This study systematically compared the immunomodulatory capacities of adipose tissue-derived multipotent stromal cells (AT-MSCs) and bone marrow-derived multipotent stromal cells (BM-MSCs) derived from age-matched donors. It was found that BM-MSCs and AT-MSCs show functionally similar immunomodulatory effects, but with a different dose-response curve, in favor of AT-MSCs. AT-MSCs can be considered as a good alternative to BM-MSCs for immunomodulatory therapy.
Keywords: Adult human bone marrow, Cellular therapy, Bone marrow, Bone marrow stromal cells, Immunosuppression, Marrow stromal stem cells, Mesenchymal stem cells
Abstract
Adipose tissue-derived multipotent stromal cells (AT-MSCs) are studied as an alternative to bone marrow-derived multipotent stromal cells (BM-MSCs) for immunomodulatory treatment. In this study, we systematically compared the immunomodulatory capacities of BM-MSCs and AT-MSCs derived from age-matched donors. We found that BM-MSCs and AT-MSCs share a similar immunophenotype and capacity for in vitro multilineage differentiation. BM-MSCs and AT-MSCs showed comparable immunomodulatory effects as they were both able to suppress proliferation of stimulated peripheral blood mononuclear cells and to inhibit differentiation of monocyte-derived immature dendritic cells. However, at equal cell numbers, the AT-MSCs showed more potent immunomodulatory effects in both assays as compared with BM-MSCs. Moreover, AT-MSCs showed a higher level of secretion of cytokines that have been implicated in the immunomodulatory modes of action of multipotent stromal cells, such as interleukin-6 and transforming growth factor-β1. This is correlated with higher metabolic activity of AT-MSCs compared with BM-MSCs. We conclude that the immunomodulatory capacities of BM-MSCs and AT-MSCs are similar, but that differences in cytokine secretion cause AT-MSCs to have more potent immunomodulatory effects than BM-MSCs. Therefore, lower numbers of AT-MSCs evoke the same level of immunomodulation. These data indicate that AT-MSCs can be considered as a good alternative to BM-MSCs for immunomodulatory therapy.
Introduction
Multipotent stromal cells (MSCs), originally detected in bone marrow, are nonhematopoietic progenitor cells that are defined by their ability to adhere to plastic surfaces and their capacity to differentiate toward different mesodermic lineages, including adipocytes, osteocytes, and chondrocytes [1]. An important characteristic of MSCs is their immunomodulatory capacity. MSCs interfere with differentiation and functions of multiple immunomodulatory cells. In vitro, MSCs suppress the proliferation of T cells upon allogeneic and mitogenic stimulation [2, 3], they inhibit monocyte-derived dendritic cell differentiation and maturation [4, 5] and the proliferation of B cells and natural killer cells [6, 7], and they promote the generation of regulatory T cells [8–10]. Multiple factors have been implicated in the immunomodulatory effects of MSCs, including indoleamine 2,3-dioxygenase (IDO), interleukin-6 (IL-6), prostaglandin E2 (PGE2), and transforming growth factor-β1 (TGF-β1) [9, 11–14].
The potential therapeutic application of MSCs for immunomodulation has been the subject of many studies over the years. In experimental models, administration of MSCs resulted in prolonged skin graft survival in baboons [15] and in prevention of both graft-versus-host disease (GvHD) [16] and the development of experimental autoimmune encephalomyelitis in mice [17]. The use of MSCs as a cellular therapy is currently explored in clinical trials, aiming at the promotion of engraftment following hematopoietic stem cell transplantation [18] and treatment of GvHD [19] and Crohn's disease [20].
Expanded bone marrow-derived MSCs have been most widely used for clinical applications, but alternative sources or subpopulations are currently under investigation [21, 22]. The frequency of MSCs in bone marrow is low (0.001%–0.01% of the total mononuclear cell fraction [23]), and aspirating bone marrow is an invasive procedure. Adipose tissue is an interesting alternative to bone marrow, since it contains an approximately 500-fold higher frequency of MSCs [24, 25] and tissue collection is simple. Moreover, 400,000 liposuctions a year are performed in the U.S. alone, where the aspirated adipose tissue is regarded as waste [26] and could be collected without any additional burden or risk for the donor. Adipose tissue-derived MSCs (AT-MSCs) are already applied in clinical trials [21, 22], but a systematic comparative study of bone marrow-derived MSCs (BM-MSCs) and AT-MSCs has not been performed. Studies that compared MSCs derived from different sources focused on phenotype, transcription profiling, and differentiation potential [27–33]. A few studies compared the immunomodulatory properties [30, 32, 34–36] and showed qualitative similarities between AT-MSCs and BM-MSCs. Quantitative analysis could not be performed in these studies, since different patient groups and different age groups were analyzed. Age is an important variable, since the composition of the MSC population and the functional properties of MSCs change with aging [37, 38].
In this study we systematically compared the in vitro immunomodulatory capacities of BM-MSCs and AT-MSCs derived from age-matched donors to further define the advantages and disadvantages related to the use of the different tissue sources for MSC therapy. We show that both AT-MSCs and BM-MSCs are able to suppress proliferation of stimulated peripheral blood mononuclear cells (PBMCs) and to inhibit differentiation of monocyte-derived immature dendritic cells (iDCs). However, AT-MSCs show more potent immunomodulatory effects, a difference that is related to higher levels of cytokine secretion.
Materials and Methods
Patients
Adipose tissue was dissected as medical waste from nine cadaveric pancreata that were donated for islet transplantation in our center, according to the institutional guidelines regarding the use of waste material. Bone marrow from a group of nine age-matched donors was obtained from the iliac crests of healthy donors and from orthopedic patients after written informed consent was obtained, according to procedures that were approved by the medical ethical committee. The mean age of bone marrow donors was 49.2 ± 13.2 years, and the mean age of adipose tissue donors was 54.2 ± 16.0 years (Table 1).
