This review discusses the current status of the cardioregenerative field. In particular, the study addresses the current knowledge of cardiac stem/progenitor cells as the regenerative substrate in the adult heart and their use in preclinical and clinical studies to repair the injured myocardium.
Keywords: Adult stem cells, Cardiac stem/progenitor cell, Self-renewal
Abstract
Acute myocardial infarction leads to irreversible loss of cardiac myocytes, thereby diminishing the pump function of the heart. As a result, the strenuous workload imposed on the remaining cardiac myocytes often gives rise to subsequent cell loss until the vicious circle ends in chronic heart failure (CHF). Thus, we are in need of a therapy that could ameliorate or even reverse the disease progression of CHF. Endogenous regeneration of the mammalian heart has been shown in the neonatal heart, and the discovery that it may still persist in adulthood sparked hope for novel cardioregenerative therapies. As the basis for cardiomyocyte renewal, multipotent cardiac stem/progenitor cells (CSCs) that reside in the heart have been shown to differentiate into cardiac myocytes, smooth muscle cells, and vascular endothelial cells. These CSCs do have the potential to actively regenerate the heart but clearly fail to do so after abundant and segmental loss of cells, such as what occurs with myocardial infarction. Therefore, it is vital to continue research for the most optimal therapy based on the use or in situ stimulation of these CSCs. In this review, we discuss the current status of the cardioregenerative field. In particular, we summarize the current knowledge of CSCs as the regenerative substrate in the adult heart and their use in preclinical and clinical studies to repair the injured myocardium.
Introduction
Since the 20th century, remarkable progress has been made in the treatment of coronary artery disease. Most of the cardiovascular risk factors, which were unraveled by observations in large cohorts such as the Framingham study, served as a substrate for pharmacological intervention [1, 2]. Besides these milestones in preventive cardiology, the last three decades were marked by several major breakthroughs in the treatment for acute myocardial infarction (AMI), such as the introduction of the coronary care unit, pharmacological reperfusion (i.e., thrombolysis), pharmacological interventions (e.g., beta blockade, ACE inhibitors, antiplatelet drugs, and statins), and improvements in interventional cardiology (i.e., primary percutaneous coronary intervention) [3]. As a result, our ability to successfully treat the acute moment of the disease came at the expense of a vast increase in patients left behind with a chronic condition. In particular, the chronic sequels of AMI such as congestive heart failure (CHF) or life-threatening cardiac arrhythmias [4] are not only frequent, they also lack effective therapy that could stop or even reverse disease progression. This would avoid the necessity of current last resource measures, which include heart transplantation (infrequently performed because of donor shortage) [5] or left ventricular assist devices either as bridge-to-transplant or destination therapy [6].
As the average life expectancy rises in the developed world combined with its population persistently subjecting itself to traditional risk factors, we are faced with an increase of epidemic magnitude in chronic heart disease that requires vast amounts of human resources and our available health care budget. In the U.S. alone, a total of 8 million people have endured an AMI, with an estimate of 785,000 new cases of AMI annually [7]. Of those, roughly 5.7 million patients have CHF accounting for approximately $30 billion annually in health care costs in 2008, with a predicted triplicate in costs rising to $97 billion annually in 2030 [7]. Given the initial loss of functional cardiac myocytes as the trigger of a subset of adverse remodeling processes that eventually lead to CHF [8], it is imperative to develop new treatments that either (a) further reduce the loss of cardiac myocytes during AMI, or, ideally, (b) can replace lost cardiac myocytes with newly generated counterparts. The latter option can be regarded as the holy grail of cardiac regenerative medicine and has been a controversial subject for centuries [9]. Luckily, it was nature itself that reaffirmed the validity of this regenerative paradigm, evidenced by various research groups that the adult mammalian heart, by itself, possesses an intrinsic form of cellular homeostasis that permits regeneration and formation of new cardiac myocytes and vasculature and subsequent replacement of lost cardiac myocytes for physiological turnover [10–13]. These exciting findings were received with initial skepticism since it was in sharp contrast with the previously embraced paradigm that relied on the notions that (a) all cardiac myocytes are terminally differentiated and thus incapable of re-entering the cell cycle (“the heart is a postmitotic organ”) and (b) there are no stem and/or progenitor cells in the heart that can differentiate into functional cardiac myocytes. One of the presumed causal factors accounting for endogenous cardiac regeneration are the tissue-specific stem/progenitor pool in the heart that creates offspring capable of differentiating into mature functional cardiac myocytes and vasculature [10]. This endogenous repair mechanism is clearly not sufficient to repair large segmental loss, such as that which occurs in a myocardial infarction (MI). Indeed, a common challenge raised by the skeptics is to question why an MI evolves into a scar if regenerating cardiac stem/progenitor cells (CSCs) are present in the myocardium? What this question overlooks is that the sudden obstruction of a main parenchymal artery of any organ, no matter how abundant its resident stem cells (e.g., testicle, bone marrow, skin, intestine, etc.), always evolves into a scar. This is so because during the evolution of long-lived organisms, the presence of adult stem cells is likely to have been selected to not regenerate catastrophic acute segmental cell losses but as a mechanism to repair minor lesions and maintain the normal wear and tear of the tissue.
In this review, we aim to summarize the evidence as to whether the heart has intrinsic regenerative capacity, and if so, to what extent regeneration is based on its own tissue-specific stem/progenitor cells. Second, we discuss the current status of the results achieved thus far in preclinical studies and in two pioneering first-in-human clinical trials that used these CSCs as the basis for cardiac regeneration in the injured heart.
