Abstract
Background
Phonatory onset is important for speech and voice and may be substantially impaired in people with Parkinson’s Disease (PD). However, the physiologic contributions of laryngeal and respiratory control to phonatory onset in PD are not well understood. Acoustic measurement of phonatory onset in neurological disease has been limited due to the confounding effects of dysarthria and the limited yield of physiologic detail.
Objective
The purpose of this study was to test whether air flow measures would be useful to characterize respiratory and laryngeal contributions to phonatory onset, whether acoustic and air flow measures of phonatory onset were aberrant in PD, and whether deficits were significantly associated with voice severity.
Methods
Twenty-one PD participants were tested and compared with 25 healthy controls. Testing included acoustic and air flow measures of phonatory onset during syllable production ([pa]) and measures of voice severity.
Results
Air flow assessment was possible for all participants; acoustic assessment was only possible for 86% of PD participants. Air flow and acoustic measures revealed shorter phonatory onset times for PD participants than controls. Air flow measures also revealed that PD participants expelled less lung air volume per syllable. Aberrant timing of phonatory onset and reduced lung air volume were associated with increased voice severity.
Conclusions
These findings suggest that air flow measures may be useful to assess the laryngeal and respiratory contributions to phonatory onset. These results also suggest that both respiratory and laryngeal control deficits may contribute to phonatory errors in PD, and that phonatory onset deficits are associated with voice severity.
Keywords: Speech, voice, air flow, volume, acoustic, larynx
INTRODUCTION
Parkinson’s disease (PD) results in detrimental effects on sensory and motor functions of the limbs and airway [1–6]. PD participants exhibit degradation in force recruitment, displacement, velocity, and acceleration of limb movements [7, 8]. Similarly, PD may be associated with abnormal patterns of laryngeal and respiratory muscle activity, reduced vital capacity, phonatory onset and offset errors, and increased effort required for speech and voice [9–15]. Voicing errors related to deficits in laryngeal and respiratory sensori-motor control may contribute to linguistic confusion, and further communicative impairment. However, no physiological studies have examined the laryngeal and respiratory contributions to phonatory onset in PD compared with healthy controls.
Assessing the laryngeal and respiratory contributions to phonatory onset is challenging, in part, because the larynx is difficult to access. Yet such assessment is vital to understand how changes in physiology may relate to neurological disease. Acoustic measures of voice onset time (VOT) have been employed to examine the timing of phonatory onset in neurological disease and aging [10–12, 16–20]. VOT for the syllable [pa] is defined as the time (ms) from the release of the consonant [p] to the first cycle of voicing in the vowel [a]. However, VOT measurement is time consuming and technically challenging, especially when the acoustic signal is contaminated by dysphonic noise as may be commonly observed in neurological diseases [21, 22]. Therefore, acoustic measurement of VOT has been used sparingly in clinical practice. Moreover, VOT yields a limited amount of detail from which to make physiological inferences. In contrast, aerodynamic measures may provide a useful alternative or supplement to assess the control of phonatory onset.
Analysis of low-pass filtered translaryngeal air flow has been fruitful in characterizing both the respiratory and laryngeal subsystems during phonatory onset in PD [2, 23]. During phonatory onset, there is a nonlinear declination or decay in translaryngeal air flow from peak air flow (following plosive release, [p]) to air flow during “steady state” phonation ([a]) as shown in Fig. 1. This declination in translaryngeal air flow may be modeled using the exponential decay equation y = a + b*e−k*t, where the decay term k indicates how soon the air flow waveform reaches the steady state, and thus reflects the timing of phonatory onset. Therefore, a difference in the magnitude of the k term would reflect a difference in the timing of phonatory onset. In addition, the translaryngeal air flow signal (cc/sec) may be integrated to estimate lung air volume (cc) expelled per syllable. Therefore, this aerodynamic analysis may provide information about both laryngeal and respiratory contributions to phonatory onset during speech.