Table 1.
Ages and origins of the multipotent stromal cell donors
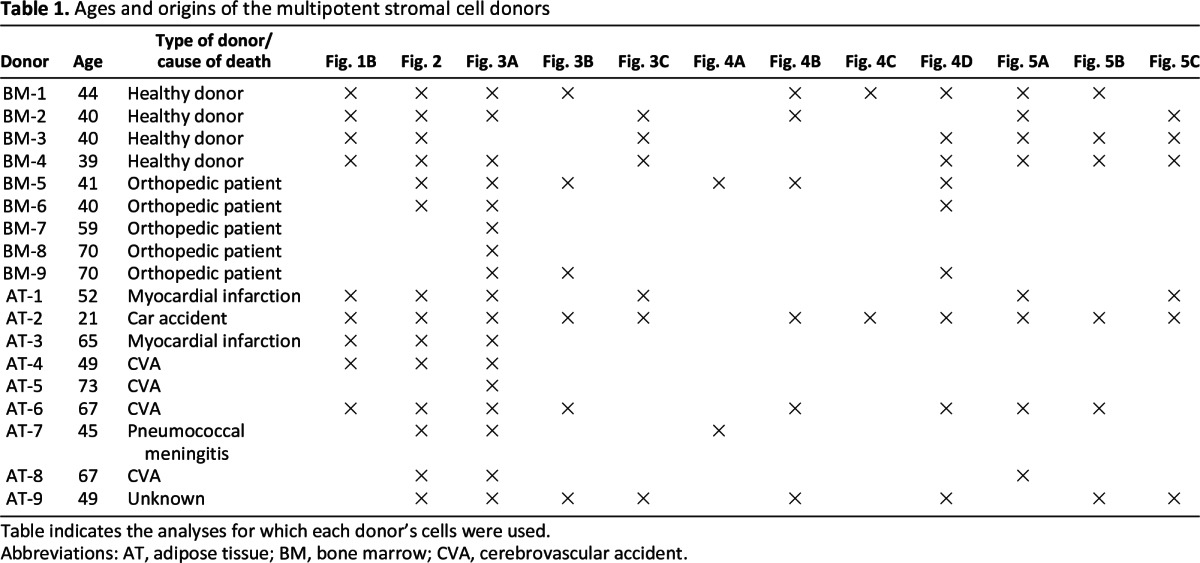
Table indicates the analyses for which each donor's cells were used.
Abbreviations: AT, adipose tissue; BM, bone marrow; CVA, cerebrovascular accident.
Generation of MSCs From Bone Marrow and Adipose Tissue
Bone marrow-derived mononuclear cells were isolated using a Ficoll-Paque density gradient (1.077 g/cm3) and plated at 1.3 × 105/cm2 in proliferation medium (Dulbecco's modified Eagle's medium-low glucose [DMEM-LG]; Invitrogen, Paisley, U.K., http://www.invitrogen.com) supplemented with 10% fetal calf serum (FCS) (Greiner Bio-One, Alphen a/d Rijn, The Netherlands, http://www.gbo.com/en) and penicillin/streptomycin (P/S) (Invitrogen). Adipose tissue was mechanically disrupted and subsequently digested using collagenase (2 mg/ml; Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands, http://www.sigmaaldrich.com) in proliferation medium. After digestion, the cell suspension was centrifuged to separate the adipocytes from the stromal vascular fraction (SVF). The SVF was filtered, and the cells were plated in culture flasks in culture medium at a density of 1.4 × 104/cm2.
Cultures were incubated at 37°C and 5% CO2. After 3–4 days nonadherent cells were removed, and medium was refreshed every 3–4 days until confluence was reached. The MSC monolayer was detached using trypsin/EDTA (Invitrogen), and cells were reseeded at 4,000 cells per cm2 for further expansion. The MSCs were characterized by immune phenotyping and used in further experiments at passages 2–5.
Isolation of Human Peripheral Blood Mononuclear Cells and Monocytes
Human PBMCs were isolated from buffy coats obtained from healthy donors from Sanquin Blood Supply (Leiden, The Netherlands, http://www.sanquin.nl/en/) using a Ficoll-Paque density gradient. Monocytes were purified from the freshly prepared mononuclear cell fraction by magnetic activated cell sorting (MACS) using CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com). Cells were separated with a MACS LS column according to the manufacturer's recommendations.
Differentiation Experiments
BM-MSCs and AT-MSCs were tested for their capacity to differentiate toward the osteogenic and adipogenic lineages. One hundred thousand MSCs were plated in 24-well plates and incubated with the appropriate medium for induction of differentiation as stated below. All cultures were refreshed weekly.
For osteogenic differentiation, MSCs were cultured in α-minimal essential medium (α-MEM) (Invitrogen) supplemented with l-glutamine (200 nM; Invitrogen), P/S and 10% FCS, 10−7 M dexamethasone, and 50 μg/ml vitamin C (both from Sigma-Aldrich). After the first week, 5 mM β-glycerophosphate (Sigma-Aldrich) was added. For adipogenic differentiation, MSCs were incubated with medium consisting of α-MEM supplemented with l-glutamine, P/S and 10% FCS, 10−7 M dexamethasone, insulin (10 μg/ml), indomethacin (5 μM), and 3-isobutyl-1-methylxanthine (5 μM) (all from Sigma-Aldrich).
After 3 weeks of culture, cells from all differentiation cultures were stained for alkaline phosphatase activity with Fast Blue (Sigma-Aldrich), and calcium deposition was determined with alizarin red (MP Biomedicals LLC, Illkirch Cedex, France, http://www.mpbio.com) staining. Formation of lipid droplets was visualized using Oil Red O staining (Sigma-Aldrich).
