
The mechanisms triggering the reconstitution of hematopoietic inductive BM microenvironment in SHIP KO mice warrant future investigation. We speculate that the significantly lower multipotent stromal cell counts and their impaired function (e.g. defective adipogenesis and elevated apoptosis) in SHIP KO mice may contribute to the reconstitution. SHIP KO PαS cells are more prone to apoptosis and form fewer adipocytes in an in vitro adipogenesis assay compared to WT PαS cells. Consistently, our in vivo transplantation data also suggest defective adipogenesis of SHIP deficient BM multipotent stromal cells. A small percentage of SHIP KO stromal cells can incorporate into the SHIP KO recipient mice (Figure 7F). However, these cells could never differentiate into mature adipocytes (Figure S2F). Hematopoiesis could also be regulated by osteoblastic niches. SHIP KO PαS cells appeared to have impaired ability to form osteoblastic nodule upon osteogenic induction in an in vitro assay (Figure S5D and S5E). However, a previous study by Takeshita et al. showed that SHIP does not modulate bone formation (osteogenesis) in vivo (Takeshita et al., 2002). Instead, due to increased number of hyper-resorptive osteoclasts, the 3D trabecular volume fraction, trabecular thickness, number and connectivity density of long bones are all slightly reduced in the SHIP KO mice (Takeshita et al., 2002). In current study, we carefully examined the bone structure. The thickness of the cortical bone was significantly reduced in the SHIP KO mice as previously reported (Takeshita et al., 2002). However, we could not detect significant increase or decrease in trabecular bone in the femur and caudal vertebra in the SHIP KO mice.
By using a specific non-hematopoietic cell type that is genetically labeled in the BM transplantation, the non-hematopoietic reconstitution in SHIP KO mice described here can be employed to visualize the donor cells’ anatomic location after engraftment, and to functionally assess hematopoietic outcome in the recipients when this specific cell type is excluded from the BM transplantation. Based on this model, the identity of the cells involved in a functional HSC microenvironment shall be investigated in the future. In a therapeutic setting, SHIP inhibition in patient’s BM may be induced shortly before radiation therapy and transplantation of healthy donor BM cells. Hematopoiesis in the recipient patient may normalize once donor-derived non-hematopoietic cells have successfully engrafted and thrived,. Taken together, our investigation suggests that non-hematopoietic reconstitution in SHIP KO mouse BM may provide a model to functionally study cell types involved in hematopoietic niches. More importantly, selective inhibition of SHIP in patients may improve treatment outcome by targeting both malignant hematopoietic cells and the aberrant BM microenvironment.
EXPERIMENTAL PROCEDURES
Mouse maintenance and genotyping
The SHIP KO mice were purchased from the Jackson Laboratory and maintained on a mixed genetic background (129/C57BL/6). SHIP heterozygous mice were backcrossed with either CD45.2+ C57BL/6 mice or CD45.1+ C57BL/6 mice (both from Jackson). Over a period of two years of backcrossing, we generated both CD45.2+ SHIP KO mice in C57BL/6 background and CD45.1+ SHIP KO mice in C57BL/6 background. Every donor and recipient mouse used in the transplantation experiments was carefully genotyped for its CD45.1 and/or CD45.2 expression before actual experiment. See also Supplemental Experimental Procedures.
BM cell transplantation
Age-matching CD45.2+ C57BL/6 and CD45.1 (B6.SJL-Ptprca/BoyAiTac) mice were purchased from Taconic (Hudson, NY). Donor whole BM cells were collected and transplantations were performed following twice 5.2 Gy 3h apart lethal γ-irradiation of recipient mice. See also Supplemental Experimental Procedures.
PαS cell isolation and characterization
PαS cells were isolated and cultivated using reagents and methods as described by Morikawa et al. (Morikawa et al., 2009). See also Supplemental Experimental Procedures.
Determination of non-hematopoietic chimerism in the BM of SHIP KO recipients
Conventional PCR genotyping was performed to detect the presence of WT and SHIP KO bands in hematopoietic and non-hematopoietic cells from the BM of SHIP KO recipients. Quantitative real-time PCR was performed using a CFX96 Thermal Cycler and iQ SYBR Green Supermix or iQ Multiplex Powermix from Bio-Rad. All primers were synthesized by Integrated DNA Technologies. See also Supplemental Experimental Procedures.
Statistics
Analysis of statistical significance for indicated data sets was performed using the Student’s t-Test capability on Microsoft Excel and GraphPad Prism.