Fig. 1.
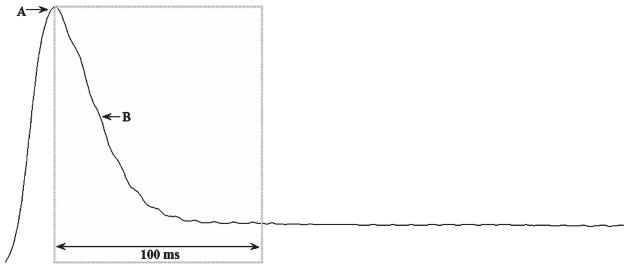
Air flow waveform for the spoken production of the syllable [pa]. A 100 ms analysis window outlined by the rectangle begins at peak translaryngeal air flow (A) and extends into steady state phonation. The declination in air flow (B) displayed in this window is associated with the onset of phonation.
Impaired control of phonatory onset may significantly impact voicing and communication [9–12, 16]. However, acoustic VOT analysis may be limited in clinical assessment of neurological diseases, including PD [21, 22]. Therefore, the purpose of the present study was to compare the laryngeal and respiratory contributions to phonatory onset in PD participants with healthy controls using the declination in the air flow signal and acoustic measures of VOT. It was hypothesized that the PD participants would exhibit shorter VOT and a larger decay term k in the signal compared with controls, reflecting a shorter time for phonatory onset. The PD participants were also hypothesized to expel less lung air volume per syllable than controls. The k (decay term) and lung air volume expelled per syllable were hypothesized to be correlated with VOT, and all three measures were hypothesized to be correlated with clinical indices of voice severity.
MATERIALS AND METHODS
Participants
This investigation was conducted in accordance with NIH regulations for the ethical treatment of human subjects. The protocol in this investigation was approved by the local institutional ethics committee for the safety of human subjects. Participants were informed of the general purposes of the study and written informed consent was obtained prior to enrolling any participants in the study. A total of 46 adults were enrolled in the present study including 21 individuals (10 men, 11 women) with PD, and 25 individuals (14 men, 11 women) as healthy age-matched controls. Mean and standard deviation of age were 72 (7) years for PD, and 76 (5) years for control participants.
Inclusion in the PD group was limited to participants with no history of other neurological or psychiatric disease and otherwise good health. Mean time since PD onset was 6.5 (5) years, and Hoehn & Yahr score [24] was 3 (0.7) and PD participants were tested a minimum of 12 hours since taking their last dose of anti-PD medication. Control subjects were in good general health, with normal breathing, speech, swallow, and voice, and with no history of neurological or psychiatric disease. All participants were non-smokers. Clinical voice assessment was also completed for each individual by a certified speech language pathologist to index voice severity using the Consensus Auditory Perceptual Evaluation of Voice (CAPE-V) [25]. The CAPE-V is an auditory-perceptual assessment tool to describe the severity of a voice problem. Mean and standard deviation of global CAPE-V scores were 49 (18) for PD, and 8 (8) for controls.
Speech analysis and digital signal processing
The air flow and acoustic procedures below were described previously [2, 23]. While comfortably seated in an exam chair, participants were instructed to say [pa] at a rate of 2 syllables per second at a comfortable pitch and loudness level. Translaryngeal air flow was channeled through a Puritan-Bennett full-face respiratory mask (model 5253) and a Hans Rudolph pneumotachometer (model R4719). A Honeywell Microswitch pressure transducer (model 163PC01D36) was used to sample the pressure drop through the pneumotachometer. The air flow signal was conditioned and filtered by Biocommunication Electronics 201 bridge amplifiers (LP -3 dB @ 50 Hz, Butterworth 3-pole). A 500 cc/sec flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Air flow was digitized at 1,000 Hz per channel at 16 bits of vertical resolution (±10 V ADC) using ADInstruments PowerLab/16sp and Chart (v5.41), and saved as ASCII files. The speech acoustic signal was transduced using a Sony condenser microphone positioned 15 cm from the mouth and digitized at 20 kHz.