Evidence for Cardiomyocyte Regeneration in the Heart
Regeneration of the Heart in Amphibians and Fish
The impression that cardiac regeneration does not occur in humans was emphasized by observations in certain species, such as the newt or zebrafish, which can easily regenerate large parts of organs or body parts, including the heart. In 1768, the biologist Lazzaro Spallanzani reported complete regeneration of the salamander limb after its removal. The capacity to fully regenerate the excised apex of the left ventricle, corresponding with a loss of ∼20% of total cardiac myocytes, without any apparent signs of scar formation was described for the newt [14, 15] and, more recently, the zebrafish [16]. The relative ease of genetically altering the zebrafish enabled scientists to show that it was in fact the cardiac myocytes adjacent to the resection wound that responded by a process of dedifferentiation and breakdown of their contractile apparatus, before activating a set of early cardiac transcription factors such as GATA4 [17–19]. Thus, these studies provide evidence of naturally occurring regeneration of the heart and the underlying mechanism involved. The question remains as to what extent mechanisms behind heart regeneration in these species could also be present in mammals, and if so, which factors lead to an apparent dormant state thereof in the adult mammalian heart.
Regeneration in the Neonatal Mammalian Heart
During embryonic development, the mammalian heart shows remarkable capacity for regeneration. Drenckhahn et al. [20] used a cardiomyocyte-specific conditional knockout of the X-linked holocytochrome c synthase (Hccs) gene to create female progeny that, by random X chromosome inactivation, had a mosaic heart. As expected, early female embryos displayed a ratio of 50:50 between normal and Hccs-null cardiac myocytes. During embryonic development, proliferating functional cardiac myocytes gradually replaced dysfunctional Hccs-null cardiac myocytes. As a result, by birth, approximately 90% of all cardiac myocytes were the progeny of cardiac myocytes with one functional Hccs allele. This regenerative response was also observed in the first days of the neonatal mouse heart and lost by 7 days of age [21]. With the use of a tamoxifen-inducible Cre recombinase under control of the α-myosin heavy chain promoter, newly generated cardiac myocytes in the neonatal heart were shown to have originated from pre-existing cardiac myocytes [21]. These findings were reinforced by the observation of a marked decline in telomerase reverse transcriptase (Tert)-green fluorescent protein (GFP)-expressing cells in the adult heart compared with the neonatal heart. Interestingly, among Tert-GFP+ cells were both Sca-1+ CSCs, as well as mature cardiac myocytes [22].
Regeneration in the Adult Mammalian Heart
With the exception of some tissues such as the liver, skin, and intestine, mammals have largely lost their regenerative potential following embryonic and the early postnatal period [20]. After an AMI, massive loss of cardiac myocytes is replaced by fibrosis and subsequent scar formation [23]. Distinguishing between the albeit very limited presence or absence of a regenerative potential of the adult mammalian heart is of utter importance since closely mimicking or augmenting a biological process already present in nature is easier than initiating a new process that does not play a role in normal cellular homeostasis and/or turnover. Until the last decade, two main clinical observations served as the basis for the old paradigm that the heart is a postmitotic organ [24]: (a) until then, observations on functionally significant myocardial regeneration in the mammal heart had not been documented, and (b) the occurrence of primary tumors arising from the myocardium has been rarely observed in the adult mammalian heart [24]. Since then, there has been a slow but steady reconsideration of this paradigm after a series of reports on the presence of cardiomyocyte renewal in the adult mammalian—including human—heart [11–13, 25–27]. In 2009, the Bergman group [13] elegantly rendered the vast increase in atmospheric 14C levels—based on post-World War II nuclear bomb testing—into a pulse-chase experiment of global magnitude to determine the age of cardiac myocytes in relation to the age of the given individual. After the Partial Test Ban in 1962, the increased levels of 14C in the atmosphere declined rapidly as it was absorbed in the biosphere. Thus, as DNA was synthesized within this given time period, the levels of 14C incorporated in the DNA corresponded with the registered levels of 14C in the atmosphere, providing the Bergman group the necessary means to accurately establish the date of DNA synthesis. If indeed the postmitotic heart lacked any regenerative potential, the age of all cardiac myocytes should coincide within the time frame of the fetal development and early postnatal period. In contrast, it showed that the adult human heart contained cardiac myocytes that were generated throughout the human life span. Correcting for polyploidization as the basis for newly synthesized DNA in older cardiac myocytes without cell division (cytokinesis), the investigators predicted an approximate annual turnover rate of cardiac myocytes in the order of 1% at the age of 25, declining toward 0.45% by the age of 75 [13].
Recently, the second report based on this approach to estimate the rate of cardiomyocyte turnover in the adult human heart came from the Anversa group [28]. Strikingly, they calculated a 16-fold higher rate in which the myocyte fraction of the heart is completely replaced approximately eight times during the human life span. These findings are in sharp contrast with the 50% replacement of cardiac myocytes during life [13], the preclinical data in rodents by the Field group [29], in which DNA synthesis in the adult heart was virtually absent (0.0006%), as well as the group of Lee [30], where a low rate (0.76%) of cardiac myocyte turnover was observed during normal aging.
In conclusion, in contrast with previous studies that showed that cardiomyocyte renewal is virtually absent, multiple research groups have independently shown the presence of a regenerative mechanism in the adult mammalian heart. There is accumulating evidence that the adult human heart is characterized by DNA synthesis and formation of cardiac myocytes. The actual turnover rate of cardiac myocytes, however, varies widely by more than 1 order of magnitude.