Suppression of PBMC Proliferation
The immunosuppressive capacity of MSCs was tested in a coculture of MSCs and PBMCs. MSCs were plated in graded doses in a 96-well flat-bottomed plate in DMEM-LG (Invitrogen) supplemented with 10% FCS (Greiner Bio-One) and P/S (Invitrogen). Following adherence overnight, PBMCs (1.0 × 105/well) were added and stimulated with human T-activator CD3/CD28 Dynabeads (Invitrogen) in a bead:PBMC ratio of 1:5. After 5 days of culture, cells were pulsed with [3H]-thymidine (0.5 μCi per well) and incubated for 16 hours at 37°C. The cultures were harvested on a glass fiber filter, and thymidine incorporation was measured with a liquid scintillation counter (Wallac, Turku, Finland, http://www.perkinelmer.com). Data were expressed as mean corrected counts per minute of triplicate cocultures. Control experiments were performed, replacing MSCs with K562 cells.
Inhibition of Monocyte-Derived Immature Dendritic Cell Differentiation
iDCs were generated from freshly isolated CD14+ monocytes by culturing 1.0 × 106 cells in six-well plates in RPMI (Invitrogen) containing P/S, l-glutamine, and 10% FCS supplemented with the growth factors IL-4 (10 ng/ml; Invitrogen) and granulocyte macrophage colony-stimulating factor (5 ng/ml; Novartis International, Basel, Switzerland, http://www.novartis.com) for 6 days. To examine the effect of MSCs on monocyte differentiation, irradiated (60 Gy) BM-MSCs and AT-MSCs were added to the culture at an MSC:monocyte ratio of 1:5 to 1:50. All experiments were performed in duplicate. The experiments were performed in direct coculture and in a Transwell coculture system (pore size, 0.4 μM; Corning Inc., Lowell, MA, http://www.corning.com). In the Transwell experiments, MSCs were plated in the lower well, and monocytes were added in the Transwell insert. At day 6 the iDCs were harvested from the coculture and analyzed for expression of CD1a and CD14 by flow cytometry.
Flow Cytometry
After trypsinization, MSC cultures were analyzed for expression of surface markers using CD90-FITC, CD73-PE, CD45-FITC, CD34-PE, HLA-DR-PE, HLA-ABC-FITC, CD80-PE (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), and CD105-FITC (Ancell, Bayport, MN, http://www.ancell.com). The monocytes from the coculture experiments were collected at day 6 and analyzed for surface marker expression using CD1a-FITC and CD14-PE (BD Biosciences). Cells were incubated with fluorescent-labeled antibodies 30 minutes at 4°C in the dark. After being washed with phosphate-buffered saline/1% albumin they were analyzed using a FACSCanto II (BD Biosciences). The analysis of the acquired data was done with FlowJo software version 7.6.1 (Tree Star, Ashland, OR, http://www.treestar.com).
Cytokine Assay
To determine cytokine concentrations in the supernatants of PBMC proliferation cultures and the monocyte cultures, cell-free supernatant was collected at days 5 and 6, respectively, and stored at −20°C until use. Cytokine concentrations were measured using the Bio-Plex Pro Human Cytokine Th1/Th2 panel (Bio-Rad, Hercules, CA, http://www.bio-rad.com). To analyze constitutive cytokine secretion, BM-MSCs and AT-MSCs were plated at confluent cell concentrations (2.0 × 105 cells in a 12-well plate) and cultured without medium replacement. At day 7, cell-free culture supernatant was collected and stored at −20°C, and MSCs were harvested and counted. Cytokine concentrations were measured using the Bio-Plex Pro Human Cytokine 27-plex panel (Bio-Rad) or sandwich enzyme-linked immunosorbent assay (BD Biosciences).
MTT Assay
In a 96-well plate, 2.0 × 104 MSCs were plated in DMEM-LG (Invitrogen) supplemented with 10% FCS (Greiner Bio-One) and P/S (Invitrogen). After overnight adherence, the medium was replaced with 100 μl of colorless Iscove's modified Dulbecco's medium (Invitrogen) supplemented with P/S and 10% FCS. For measurement of the metabolic activity 10 μl of 12 mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) stock solution (Life Technologies, Grand Island, NY, http://www.lifetech.com) was added to each well and incubated for 4 hours at 37°C. Then 75 μl of the medium was removed and 100 μl of DMSO was added and incubated for 10 minutes at 37°C. Absorbance was read at 540 nm.
Interferon-γ Stimulation of MSCs
To analyze IDO upregulation induced by interferon-γ (IFN-γ), MSCs were plated at a concentration of 2.0 × 105 cells per well in a 12-well plate, and IFN-γ was added to the culture medium (500 U/ml; Sigma-Aldrich). At time points of 0, 4, 8, and 24 hours, RNA was extracted, and cell-free supernatant was collected and stored for further analysis at −20°C.
Gene Expression Analysis
RNA was extracted from MSCs using the RNeasy micro kit from Qiagen (Hilden, Germany, http://www.qiagen.com). cDNA was synthesized with Superscript III RT (Invitrogen). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analyses were performed on a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) using SYBR Green reagent (Roche Diagnostics, Almere, The Netherlands, http://www.roche-applied-science.com). The following primer sets were used: IDO: forward, 5′-CCTGAGGAGCTACCATCTGC-3′, and reverse, 5′-TCAGTGCCTCCAGTTCCTTT-3′; IL-6: forward, 5′-TTCAATGAGGAGACTTGCCTG-3′, and reverse, 5′-ACAACAACAATCTGAGGTGCC-3′; and β-actin: forward, 5′-AGGCATCCTCACCCTGAAGTA-3′, and reverse, 5′-CACACGCAGCTCATTGTAGA-3′. All RT-qPCR data were normalized to β-actin expression, and the data were analyzed using the δ-Ct method.