Air flow waveforms from an average of 280 spoken syllables of [pa] per participant were signal averaged for each individual and analyzed using custom written routines in MATLAB (v7.0.4). Initial and final syllables for each test trial were excluded to eliminate utterance end-effects. For each syllable, a 100 ms-wide window was selected beginning at the peak in the air flow signal through the region of the waveform during steady-state phonation (See Fig. 1). Data within this 100 ms analysis window were isolated and the waveforms were averaged for each participant. For each participant, exponential nonlinear fits of these air flow waveforms were performed to determine the decay term (k), using the exponential decay equation y = a + b*e−k*t (SigmaPlot 10.0). The magnitude of the decay term k from this nonlinear fit indicates how soon the air flow waveform reaches the steady state, and thus reflects the timing of phonatory onset.
The air flow signal (cc/sec) for each participant was then integrated to estimate lung air volume (cc) expelled per syllable using a partial sums algorithm (Minitab, v15). Acoustic measurement of voice onset time (VOT) for 10 spoken syllables of [pa] per participant was performed using MultiSpeech (KayPENTAX) as previously described [10, 16, 26] by measuring the time from the release of the consonant [p] to the first cycle of voicing in the vowel [a].
Statistical analysis
An ANCOVA design was used to test group differences to control for the covariates of age and sex [16–19, 27–30]. The Pearson product moment correlation coefficient was used to test for a correlation of the decay term k and lung air volume with VOT, and for a correlation between each of these three variables with a clinical measure of voice severity (i.e., CAPE-V). The criterion level for significance for each test was set at α = 0.05.
RESULTS
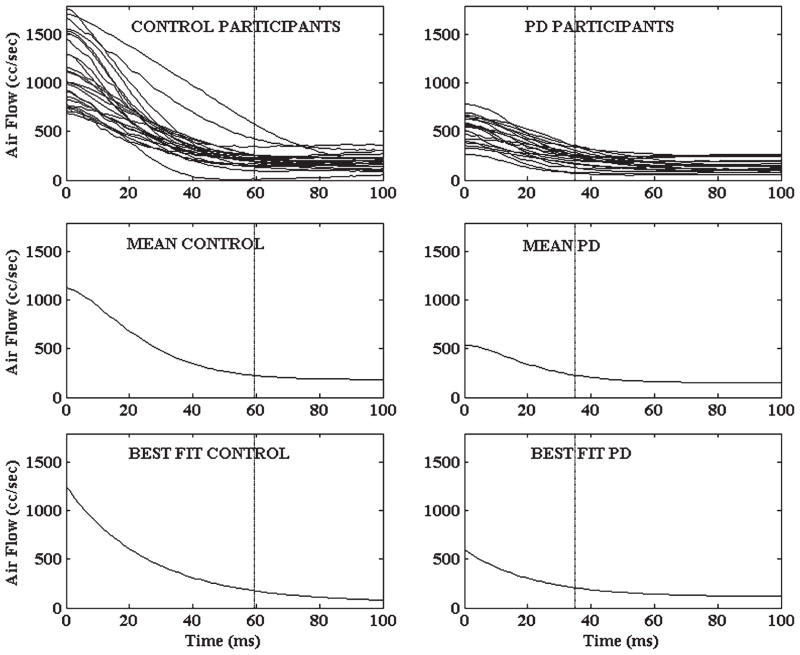
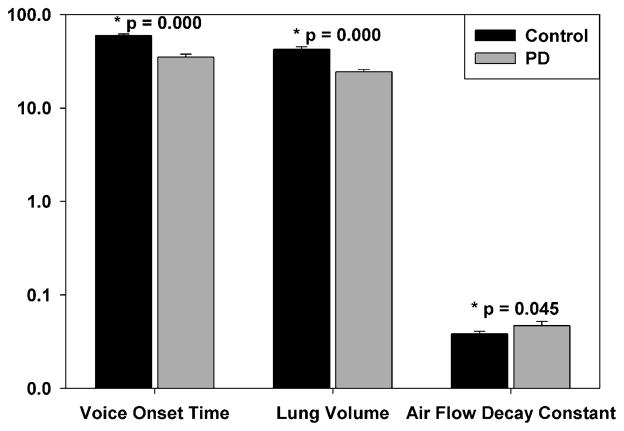
Individual participant mean air flow waveforms, group mean waveforms, and nonlinear fits for each group are displayed in Fig. 2. Nonlinear fit of the air flow waveforms yielded excellent fit for each participant with mean r2 of 0.98966 (range 0.95570 to 0.99950; all p < 0.0001). PD participants exhibited a shorter VOT, exhibited a larger air flow decay constant k, and expended less lung air volume per syllable than controls (Fig. 3). Mean VOT for each group is also plotted as a vertical reference line in Fig. 2. Aerodynamic assessment was possible for all participants. However, VOT measurements were only possible for 18/21 (86%) PD participants due to dysphonia. Ten percent of the VOT data were reanalyzed and revealed high test-retest reliability (r = 0.99, p < 0.01) with differences less than 5 ms.
Fig. 2.
Individual participant (mean) air flow declination waverforms (top row), group mean waveform (middle row), and nonlinear fit (bottom row) are displayed for control and PD participants. Nonlinear fits were modeled using the exponential decay equation y = a + b*e−k*t; the decay term k from this fit reflects the timing of phonatory onset. Group mean acoustic voice onset time (VOT) is displayed on each plot as a vertical reference line.