Cardiac Stem/Progenitor Cells
Evidence for Stem/Progenitor Cell Involvement in Cardiac Regeneration
Since the concept of cardiac regeneration in the mammalian heart slowly emerged as a reconsidered paradigm on the adult heart cellular homeostasis, one of the next questions that remained to be answered was the cellular source of these newly formed cardiac myocytes. To address this question, Hsieh et al. designed a genetic lineage tracing experiment that for the first time shed light on the cellular homeostasis that governs cardiomyocyte renewal [27]. In a double transgenic MerCreMer/ZEG-inducible cardiomyocyte reporter mouse, a tamoxifen-induced pulse caused an irreversible genetic switch—only in the cardiac myocytes—from β-galactosidase (β-gal) to the expression of GFP. Hence, if during the chase any GFP-negative stem or progenitor would form new myocytes, they would still express β-galactosidase. Two major findings emerged from this landmark report: (a) various models of myocardial injury (myocardial infarction model and chronic pressure overload) resulted in a significant increase in GFP− β-gal+ cardiac myocytes and a corresponding decrease in GFP+ β-gal− cardiac myocytes, and (b) during normal aging of the rodent heart, there was no decrease in GFP+ β-gal− cardiac myocytes, suggesting an absence of stem cell-based physiologic cardiomyocyte renewal [27]. Recent work from the same group, however, brought to light that, upon induction of MI, there is a high rate of cardiomyocyte turnover in the adult mammalian heart that originates from pre-existing GFP+ cardiac myocytes rather than noncardiomyocytes. With regard to physiological aging, using multi-isotope imaging mass spectrometry with their previous transgenic mouse model [27], they calculated that, in young adult hearts, cardiac myocytes are replaced by proliferation of dedifferentiated pre-existing cardiac myocytes at an annual rate of 0.76% [30]. In contrast with these findings, a similar genetic fate mapping approach from the Marbán group [31] showed that new cardiac myocytes not only arise from pre-existing cardiac myocytes but also from stem/progenitor cells following MI. In line with previous reports, cardiomyocyte turnover predominantly occurs through proliferation of pre-existing cardiac myocytes at an annual rate of 1.3%–4% during normal aging [31]. Taken together, it seems that, unlike zebrafish, which solely regenerate based on proliferation of dedifferentiated cardiac myocytes [17, 19], the mammalian heart appears to rely on two mechanisms for endogenous regeneration: (a) a source of stem/progenitor cells that upon differentiation and maturation reconstitute the lost cardiac myocytes as occurs in injury [27, 31], and (b) the proliferation of dedifferentiated cardiac myocytes that can re-enter the cell cycle and can give rise to mononucleated, newly formed cardiac myocytes [30, 31]. Although a major step toward a better understanding of mammalian regeneration of the heart, these studies could not pin down the exact anatomic location or molecular footprint of these stem and/or progenitor cells.
Types of Cardiac Stem/Progenitor Cells
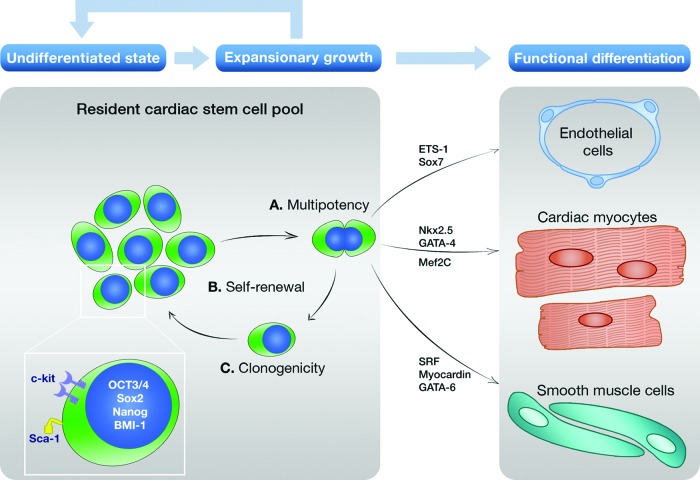
By lack of consensus, different sources of stem and/or progenitor cells have been proposed as the causal factor for cardiac regeneration. One of these sources is a compartment of endogenous stem or progenitor cells directly from the heart itself as a logical source for maintaining the cardiomyocyte pool by a continuous process by replenishing old dying cardiac myocytes with new ones (Fig. 1). Almost 10 years ago, the first report was published on an endogenous CSC from the mammalian heart with regenerative potential based on the tyrosine kinase receptor c-kit [12]. Since then, numerous reports were published on different markers used to identify CSCs in the heart, such as Sca-1 [32–36], Isl-1 [37–39], side population (SP) cells [40–44], or cardiosphere-derived cells (CDCs) [45–50]. A schematic overview of resident stem/progenitor cells in the mammalian heart is depicted in Table 1. The abundance in different types of CSCs on the one hand and the clinical observation of such limited regenerative potential in the heart on the other hand led some to the conclusion that most types are in fact one type of CSC in different phases of differentiation and/or maturation [51]. Nevertheless, we will briefly outline the molecular characteristics of the most commonly described CSCs.
Figure 1.
Cardiac stem/progenitor cell-based regeneration in the adult mammalian heart. The proposed mechanism of cardiac regeneration mediated by endogenous CSCs present in the heart is shown. This mechanism is based on the observation that cardiac stem/progenitor cells (CSCs) are multipotent (A), self-renewing (B), and clonogenic (C). In their undifferentiated state, CSCs express low levels of pluripotency markers such as OCT3/4, Sox2, and Nanog (see inset). The majority of CSCs also express key regulators such as BMI-1 that controls cell cycle inhibitors P19 and P21 to maintain and regulate their ability to proliferate. Once activated, CSCs can re-enter the cell cycle and subsequently can give rise to progeny that both maintain their own pool of undifferentiated stem cells and mature into three different lineages (see functional differentiation) under the influence of various lineage-specific transcription factors.