Results
BM-MSCs and AT-MSCs Have Similar Phenotype and Differentiation Capacity
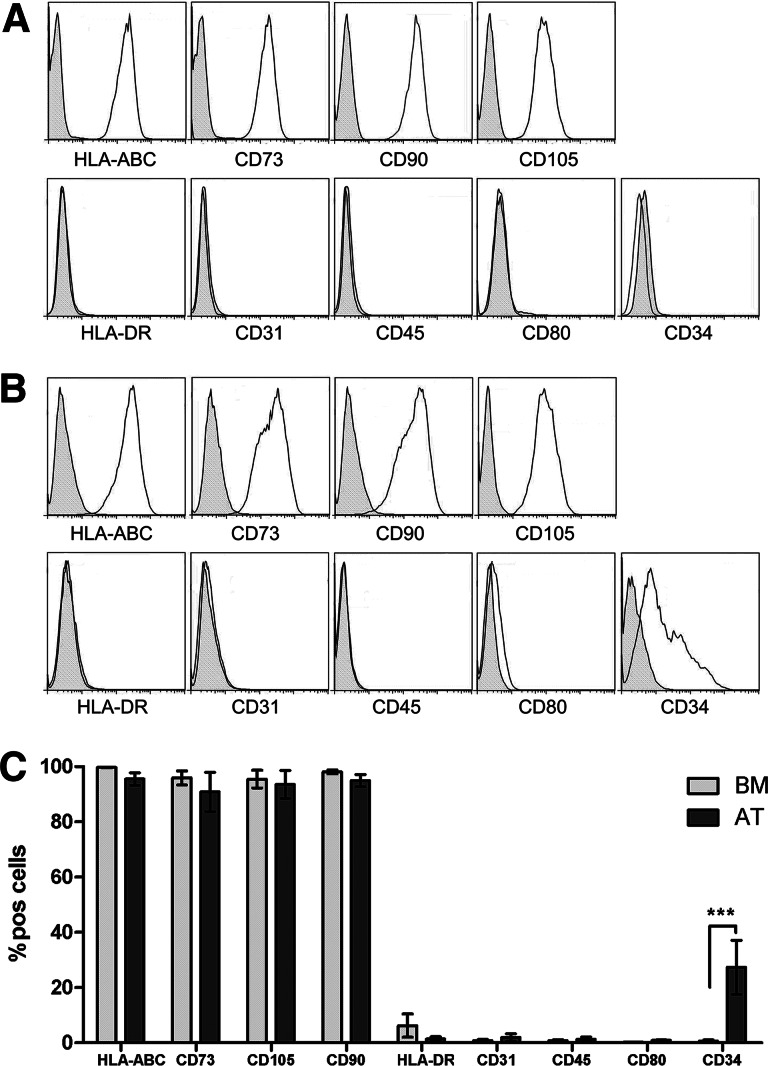
Immunophenotypic analysis of BM-MSCs and AT-MSCs showed a similar surface marker expression profile (Fig. 1A–1C). This profile is in accordance with the phenotypical definition of MSCs by the International Society for Cellular Therapy [1], with the exception of CD34, which was expressed only on a fraction of AT-MSCs (27.4% ± 9.8%, n = 4) (Fig. 1C).
Figure 1.
Bone marrow-derived multipotent stromal cells (BM-MSCs) and adipose tissue-derived multipotent stromal cells (AT-MSCs) show the same immunophenotype, except for CD34 expression. (A, B): Representative histograms of BM-MSC (A) and AT-MSC (B) fluorescence-activated cell sorting (FACS) analysis. All AT-MSC donors showed a CD34+ population. Gray histograms are isotype control. (C): Cumulative data of FACS analysis of BM-MSCs and AT-MSCs for several surface markers. The expression of CD34 was significantly higher on AT-MSCs compared with BM-MSCs (data are means from four BM-MSC and five AT-MSC donors; statistical analysis was performed using a two-way analysis of variance; *, p < .05; **, p < .01; ***, p < .001). Abbreviations: AT, adipose tissue-derived multipotent stromal cells; BM, bone marrow-derived multipotent stromal cells; HLA, human leukocyte antigen; pos, positive.
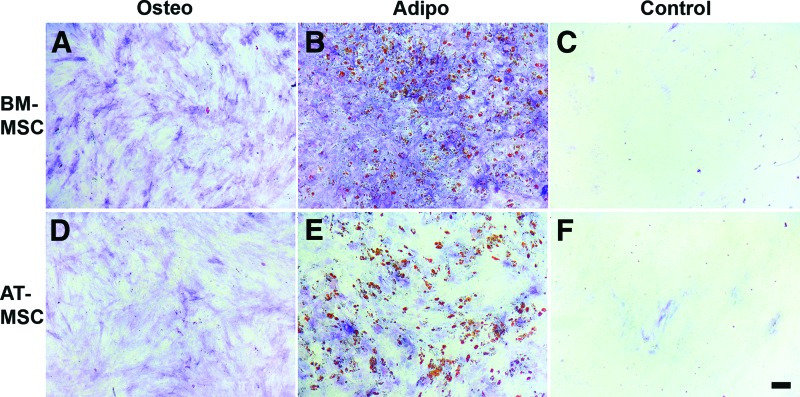
Both BM-MSCs and AT-MSCs were able to differentiate toward the osteogenic and adipogenic lineages (Fig. 2). No differentiation was observed for MSCs that were cultured in medium only.
Figure 2.