Fig. 3.
Acoustic voice onset time (ms), lung air volume expended per syllable (cc), and air flow decay constant k for control and PD participants. Bar height represents the mean value with standard error of the mean for each group. Please note use of a logarithmic scale on the vertical axis.
There were medium correlations between VOT and the decay constant k (r = −0.59, p < 0.001), and between VOT and lung air volume expended per syllable (r = 0.50, p < 0.005), indicating that larger k and smaller lung air volume were associated with shorter VOT. Lung air volume expended per syllable (r = −0.66, p < 0.001) and VOT (r = −0.60, p < 0.001) correlations with voice severity were large, indicating that smaller lung air volume and shorter VOT were associated with increased voice severity. Correlation of the k decay term with voice severity was non-significant (r = 0.28, p > 0.127).
DISCUSSION
This study presents the first data comparing the laryngeal and respiratory contributions to phonatory onset in PD with healthy controls. The results generally supported the experimental hypotheses. In summary, individuals with PD exhibited shorter phonatory onset time, exhibited a larger k constant, and expelled less lung air volume per syllable than healthy controls. Lung air volume and k were correlated with VOT; lung air volume and VOT were correlated with voice severity. However, the correlation between voice severity and the k constant was non-significant. Analysis of the air flow signal was possible for all 46 (100%) participants, but acoustic VOT measurement was only possible for 18/21 (86%) PD participants.
The finding from the present investigation that the timing of phonatory onset in PD was aberrant compared to healthy controls was consistent with the important role of the basal ganglia and related neural circuits [31] in scaling parameters of movement control (e.g., displacement, velocity) and the decreased movement magnitude (hypokinesia) and velocity (bradykinesia) in PD described in other studies [6,8]. The findings using aerodynamic and acoustic measures of phonatory onset were also consistent with previous reports that employed acoustic measures of voice onset time for [pa]. Previous investigations revealed that individuals with PD exhibited acoustic voice onset times for [pa] that were shorter than that of healthy controls [10, 16]. These and other authors [19] interpreted shorter voice onset times for [pa] to reflect a possible compensatory adjustment by the larynx in which the initial vocal fold (and arytenoid) position may be postured closer to the target prior to movement onset. Such an early adjustment may serve to reduce the magnitude of distance required to reach the target position in an attempt to compensate for reduced movement velocity. Similarly, patterns of reduced magnitude of orofacial movement and shorter voice onset time in older and neurologically impaired individuals have been interpreted as a possible attempt to compensate for reduced movement velocity [17, 18, 20].