Table 1.
Resident stem/progenitor cells in the mammalian heart
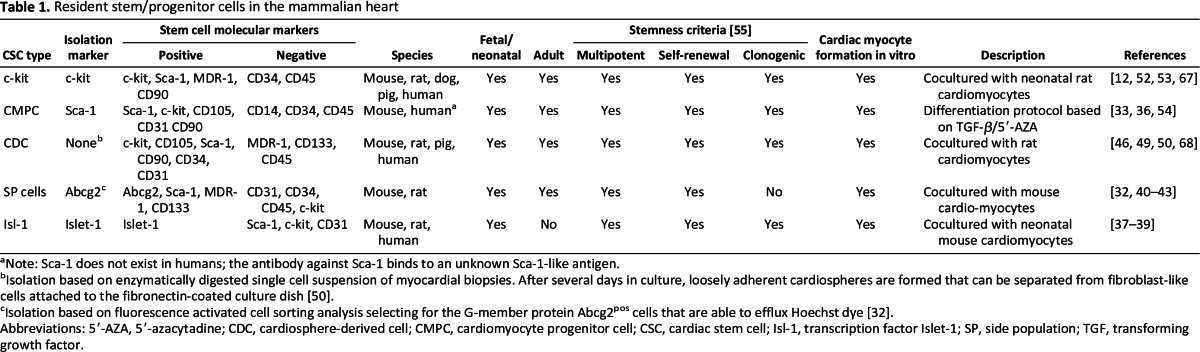
aNote: Sca-1 does not exist in humans; the antibody against Sca-1 binds to an unknown Sca-1-like antigen.
bIsolation based on enzymatically digested single cell suspension of myocardial biopsies. After several days in culture, loosely adherent cardiospheres are formed that can be separated from fibroblast-like cells attached to the fibronectin-coated culture dish [50].
cIsolation based on fluorescence activated cell sorting analysis selecting for the G-member protein Abcg2pos cells that are able to efflux Hoechst dye [32].
Abbreviations: 5′-AZA, 5′-azacytadine; CDC, cardiosphere-derived cell; CMPC, cardiomyocyte progenitor cell; CSC, cardiac stem cell; Isl-1, transcription factor Islet-1; SP, side population; TGF, transforming growth factor.
c-kitpos Lineageneg Cardiac Stem Cells
The most extensively studied CSC is based on the presence of the c-kit receptor that can be activated by stem cell factor. In 2003, Beltrami et al. [12] described the isolation of c-kitpos lineage-negative CSCs in the adult mammalian rat heart. The criteria for properties of bona fide stem cells were shown for these CSCs: being self-renewing, clonogenic, and multipotent. In in vitro and in vivo experiments, these c-kitpos CSCs were able to differentiate toward cardiac myocytes, smooth muscle cells, and vascular endothelial cells. In 2005, the in vivo potential was further demonstrated by Dawn et al. [52] showing that a few GFPpos c-kitpos CSCs had formed GFPpos cardiac myocytes in the infarcted myocardium in rats. Next, Bearzi et al. [53] showed that the adult human heart also contained c-kitpos CSCs. Upon successful isolation and characterization in vitro, these human CSCs were tested in an infarction model based on immunodeficient mice. The observation of a chimeric heart containing human CSC-derived cardiac myocytes dispersed in between the rodent myocardium further strengthened the regenerative potential of these CSCs.
Sca-1+ Cardiomyocyte Progenitor Cells
In 2003, a supposed different population was documented by Oh et al. [33] based on stem-cell antigen 1 (Sca-1). In 6- to 12-week-old mice, these Sca-1+ CSCs were able to differentiate toward cardiac myocytes upon induction with the cytosine analog 5′-azacytadine. When tested in an experimental model of myocardial infarction, Sca-1+ were infused intravenously and homed toward the infarcted myocardium, where in vivo differentiation toward cardiac myocytes was observed. Despite the fact that Sca-1 does not exist in humans, the Doevendans and Goumans group [36] reported the successful isolation of a cardiac progenitor cell population in the human fetal and adult heart based on a antibody directed against the mouse Sca-1 epitope. Akin to its rodent counterparts, these human Sca-1+ cardiomyocyte progenitor cells (CMPCs) showed a capability for self-renewal and multipotency by differentiating toward cardiac myocytes and/or vascular tube-like endothelial cells positive for PECAM-1. When tested in immunodeficient mice for their regenerative capacity, fetal human Sca-1+ CMPCs improved cardiac function following infarction and showed in vivo differentiation toward a cardiomyocyte-like phenotype based on the presence of troponin I [54].
Side Population Cells
Contrary to its name, cardiac side population (SP) cells have gained an extensive body of evidence as a distinct entity of cells capable of producing progeny that can renew cardiac myocytes during normal development and disease. SP cells are isolated based on their ability to efflux DNA-binding dyes through an ATP-binding cassette (ABC) transporter.
In 2002, Hierlihy et al. [40] described the isolation of cells with stem cell-like behavior that resided in the adult heart that appeared on the “side” on fluorescence-activated cell sorting because these cells were able to efflux Hoechst 33342 using the ABC reporter Abcg2. Since then, the existence of cardiac SP cells have been confirmed by several independent groups [32, 41–43]. Using other molecular markers to identify SP cells is based on Sca-1, expressed by 80%–90% of SP cells [44]. However, they constitute less than 1% of all Sca-1+ cells in the heart [44]. Hence, given the large overlap with the more heterogeneous Sca-1+ CMPCs that do not show signs of clonogenicity, it is conceivable that these SP cells could in fact be the active cell compartment isolated by the groups that investigate Sca-1+ CMPCs.