BM-MSCs and AT-MSCs both differentiate toward the osteogenic and adipogenic lineages. Confluent cultures of BM-MSCs (A–C) and AT-MSCs (D–F) were maintained in osteogenic differentiation medium (A, D), adipogenic differentiation medium (B, E), or control medium (C, F). After 3 weeks of culture in differentiation medium, both BM-MSCs and AT-MSCs were positive for alkaline phosphatase activity (A, D) and lipid droplets were formed (B, E). Control cultures in proliferation medium did not show alkaline phosphatase activity or the formation of lipid droplets. Multipotent stromal cell populations from six BM-MSC donors and from eight AT-MSC donors were tested for their differentiation capacity; representative pictures are shown for BM-MSCs and AT-MSCs. Scale bar = 50 μm. Abbreviations: Adipo, adipogenic; AT-MSC, adipose tissue-derived multipotent stromal cells; BM-MSC, bone marrow-derived multipotent stromal cells; Osteo, osteogenic.
AT-MSCs Are More Potent in Their Suppression of PBMC Proliferation Than BM-MSCs
Functional Suppression
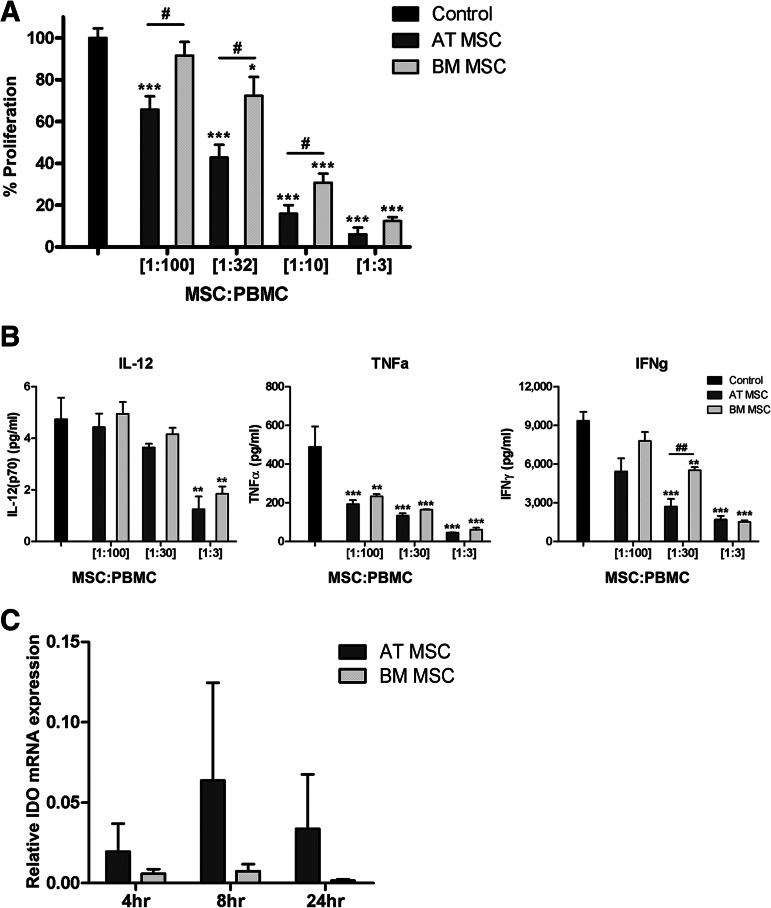
Both BM-MSCs and AT-MSCs were able to suppress the proliferation of PBMCs in a dose-dependent fashion (Fig. 3A). However, addition of equal numbers of AT-MSCs resulted in significantly higher levels of suppression of PBMC proliferation compared with BM-MSCs (Fig. 3A). Approximately three times as many BM-MSCs were needed to obtain the suppressive effect that was observed with AT-MSCs. At an MSC:PBMC ratio of 1:3, both BM-MSCs and AT-MSCs showed almost complete inhibition of PBMC proliferation. Control experiments, replacing MSCs with K562 cells, showed that the effect on proliferation was not due to cell crowding or exhaustion of the culture medium (data not shown).
Figure 3.
AT-MSCs are more potent in suppressing PBMC proliferation compared with BM-MSCs. (A): MSCs suppressed PBMC proliferation in a dose-dependent fashion. AT-MSCs showed a significantly stronger suppression of proliferation at MSC:PBMC ratios of 1:100, 1:32, and 1:10 (two separate experiments; n = 9 for AT-MSCs, n = 8 for BM-MSCs). (B): From one experiment, culture supernatants of PBMC proliferation in the presence and absence of MSCs were assayed for cytokine concentrations at day 5 of coculture (n = 3 for both groups). Statistical analysis was performed using Student's t test (data are mean ± SEM; * indicates compared with control: *, p < .05; **, p < .01; ***, p < .001; # indicates AT-MSCs compared with BM-MSCs: #, p < .05; ##, p < .01). (C): After IFN-γ stimulation of MSCs, both BM-MSCs and AT-MSCs showed IDO mRNA upregulation, with an optimum at 8 hours. IDO mRNA expression is shown relative to β-actin mRNA expression. Statistical analysis was performed using Student's t test (n = 3 for both groups). Abbreviations: AT MSC, adipose tissue-derived multipotent stromal cells; BM MSC, bone marrow-derived multipotent stromal cells; IDO, indoleamine 2,3-dioxygenase; MSC, multipotent stromal cells; PBMC, peripheral blood mononuclear cells.
Cytokine Production
In the presence of increasing amounts of both BM-MSCs and AT-MSCs, the concentrations of the proinflammatory cytokines IL-12, tumor necrosis factor-α (TNF-α), and IFN-γ in the culture supernatants of PBMC-MSC coculture decreased in a dose-dependent fashion (Fig. 3B). At an MSC:PBMC ratio of 1:30, the IFN-γ concentrations in the culture supernatant were significantly lower (p < .01) in the presence of AT-MSCs compared with BM-MSCs (Fig. 3B).