In addition, previous studies identified a significant association between respiratory volume and voice onset time. In these studies, it may be that smaller scaling of vital capacity, accompanied by a higher, and possibly more cranial diaphragm position, imposing less caudally directed opposition by the diaphragm and respiratory trunk to vocal fold medialization, may have accounted for shorter time for voice onset [32]. Given that many individuals with PD exhibited reduced vital capacity during speech [33, 13], and that voice onset time is correlated with vital capacity [32], it was reasonable to expect that individuals with PD in the present study may also have reduced lung volume during syllable production accompanied by shorter time for the arytenoids/vocal folds to approach the midline. In the present study, shorter voice onset times and larger k (i.e., phonatory onset began sooner), and smaller lung volume expelled per syllable during [pa] production in PD, including medium correlations between VOT and k with lung air volume, were observations that were consistent with this expectation. These observations may suggest reduced scaling of movement parameters (e.g., displacement, velocity) related to phonatory onset, and may represent an attempt to compensate for such movement deficits.
However, the possible “compensations” described above are not necessarily advantageous. The phonemic identity of [pa] (as in “pot”) depends in part upon an appreciably longer phonatory onset time than would be required for [ba] (as in “bought”). If the timing of vocal fold medialization, or phonatory onset, occurs too soon for [pa], it may be perceived by the listener as [ba], resulting in potential linguistic confusion. These voicing errors using acoustic measures have been previously described as a primary feature of dysarthria in PD [11, 12]. Aerodynamic analysis may be helpful to identify the laryngeal and respiratory contributions to these phonatory onset errors. Lung air volume and VOT correlations with voice severity were large, indicating that smaller lung air volume and shorter VOT were associated with increased voice severity. Accordingly, individuals with aberrant laryngeal and respiratory control also exhibited increased voice severity.
Aerodynamic analysis of phonatory onset may be a particularly helpful complement to traditional acoustic measures of VOT given the dependence of VOT measurement on a clear acoustic signal. Aerodynamic analysis for the present study was based on the physiology of the low-pass filtered translaryngeal air flow signal. Acoustic VOT may often be difficult to extract from the acoustic signal of a speaker with dysarthria, and this limitation is particularly problematic when attempting to study individuals with neurological disorders [21]. In the present study, aerodynamic analysis was possible for all participants, but acoustic VOT measurement was only possible for 18/21 (86%) PD participants. Therefore, the air flow analysis employed in the present investigation may be a valuable alternative or supplement to acoustic analyses in future work.
However, aerodynamic and acoustic analyses are limited as they do not permit direct observation of the kinematics of arytenoid/vocal fold medialization for the onset of vocal fold vibration. Therefore, future studies to compare acoustic, perceptual, and videoendoscopic or high speed video measures of phonatory onset and arytenoid/vocal fold medialization with these aerodynamic measures are warranted [34]. Given the degree to which the magnitude and timing of arytenoid/vocal fold medialization may covary with vital capacity, future studies should also carefully consider inclusion of volume-calibrated measures of respiratory function. Finally, in this study syllable production ([pa]) revealed compelling results, yet other and more complex speech-related tasks may provide a more representative view of the variety of potential speech-related changes that accompany PD.