Cardiosphere-Derived Cells
In 2004, Messina et al. [50] reported the isolation of adult CSCs that grow in self-adherent clusters—designated as cardiospheres (CSs)—and are comprised of a mixture of differentiating progenitor cells, cardiac myocyte-like cells, and/or vascular cells. Messina et al. [50] suggested that these various cell types are all the progeny of a small subset of undifferentiated cells within CSs, which are self-renewing and clonogenic, and express different stem cell markers such as c-kit and Sca-1. These CDCs were isolated from the adult murine and human heart and could be easily expanded in vitro based on this cardiosphere-forming isolation protocol.
Taken together, the last decade of intense research led to a vast increase in our knowledge on the existence of different types of CSCs that show true characteristics of stem-progenitor cells [55] and their role in cardiomyocyte renewal in the mammalian heart. However, in vitro study results obtained with CSCs should be interpreted with the necessary caution because they more likely reflect the presence of a regenerative potential of CSCs rather than the actual role thereof in tissue cellular homeostasis. As contemplated by Simons and Clevers [56], current methods such as the search for a unique CSC-specific molecular marker and quantitative analysis based on immunohistochemistry provide only a small glimpse of CSC behavior at best. Thus, in order to reliably asses the true regenerative role of CSCs, we need to advance the CSC research field by the introduction of research methods such as lineage tracing based on inducible genetic labeling that can clearly and unambiguously provide insights in CSC dynamics and behavior in vivo.
Cardiac Stem Cells as the Basis of Cardiac Regenerative Therapy
Cardiac Stem Cell Therapy: Preclinical Results
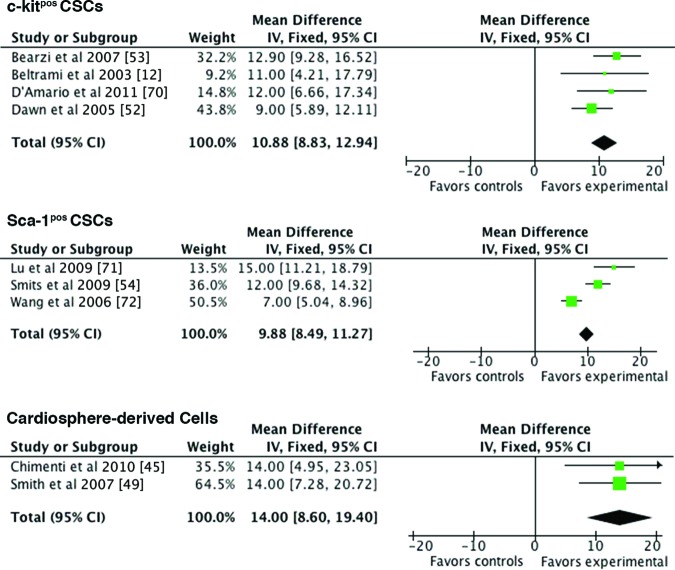
Despite the uncertainties pertaining to normal CSC behavior and dynamics in vivo, most reports on CSCs also include experimental data on the use of these cells as the treatment for left ventricular improvement in acute and/or chronic MI. Unlike earlier studied sources for stem cell therapy, such as bone marrow mononuclear cells, which do show a modest beneficial effect albeit via other mechanisms than true regeneration [57], most types of CSCs show signs of formation of new cardiac myocytes besides formation of new vasculature. As shown in the Forrest plot (Fig. 2), two important findings emanate from the cumulative evidence regarding usage of CSCs as novel cardioregenerative treatment in rodents: (a) overall, there is an absolute increase in left ventricular ejection fraction (LVEF) based on CSCs by ∼12%, and (b) the type of CSC, based on different molecular markers (e.g., c-kit, MDR1, Sca-1), does not seem to show superiority of one stem cell marker over the other.
Figure 2.
Preclinical studies on the efficacy of exogenous CSC delivery in myocardial infarction in rodents. Shown is a Forrest plot showing the pooled results of the mean difference on left ventricular ejection fraction (LVEF) at follow-up (21–28 days) compared with the baseline of exogenous CSC delivery in rodent models of acute myocardial infarction. Different CSC therapy led to a roughly equal improvement in LVEF for c-kitpos CSCs (+10.88%; [95% CI: 8.83–12.94]), Sca-1pos CSCs (+9.88%; [95% CI: 8.49–11.27]), or CDCs (+14.00%; [95% CI: 8.6–19.40]) at follow-up. Abbreviations: CI, confidence interval; CSC, cardiac stem/progenitor cell; IV, inverse variance; Fixed, fixed effects analysis.
The translational approach as currently practiced requires the use of larger animal models that more closely mimic human disease to establish whether the beneficial effects found in rodents actually hold in a (pre)clinical setting [58]. In particular, pigs are often used for this purpose since they fulfill this criterion by their close resemblance in physiology and anatomy to the human cardiovascular system. To date, there are only two published reports on the usage of exogenously administered CSCs in a large animal model, both testing the use of CDCs. In 2009, the group proved the short-term safety of intracoronary delivery of CDCs. Regarding treatment efficacy, they used a porcine model of ischemic cardiomyopathy, in which 300,000 CDCs per kg was compared with vehicle alone (calcium-free phosphate-buffered saline with 100 units/ml heparin and 250 μg/ml nitroglycerin). Strikingly, CDC treatment led to considerable formation of new cardiac myocytes and a significant reduction of infarct scar tissue [46]. Next, in 2011 the Marbán group extended these data in large animals by a new comparison between CDCs, the CSs themselves, or placebo in a mini-pig model of chronic MI. In total, 10 × 106 cells were injected intramyocardially 4 weeks after an anteroseptal myocardial infarction. After 4 weeks of follow-up, the differences in LVEF between placebo and CDCs and between placebo and CSs were approximately 7% and 4%, respectively (LVEF at follow-up 40 ± 7% vs. 47 ± 5 vs. 44 ± 5) [59].