The upregulation of IDO expression in MSCs following IFN-γ stimulation is regarded as an important mechanism for the MSC-induced suppression of PBMC proliferation. Therefore, we investigated the level and speed of induction of IDO expression in response to IFN-γ stimulation in BM-MSCs and AT-MSCs. Following IFN-γ stimulation, expression of IDO mRNA was induced in both BM-MSC and AT-MSC populations. Between 4 and 24 hours after IFN-γ stimulation, the average IDO expression in AT-MSCs was higher than in BM-MSCs. However, because of the high variation, the difference did not reach statistical significance. At 8 hours, the highest expression levels of IDO were observed for both AT-MSCs and BM-MSCs (Fig. 3C).
AT-MSCs Are More Potent in Inhibiting Dendritic Cell Formation Than BM-MSCs
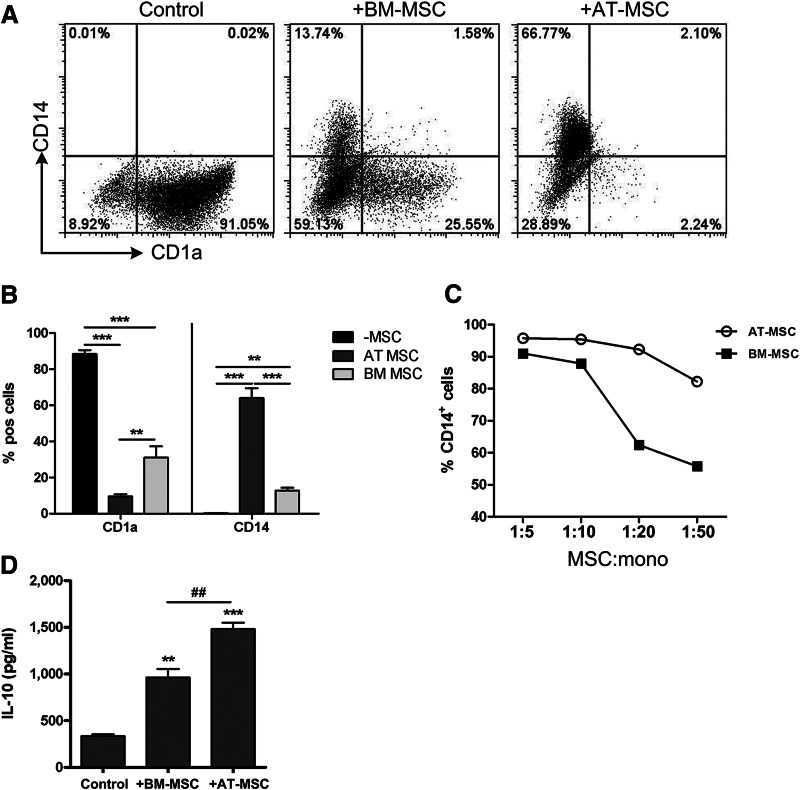
BM-MSCs and AT-MSCs were further tested for their capacity to inhibit the differentiation of CD1a−CD14+ monocytes toward CD1a+CD14− iDCs. Both AT-MSCs and BM-MSCs exhibited this inhibitory effect at an MSC:monocyte ratio of 1:10 (Fig. 4A, 4B). Also in this immunomodulation assay, a clear difference in the dose-response relation was observed, in favor of AT-MSCs (Fig. 4C). At an MSC:monocyte ratio of 1:20, AT-MSCs showed the same level of inhibition as BM-MSCs showed at a ratio of 1:5.
Figure 4.
AT-MSCs are more potent inhibitors of monocyte differentiation than BM-MSCs. (A): Representative dot plots of monocyte differentiation toward immature dendritic cells in the absence and presence of BM-MSCs and AT-MSCs (MSC:monocyte ratio of 1:10). (B): Cumulative data of CD1a and CD14 expression on differentiated monocytes. Data are means from three different BM-MSCs and three different AT-MSCs (MSC:monocyte ratio of 1:10); statistical analysis was performed using Student's t test (*, p < .05; **, p < .01; ***, p < .001). (C): Dose-response curves of the percentage of CD14+ cells in the presence of BM-MSCs (■) or AT-MSCs (○). (D): IL-10 concentrations in culture supernatants from day 6 of the monocyte differentiation in the presence of BM-MSCs and AT-MSCs are increased compared with the differentiation without MSCs. Data are means from three different experiments with six different BM-MSCs and three different AT-MSCs (MSC:monocyte ratio of 1:10); statistical analysis was performed using Student's t test (* indicates compared with control: **, p < .01; ***, p < .001; # indicates BM-MSCs compared with AT-MSCs: ##, p < .01). Abbreviations: AT-MSC, adipose tissue-derived multipotent stromal cells; BM-MSC, bone marrow-derived multipotent stromal cells; IL, interleukin; mono, monocyte; MSC, multipotent stromal cells; pos, positive.
Addition of BM-MSCs and AT-MSCs increased the concentrations of the anti-inflammatory cytokine IL-10 in supernatants of cultures from monocytes that were differentiated toward iDCs (Fig. 4D). Again, addition of AT-MSCs resulted in a significantly higher concentrations of IL-10 than addition of BM-MSCs (1,481.0 ± 118.8 pg/ml vs. 961.8 ± 159.5 pg/ml, n = 3, p < .01).