CONCLUSION
The laryngeal and respiratory contributions to control of phonatory onset in PD are not well understood. In the present study, the application of physiological measures of speech aerodynamics provided a non-invasive index to detect significant changes in the control of phonatory onset. The results of the present investigation led to the conclusion that PD participants exhibited evidence of aberrant (i.e., shorter) timing of phonatory onset, reduced lung air volume expelled per syllable, and that these deficits were strongly associated with increased voice severity. These present findings demonstrated the important laryngeal and respiratory contributions to phonatory onset, the important role of the basal ganglia and related neural circuits to control these movement parameters, and how these important functions are affected in PD. Air flow measures of phonatory onset may be useful within a more comprehensive battery of measures to assess the dynamics of phonatory control in people with PD.
Acknowledgments
Dr. Hammer’s research is supported through NIH grants DC010900, RR025012, and RR023268.
Footnotes
CONFLICT OF INTEREST
The author has no conflict of interest to report.
References
- 1.Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE Transactions on Biomedical Engineering. 2009;56:1154–1159. doi: 10.1109/TBME.2008.2007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson’s disease: Implications for speech respiratory and phonatory control. Experimental Brain Research. 2010;201:401–409. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer MJ, Murphy CA, Abrams TM. Airway Somatosensory Deficits and Dysphagia in Parkinson’s Disease. Journal of Parkinson’s Disease. 2013;3(1):39–44. doi: 10.3233/JPD-120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider JS, Diamond SG, Markham CH. Deficits in orofacial sensorimotor function in Parkinson’s disease. Annals of Neurology. 1986;19:275–282. doi: 10.1002/ana.410190309. [DOI] [PubMed] [Google Scholar]
- 5.Schneider JS, Diamond SG, Markham CH. Parkinson’s disease: Sensory and motor problems in arms and hands. Neurology. 1987;37:951–956. doi: 10.1212/wnl.37.6.951. [DOI] [PubMed] [Google Scholar]
- 6.Turner RS, Grafton ST, McIntosh AR, DeLong MR, Hoffman JM. The functional anatomy of parkinsonian bradykinesia. NeuroImage. 2003;19:163–179. doi: 10.1016/s1053-8119(03)00059-4. [DOI] [PubMed] [Google Scholar]
- 7.Desmurget M, Grafton ST, Vindras P, Gréa H, Turner RS. The basal ganglia network mediates the planning of movement amplitude. European Journal of Neuroscience. 2004;19:2871–2880. doi: 10.1111/j.0953-816X.2004.03395.x. [DOI] [PubMed] [Google Scholar]
- 8.Robichaud JA, Pfann KD, Vaillancourt DE, Comella CL, Corcos DM. Force control and disease severity in Parkinson’s disease. Movement Disorders. 2005;20:441–450. doi: 10.1002/mds.20350. [DOI] [PubMed] [Google Scholar]
- 9.Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levadopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. Journal of Speech Language and Hearing Research. 2001;44:1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- 10.Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, Jiminez EB. Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain and Language. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- 11.Ludlow CL, Bassich CJ. The results of acoustic and perceptual assessment of two types of dysarthria. In: Berry W, editor. Clinical Dysarthria. College-Hill; San Diego, CA: 1983. pp. 121–153. [Google Scholar]
- 12.Ludlow CL, Bassich CJ. Relationships between perceptual ratings and acoustic measures of hypokinetic speech. In: McNeil MR, Rosenbek JC, Aronson AE, editors. The Dysarthrias. College Hill; San Diego, CA: 1984. pp. 163–195. [Google Scholar]
- 13.Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. Journal of Speech and Hearing Research. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- 14.Tamaki A, Matsuo Y, Yanagihara T, Abe K. Influence of thoracoabdominal movement on pulmonary function in patients with Parkinson’s disease: Comparison with healthy subjects. Neural Rehabilitation and Neural Repair. 2000;14:43–47. doi: 10.1177/154596830001400105. [DOI] [PubMed] [Google Scholar]