In 2005, Linke et al. [60] described the activation of CSCs in dog heart in response to hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1). Upon induction of myocardial infarction, intramyocardial HGF/IGF-1 injections in the border zone of the infarct led to the formation of new cardiac myocytes and coronary vessels within the infarct. Analogous to this approach, another study in the porcine model by Ellison et al. [61] showed activation of the endogenous cardiac stem cell compartment based on these two previously established activators of CSCs [60]. A growth factor cocktail of IGF-1 and HGF was administered intracoronary 30 minutes after AMI. As a result, the growth factor-treated animals showed a preserved LVEF compared with placebo, at 2 months follow-up. The thymidine analog bromodeoxyuridine was used to visualize cell generation, which revealed extensive new cardiomyocyte formation that coincided with the activation, proliferation, and differentiation of the endogenous CSC compartment [61].
Cardiac Stem Cell Therapy: Clinical Results
Table 2 provides an overview of the current research programs of the different types of CSC therapy. Despite the scarcity of experimental data in large animal models for CSCs, clinical trials are already under way [62, 63]. The first trial, SCIPIO (cardiac Stem Cells In Patients with Ischemic Cardiomyopathy), initiated by the combined effort of Anversa and Bolli, is an open-label phase I trial that randomly allocated 16 patients with postinfarction left ventricular (LV) dysfunction (LVEF ≤ 40%), who had undergone coronary artery bypass grafting, to intracoronary infusion of 0.5–1 × 106 c-kit-positive, lineage-negative cardiac stem cells or standard care as usual [62]. The delivery of c-kitpos CSCs was approximately 4 months after surgery. LVEF increased by 8% at 4 months after c-kitpos CSC delivery, whereas the patients receiving standard care as usual did not show any signs of change. Strikingly, a progressive improvement in LVEF was observed in the first eight patients who reached the 1-year follow-up with an increase in 12% compared with controls [62].
Table 2.
Update on research development programs of CSC therapy for ischemic heart disease
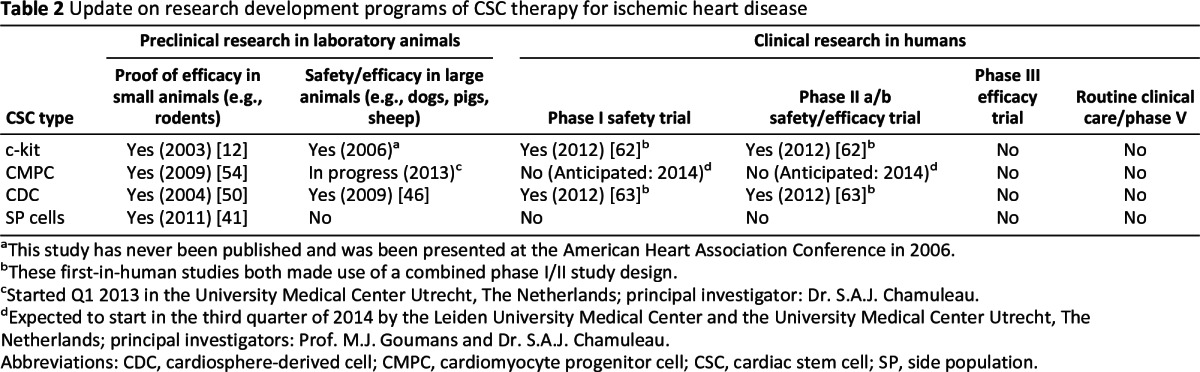
aThis study has never been published and was been presented at the American Heart Association Conference in 2006.
bThese first-in-human studies both made use of a combined phase I/II study design.
cStarted Q1 2013 in the University Medical Center Utrecht, The Netherlands; principal investigator: Dr. S.A.J. Chamuleau.
dExpected to start in the third quarter of 2014 by the Leiden University Medical Center and the University Medical Center Utrecht, The Netherlands; principal investigators: Prof. M.J. Goumans and Dr. S.A.J. Chamuleau.
Abbreviations: CDC, cardiosphere-derived cell; CMPC, cardiomyocyte progenitor cell; CSC, cardiac stem cell; SP, side population.
The second trial, CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction), initiated by Eduardo Marbán, was a prospective, randomized phase I safety trial that investigated the effect of intracoronary admission of CDCs in patients with a recent acute myocardial infarction (with LV ejection fraction of 25%–45%) on major cardiac and noncardiac adverse events and formation of neoplasia [63]. In total, 25 patients were enrolled, of whom 17 received CDCs 1.5–3 months after the index event. Eight patients served as controls and received guideline-based care as usual. During follow-up, four patients (24%) in the CDC group experienced serious adverse events compared with one patient in the control group (13%). Regarding functional analysis at 6 months, MRI analysis of patients treated with CDCs showed a significant reduction in scar mass, increased viable heart mass, and regional systolic wall thickening. However, changes in end-diastolic volume, end- systolic volume, and LVEF did not differ between groups at 6 months [63].