AT-MSCs Secrete Higher Concentrations of Cytokines Compared With BM-MSCs
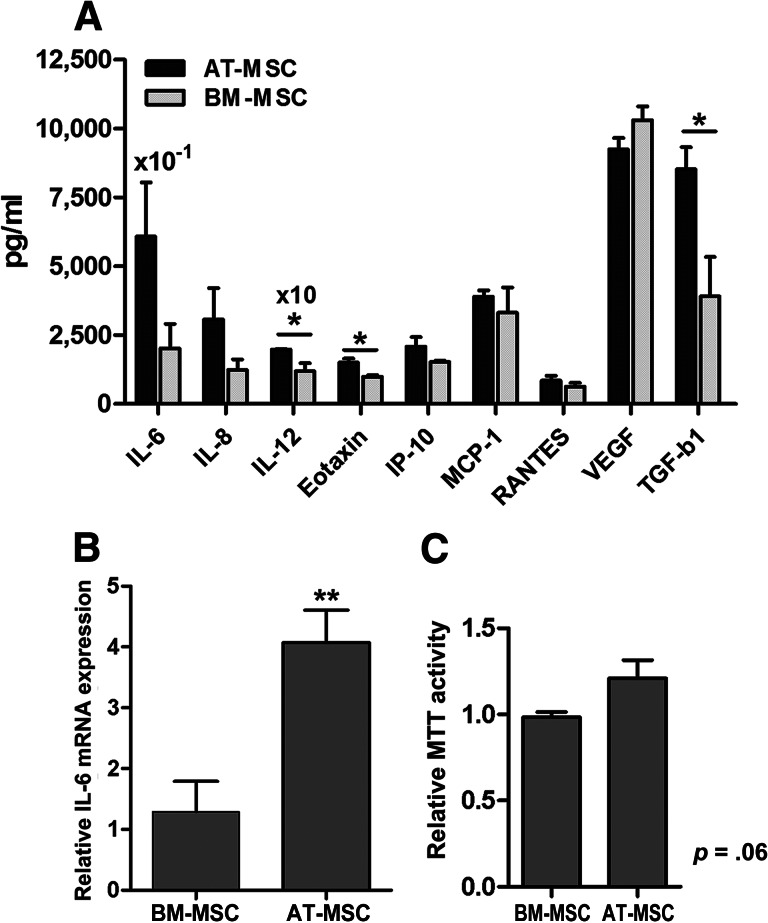
To investigate whether the difference in potency in the various immunomodulatory assays could be explained by a generally increased cytokine secretion by AT-MSCs compared with BM-MSCs, cytokine profiling was performed in culture supernatant of unstimulated MSCs. After 7 days of culture, no differences were observed in the number of cells between AT-MSC and BM-MSC cultures. Culture supernatants from AT-MSC cultures generally contained higher concentrations of cytokines than culture supernatants from BM-MSCs, and the reverse phenomenon was never observed. The differences were significant for the cytokines IL-6, IL-8, IL-12, Eotaxin, and TGF-β1 (Fig. 5A). The largest differences were found for IL-6 and TGF-β1. For IL-6, this difference in cytokine concentrations was confirmed at the transcriptional level (Fig. 5B).
Figure 5.
AT-MSCs secrete higher levels of cytokines compared with BM-MSCs. (A): Cytokine concentrations measured in culture supernatant were higher for AT-MSCs compared with BM-MSCs. A representative experiment is shown from three separate experiments (n = 4 for both groups; IL-6 values represent 1.0 × 10−1 of the measured concentrations; IL-12 values shown are 10× the measured concentrations). (B): IL-6 mRNA expression was also increased in AT-MSCs compared with BM-MSCs (n = 3 for both groups). (C): AT-MSCs showed a slightly enhanced MTT activity; MTT activity of AT-MSCs is shown relative to that of BM-MSCs (n = 3 for both groups). Data are means ± SEM from three different experiments; statistical analysis was performed using Student's t test (*, p < .05; **, p < .01). Abbreviations: AT-MSC, adipose tissue-derived multipotent stromal cells; BM-MSC, bone marrow-derived multipotent stromal cells; IL, interleukin; IP-10, interferon γ-induced protein 10; MCP-1, monocyte chemotactic protein-1; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; TGF-b1, transforming growth factor-β1; VEGF, vascular endothelial growth factor.
We hypothesized that the generally enhanced levels of multiple cytokines in AT-MSCs are the result of a higher metabolic activity in AT-MSCs. To test this hypothesis, we directly analyzed the metabolic activity of BM-MSCs and AT-MSCs using an MTT assay. Indeed, the AT-MSCs showed a higher average metabolic activity compared with BM-MSCs; however, this observation reached only borderline significance (p = .06) (Fig. 5C).
Discussion
AT-MSCs have been considered as an alternative to BM-MSCs for clinical application [16, 30, 39]. In this study we performed an in vitro systematic comparison of BM-MSCs and AT-MSCs from age-matched donors. We found a similar phenotype and capacity for multilineage in vitro differentiation toward the osteogenic and adipogenic lineages. We observed that both BM-MSCs and AT-MSCs exhibited the capacity to inhibit PBMC proliferation as well as monocyte differentiation toward iDCs. However, the dose-response curve for AT-MSCs was clearly different from that for BM-MSCs. To acquire the same level of suppression, fewer AT-MSCs were required than BM-MSCs. Furthermore, AT-MSCs secreted higher levels of cytokines than BM-MSCs, which corresponded to a higher metabolic activity.
The expression of surface marker CD34 on a subpopulation of the AT-MSCs has been described previously. This expression decreases during culture expansion [40–42], explaining the observed variation. The relevance of CD34 expression on AT-MSCs is currently unclear. CD34+/CD31− cells have been detected at perivascular locations in adipose tissue [43]. These cells can differentiate in vitro toward endothelial cells [44], which suggests that they might play role in vasculogenesis. Such a preferred role for these CD34+/CD31− cells is not supported by the findings of Suga et al., who showed similar capabilities for capillary-network formation for CD34+ and CD34− AT-MSCs [45].