- 15.Tzelepis GE, McCool DF, Friedman JH, Hoppin FG. Respiratory muscle dysfunction in Parkinson’s disease. American Review of Respiratory Disorders. 1988;138:266–271. doi: 10.1164/ajrccm/138.2.266. [DOI] [PubMed] [Google Scholar]
- 16.Liss JM, Weismer G, Rosenbek JC. Selected acoustic characteristics of speech production in very old males. Journal of Gerontology. 1990;45:35–45. doi: 10.1093/geronj/45.2.p35. [DOI] [PubMed] [Google Scholar]
- 17.Neiman GS, Klich RJ, Shuey EM. Voice onset time in young and 70-year-old women. Journal of Speech and Hearing Research. 1983;26:118–123. doi: 10.1044/jshr.2601.118. [DOI] [PubMed] [Google Scholar]
- 18.Netsell R, Daniel B, Celesia GG. Acceleration and weakness in parkinsonian dysarthria. Journal of Speech and Hearing Disorders. 1975;40:170–178. doi: 10.1044/jshd.4002.170. [DOI] [PubMed] [Google Scholar]
- 19.Sweeting PM, Baken RJ. Voice onset time in a normal-aged population. Journal of Speech and Hearing Research. 1983;25:129–134. doi: 10.1044/jshr.2501.129. [DOI] [PubMed] [Google Scholar]
- 20.Weismer G, Jeng J-Y, Laures JS, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica. 2001;53:1–18. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- 21.Ozsancak C, Auzou P, Jan M, Hannequin D. Measurement of voice onset time in dysarthric patients: Methodological considerations. Folia Phoniatrica et Logopaedica. 2001;53:48–57. doi: 10.1159/000052653. [DOI] [PubMed] [Google Scholar]
- 22.Titze IR. Summary Statement. In: Titze IR, editor. Workshop on Acoustic Voice Analysis. National Center for Voice and Speech; Denver, CO: 1994. [Google Scholar]
- 23.Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. Journal of Neurology. 2010;257:1692–1702. doi: 10.1007/s00415-010-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 25.Kempster GB, Gerratt BR, Verdolini-Abbot K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. American Journal of Speech Language Pathology. 2009;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). [DOI] [PubMed] [Google Scholar]
- 26.Lisker L, Abramson AS. A cross-language study of voicing in initial stops: Acoustical measurements. Word. 1964;20:384–422. [Google Scholar]
- 27.Ryalls J, Zipprer A, Baldauff P. A preliminary investigation of the effects of gender and race on voice onset time. Journal of Speech, Language, and Hearing Research. 1997;40:642–645. doi: 10.1044/jslhr.4003.642. [DOI] [PubMed] [Google Scholar]
- 28.Swartz BL. Gender differences in voice onset time. Perceptual and Motor Skills. 1992;75:983–992. doi: 10.2466/pms.1992.75.2.415. [DOI] [PubMed] [Google Scholar]
- 29.Whiteside SP, Irving CJ. Speakers’ sex differences in voice onset time: Some preliminary findings. Perceptual and Motor Skills. 1997;85:459–463. doi: 10.2466/pms.1997.85.2.459. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside SP, Irving CJ. Speakers’ sex differences in voice onset time: A study of isolated word production. Perceptual and Motor Skills. 1998;86:651–654. doi: 10.2466/pms.1998.86.2.651. [DOI] [PubMed] [Google Scholar]
- 31.Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. Journal of Neurophysiology. 2003;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hoit JD, Solomon NP, Hixon TJ. Effect of lung volume on voice onset time (VOT) Journal of Speech and Hearing Research. 1993;36:516–521. doi: 10.1044/jshr.3603.516. [DOI] [PubMed] [Google Scholar]
- 33.Bunton K. Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson disease. Journal of Communication Disorders. 2005;38:331–348. doi: 10.1016/j.jcomdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Kunduk M, Yan Y, McWhorter J, Bless D. Investigation of voice initiation and voice offset characteristics with high-speed digital imaging. Logopedics, Phoniatrics, Vocology. 2007;31:139–144. doi: 10.1080/14015430500364065. [DOI] [PubMed] [Google Scholar]