However tempting it is to look at the highly anticipated functional analysis, these phase I trials can merely provide information on what they were designed to investigate, which is the safety of CSC therapy on short-term follow-up. As a reminder from history, only 7 years ago, the cardiovascular scientific community received similar initial reports that sparked intense hope that bone marrow-derived cell therapy could reduce the burden of ischemic heart disease. However, the initial report [64] of a 15% improvement in LVEF was gradually reduced to an significant increase of 2.87% published very recently in the Cochrane library that reported on the pooled analysis of in total 2,533 patients [65]. We speculate that the evidence as summarized in this review justifies CSCs as one of the currently investigated cell types with the highest potential to ameliorate the repercussion of massive cardiomyocyte loss following AMI.
Cell Therapy: Problems and Pitfalls From a Translational Perspective
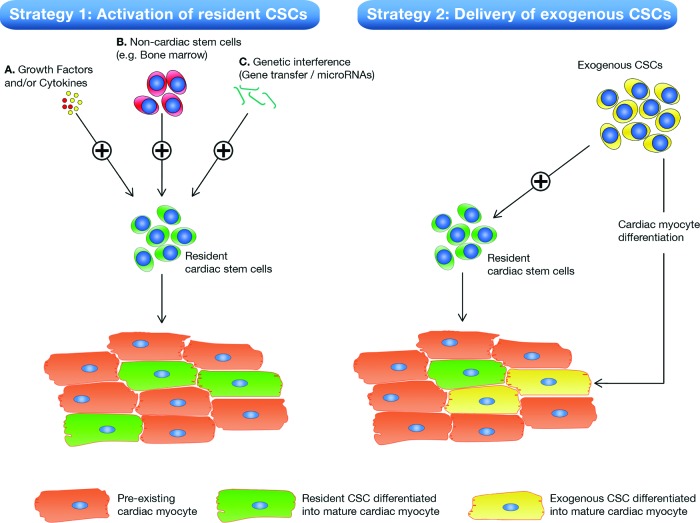
Previously, different versions of autologous cell therapy (e.g., bone marrow-derived mononuclear cells) also reached the stage of clinical testing. Most of these therapies have been proven marginally effective but failed to solve the severe health care problem that CHF imposes or to have a measurable impact in the day-to-day clinical practice of CHF treatment. Even when the preliminary results of the CADUCEUS and SCIPIO trials truly reflect the marked improvement that can be expected based on CSC therapy, this autologous cell approach is still hampered by several pitfalls that need to be resolved in the near future. First, a low engraftment of exogenously administered cells in the heart possibly dilutes the treatment effect of cell therapy. In our center, we compared the delivery efficiency of three commonly used delivery strategies (intracoronary infusion, intramyocardial injection, and surgical injection). Four hours after delivery, we could only detect ∼10% of all delivered indium111-labeled mesenchymal stem cells, regardless of delivery method [69]. Second, autologous stem/progenitor cell therapy relies on a complex infrastructure of both human expertise and costly facilities that are needed for the isolation, cleaning, and culturing of CSCs under stringent good manufacturing practice (GMP) conditions to enable successful autologous cell therapy. Third, autologous therapies, at present, still fail to satisfy the cost constraints posed by the need to make the treatment affordable to a very large number of candidate patients. Moreover, handling of the cell product should be devised in such a way that it can be prepared and administered not only in the tertiary cardiovascular centers but also in the majority of hospitals with access to a catheterization laboratory. Therefore, novel strategies activating the endogenous CSC compartment are under current investigation and aimed to “bypass” the abovementioned shortcomings of exogenous CSC therapy (Fig. 3).
Figure 3.
Novel strategies for CSC-based myocardial repair. Schematic overviews of current strategies used to make use of CSCs for myocardial repair are shown. Strategy 1 is based on activation of endogenous CSCs by various means, e.g., growth factors (A), noncardiac stem cells (B), or gene therapy (C). Upon activation, resident endogenous CSCs can proliferate and mature into newly formed cardiac myocytes (green cardiac myocytes). Strategy 2 is based on the delivery of autologous CSCs that have been isolated from small myocardial biopsies and scaled up outside the patient to sufficient numbers. Exogenous CSCs are also shown to be capable of activating the local endogenous CSC compartment. In addition, exogenously delivered CSCs are hypothesized to mature and differentiate into functional cardiac myocytes (yellow cardiac myocytes) that are electromechanically coupled with the pre-existing cardiac myocytes (orange cardiac myocytes) [31]. Abbreviation: CSC, cardiac stem/progenitor cell.
Conclusion
In this review, we have summarized the current status of the cardiac regenerative research field and highlighted the major breakthroughs that led us to rethink old paradigms with regard to the regenerative capacity of the adult heart that coincides with the presence of an endogenous stem/progenitor cell pool that shows self-renewal and multipotency both in vitro and in vivo. Next to these major breakthroughs, we have also shown controversial points in which numerous contradicting reports still preclude a broad consensus. Resolving several issues is paramount in order to advance the field toward development of novel therapies that can augment the regenerative potential of CSCs. We would like to specifically mention two that are in our view of most importance. First, the low level of naturally occurring regeneration in the human heart indicates that CSCs are part of a tightly regulated process that controls the number of progeny that can give rise to new cardiac myocytes. Which factors can activate quiescent CSCs and subsequently let these cells take place in new rounds of cell division? In addition, which factors govern the newly formed progeny toward activation of a set of cardiomyogenic transcription factors that lead to formation of new cardiac myocytes? As shown by Sluijter et al. [66], micro-RNA (miR) interference could successfully govern the differentiation efficiency of Sca-1pos CSCs toward cardiomyocyte-like cells by overexpressing miR 1 and 499.