Supplementary Material
Acknowledgments
We thank Jia Zhong and Li Zhao for maintaining mice and Nick Calderone for histology services. O.D.L. and J.L. are supported by National Institutes of Health (NIH) Training Grant HL066987 (L.E.S.). C.N.-A. is the recipient of a long-term postdoctoral fellowship from the Human Frontiers in Science Program. H.R.L. is supported by NIH grants HL085100, AI076471, HL092020, and GM076084 and a Research Scholar Grant from American Cancer Society. O.D.L. conducted all experiments with assistance from J.L.. C.N.-A. and G.P. conducted laser scanning cytometry study. S.M., L.C. and L.E.S. advised on experiments and data interpretation. O.D.L. and H.R.L. were responsible for conceiving this study, interpreting the data and manuscript preparation. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baup D, Moser M, Schurmans S, Leo O. Developmental regulation of the composite CAG promoter activity in the murine T lymphocyte cell lineage. Genesis. 2009;47:799–804. doi: 10.1002/dvg.20569. [DOI] [PubMed] [Google Scholar]
- Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- Bierkens JG, Hendry JH, Testa NG. Recovery of the proliferative and functional integrity of mouse bone marrow in long-term cultures established after whole-body irradiation at different doses and dose rates. Exp Hematol. 1991;19:81–86. [PubMed] [Google Scholar]
- Brooks R, Fuhler GM, Iyer S, Smith MJ, Park MY, Paraiso KH, Engelman RW, Kerr WG. SHIP1 inhibition increases immunoregulatory capacity and triggers apoptosis of hematopoietic cancer cells. J Immunol. 2010;184:3582–3589. doi: 10.4049/jimmunol.0902844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, Robey PG, Belkaid Y. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J Leukoc Biol. 2012;92:123–131. doi: 10.1189/jlb.1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- Desponts C, Hazen AL, Paraiso KH, Kerr WG. SHIP deficiency enhances HSC proliferation and survival but compromises homing and repopulation. Blood. 2006;107:4338–4345. doi: 10.1182/blood-2005-12-5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn CM, Kaufman DS. Donor cell leukemia: insight into cancer stem cells and the stem cell niche. Blood. 2007;109:2688–2692. doi: 10.1182/blood-2006-07-021980. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2:E65–67. doi: 10.1038/35008695. [DOI] [PubMed] [Google Scholar]
- Hazen AL, Smith MJ, Desponts C, Winter O, Moser K, Kerr WG. SHIP is required for a functional hematopoietic stem cell niche. Blood. 2009;113:2924–2933. doi: 10.1182/blood-2008-02-138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason CD, Antonchuk J, Bodner C, Humphries RK. Homeostasis and regeneration of the hematopoietic stem cell pool are altered in SHIP-deficient mice. Blood. 2003;102:3541–3547. doi: 10.1182/blood-2002-12-3939. [DOI] [PubMed] [Google Scholar]
- Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Mankani MH, Bianco P, Robey PG. Enumeration of the colony-forming units-fibroblast from mouse and human bone marrow in normal and pathological conditions. Stem Cell Res. 2009;2:83–94. doi: 10.1016/j.scr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver J, Jhanwar SC, O’Reilly RJ, Castro-Malaspina H. Host origin of the human hematopoietic microenvironment following allogeneic bone marrow transplantation. Blood. 1987;70:1966–1968. [PubMed] [Google Scholar]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- Li XM, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exp Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monjanel H, Deconinck E, Perrodeau E, Gastinne T, Delwail V, Moreau A, Francois S, Berthou C, Gyan E, Milpied N. Long-Term Follow-Up of Tandem High-Dose Therapy with Autologous Stem Cell Support for Adults with High-Risk Age-Adjusted International Prognostic Index Aggressive non-Hodgkin Lymphomas: A GOELAMS Pilot Study. Biol Blood Marrow Transplant. 2010;17:935–940. doi: 10.1016/j.bbmt.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Purton LE, Scadden DT. The hematopoietic stem cell niche. StemBook. 2008 103824/stembook1281. http://wwwstembookorg. [PubMed]
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider LR, Fuller JF, Wolf I, Liu Y, Lucas DM. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14:505–520. [PubMed] [Google Scholar]
- Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160–170. doi: 10.1038/sj.leu.2402763. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- Siqueira MF, Flowers S, Bhattacharya R, Faibish D, Behl Y, Kotton DN, Gerstenfeld L, Moran E, Graves DT. FOXO1 modulates osteoblast differentiation. Bone. 2011 doi: 10.1016/j.bone.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlo G, Spielberger R, Frankel P, Karanes C, Krishnan A, Parker P, Popplewell L, Sahebi F, Kogut N, Snyder D, et al. Total marrow irradiation: a new ablative regimen as part of tandem autologous stem cell transplantation for patients with multiple myeloma. Clin Cancer Res. 2011;17:174–182. doi: 10.1158/1078-0432.CCR-10-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, et al. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med. 2002;8:943–949. doi: 10.1038/nm752. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007a;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007b;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, Zhang Y, Mao N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.