Second, despite extensive preclinical data in young rodents, the effects of aging on behavior and viability of these CSCs in elderly patients with coronary artery disease remains largely unexplored. Cellular aging is governed by the expression of nuclear proteins that regulate cell cycle inhibition and irreversible growth arrest. Therefore, if the regenerative response of CSCs to an ischemic insult is to be further explored as a new treatment for post-MI heart failure, it is imperative to unravel which individual patient characteristics affect CSC viability and, above all, their irreversible state of cellular senescence.
In the foreseeable future, both the highly anticipated results of clinical trials involving the use of CSCs and preclinical clues that can further tailor the high potential of CSCs could pave the way for CSC-based myocardial repair on a clinical relevant scale. Given the anticipated increase in socioeconomic costs related to heart failure, any reduction in the chance to develop heart failure following an acute myocardial infarction could drastically reduce the burden of ischemic heart disease on our health care resources and, above all, improve the patients' quality of life.
Acknowledgments
This work was funded by the HGG Group B.V. and the Wijnand M. Pon Stichting.
Author Contributions
S.K.: conception and design, manuscript writing; S.J.J.L.: manuscript writing and revision; R.G., J.M.I.H.G., F.J.v.S., and J.P.G.S.: manuscript revision; P.A.D.: manuscript revision, final approval of manuscript; G.M.E.: manuscript writing and revision, final approval of manuscript; S.A.J.C.: conception and design, manuscript revision, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
References
- 1.Anderson KM, Odell PM, Wilson PW, et al. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 4.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 5.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report-2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Kirkels JH, de Jonge N, Lahpor JR. Assist devices in the new decade: From technical developments to political decisions. Eur J Heart Fail. 2010;12:217–218. doi: 10.1093/eurjhf/hfq010. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics–2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 9.Laflamme M, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison GM, Nadal-Ginard B, Torella D. Optimizing cardiac repair and regeneration through activation of the endogenous cardiac stem cell compartment. J Cardiovasc Transl Res. 2012;5:667–677. doi: 10.1007/s12265-012-9384-5. [DOI] [PubMed] [Google Scholar]
- 11.Beltrami AP, Urbanek K, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–253. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 15.Witman N, Murtuza B, Davis B, et al. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev Biol. 2011;354:67–76. doi: 10.1016/j.ydbio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 17.Jopling C, Sleep E, Raya M, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepilina A, Coon AN, Kikuchi K, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi K, Holdway JE, Werdich A, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drenckhahn JD, Schwarz QP, Gray S, et al. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell. 2008;15:521–533. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Porrello ER, Mahmoud AI, Simpson E, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson GD, Breault D, Horrocks G, et al. Telomerase expression in the mammalian heart. FASEB J. 2012;26:4832–4840. doi: 10.1096/fj.12-208843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleutjens JP, Blankesteijn WM, Daemen MJ, et al. The infarcted myocardium: Simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc Res. 1999;44:232–241. doi: 10.1016/s0008-6363(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 24.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 26.Boström P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh PCH, Segers VFM, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajstura J, Rota M, Cappetta D, et al. Cardiomyogenesis in the aging and failing human heart. Circulation. 2012;126:1869–1881. doi: 10.1161/CIRCULATIONAHA.112.118380. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 30.Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malliaras K, Zhang Y, Seinfeld J, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac Sp cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey B, Fransioli J, Gude N, et al. Sca-1 knockout impairs myocardial and cardiac progenitor cell function. Circ Res. 2012;111:750–760. doi: 10.1161/CIRCRESAHA.112.274662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuura K, Nagai T, Nishigaki N, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 36.Goumans MJ, de Boer TP, Smits AM, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1 1 cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai CL, Liang X, Shi Y, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Hierlihy AM, Seale P, Lobe CG, et al. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 41.Liang SX, Khachigian LM, Ahmadi Z, et al. In vitro and in vivo proliferation, differentiation and migration of cardiac endothelial progenitor cells (SCA1+/CD31+ side-population cells) J Thromb Haemost. 2011;9:1628–1637. doi: 10.1111/j.1538-7836.2011.04375.x. [DOI] [PubMed] [Google Scholar]
- 42.Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfister O, Mouquet F, Jain M, et al. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 44.Unno K, Jain M, Liao R. Cardiac side population cells: Moving toward the center stage in cardiac regeneration. Circ Res. 2012;110:1355–1363. doi: 10.1161/CIRCRESAHA.111.243014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li TS, Cheng K, Malliaras K, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malliaras K, Li TS, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 50.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 51.Chamuleau SA, Vrijsen KR, Rokosh DG, et al. Cell therapy for ischaemic heart disease: Focus on the role of resident cardiac stem cells. Neth Heart J. 2009;17:199–207. doi: 10.1007/BF03086247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smits AM, van Laake LW, den Ouden K, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 55.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties: Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 56.Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 57.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: Recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee ST, White AJ, Matsushita S, et al. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 60.Linke A, Müller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellison GM, Torella D, Dellegrottaglie S, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 65.Clifford DM, Fisher SA, Brunskill SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 66.Sluijter JP, van Mil A, van Vliet P, et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 67.Tallini Y, Greene M, Craven M, et al. c-Kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simpson D, Mishra R, Sharma S, et al. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation. 2012;126:S46–S53. doi: 10.1161/CIRCULATIONAHA.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Spoel TI, Vrijsen KR, Koudstaal S, et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: A study on delivery efficiency. J Cell Mol Med. 2012;16:2768–2776. doi: 10.1111/j.1582-4934.2012.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.D'Amario D, Cabral-Da-Silva M, Zheng H, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Lu G, Haider H, Jiang S, et al. Sca-1+ stem cell survival and engraftment in the infarcted heart: Dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Hu Q, Nakamura Y, et al. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]