Several studies have compared BM-MSCs and AT-MSCs, but mostly small groups of donors and different patient groups and age groups were investigated. Those studies reported that AT-MSCs and BM-MSCs both exhibit the capacity to inhibit PBMC proliferation but indicated no differences between the MSC populations [30, 32, 34–36]. We also showed that both populations are able to inhibit PBMC proliferation, but with a different dose-response curve. Others used MSC concentrations at the plateau phase of the dose-response curve and MSCs from different patient groups and age. Therefore they did not find differences in potency between AT-MSCs and BM-MSCs. We showed that differences were found only at concentrations in the linear phase of the dose-response curve.
The more potent suppression of PBMC proliferation by AT-MSCs than by BM-MSCs was also reflected in the cytokine concentrations measured in the coculture supernatants of the PBMC-MSC cocultures. Our data suggest that both BM-MSCs and AT-MSCs induce a shift from a Th1 toward a Th2 response, since concentrations of the proinflammatory cytokines IL-12, TNF-α, and IFN-γ were significantly reduced in culture supernatants of PBMCs cultured in the presence of either type of MSCs. This effect was stronger in the presence of AT-MSCs than in the presence of BM-MSCs, and for IFN-γ this difference between AT-MSCs and BM-MSCs became statistically significant.
IFN-γ has also been implicated in the initiation of the immunosuppressive function of MSCs. Activation of MSCs by IFN-γ is involved in the inhibitory effect of MSCs on T-cell proliferation through induction of expression of IDO, an enzyme that catabolizes tryptophan [13]. The level of induced IDO expression differs between MSC populations and has been correlated to their immunosuppressive potential [46]. We found that, in response to IFN-γ, IDO expression is induced to higher levels in AT-MSCs than in BM-MSCs. The more potent inhibitory effect of AT-MSCs on PBMC proliferation can be explained by this stronger response to IFN-γ; that is, the IFN-γ that is produced by the activated PBMCs causes a stronger or more adequate response of the AT-MSCs resulting in stronger inhibition of the PBMC proliferation, finally resulting in lower concentrations of IFN-γ and other proinflammatory cytokines. We have shown in vitro that MSCs inhibit monocyte differentiation toward iDCs and rather skew monocytes toward a tolerogenic cell type, with increased secretion of the immunomodulatory cytokine IL-10 [47]. In our monocyte-MSC cocultures, the secretion of the immunomodulatory cytokine IL-10 was more pronounced in the presence of AT-MSCs than in the presence of BM-MSCs. This indicates that the monocytes have become more tolerogenic in the presence of AT-MSCs. Since activated monocytes have been shown to increase their IL-10 expression upon IL-10 exposure, creating a positive feedback loop [48], this difference in effect of AT- versus BM-MSCs might even be further enhanced.
Compared with BM-MSCs, AT-MSCs constitutively secrete higher levels of multiple cytokines that have been implicated in MSC-mediated immunomodulation, such as IL-6 and TGF-β1 [5, 11, 49, 50]. We have shown that IL-6 is a key factor for the MSC-induced inhibition of monocyte differentiation toward iDCs and the induction of IL-10 secretion by monocytes [47]. The higher IL-6 secretion by AT-MSCs can therefore explain the more pronounced inhibition of iDC generation and the increased IL-10 protein concentrations that were observed in the monocyte cultures in the presence of AT-MSCs. TGF-β1 has been implicated in the generation of regulatory T cells by MSCs [11, 14]. Therefore, we hypothesize that the observed higher TGF-β1 production by AT-MSCs may also be associated with an increased ability to induce regulatory T cells.
The overall explanation for the observed functional superiority of AT-MSCs may be their higher metabolic activity, resulting in production of higher levels of cytokines that are involved in the immunosuppressive mechanisms of MSCs. It is therefore conceivable that this also holds true for other factors that are involved in immunomodulation by MSCs, such as PGE2 [30, 51], galectin-1 [52, 53], and HLA-G5 [54].
Conclusion
Our data show that BM-MSCs and AT-MSCs share a similar immunophenotype and capacity for in vitro multilineage differentiation. Also, functionally, BM-MSCs and AT-MSCs show similar immunomodulatory effects, but with a different dose-response curve, in favor of AT-MSCs. We show that the differences in immunomodulatory properties between BM-MSCs and AT-MSCs are primarily due to the quantitative aspects of the suppression, which are likely related to their respective metabolic activity. These data support the notion that AT-MSCs could be a promising alternative to BM-MSCs for future clinical immunomodulatory applications.
Acknowledgments
We thank Esther Steeneveld and Marten Engelse for providing the adipose tissue from donor pancreas. This work received financial support from the Netherlands Organization for Scientific Research ZonMW Translational Adult Stem Cell program no. 11.600.1016.
Author Contributions
S.M.M.: conception and design, collection and analysis of data, interpretation of data, manuscript writing; J.J.Z.: collection of study samples; W.E.F.: interpretation of data, manuscript writing; H.R.: conception and design, interpretation of data, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 3.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33:593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 5.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 6.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 7.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 8.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 9.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 10.Crop M, Baan CC, Korevaar SS, et al. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19:1843–1853. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- 11.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 13.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 14.Fu S, Zhang N, Yopp AC, et al. TGF-beta Induces Foxp3 + T-regulatory cells from CD4+CD25- precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 16.Yañez R, Lamana ML, Garcia-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 17.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 18.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. doi: 10.1182/blood-2007-04-087056. [DOI] [PubMed] [Google Scholar]
- 19.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 20.Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 21.Ra JC, Shin IS, Kim SH, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 22.Fang B, Song Y, Liao L, et al. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39:3358–3362. doi: 10.1016/j.transproceed.2007.08.103. [DOI] [PubMed] [Google Scholar]
- 23.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann NY Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 24.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 25.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 2004. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 29.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 30.Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's jelly and bone marrow sources. Cell Immunol. 2010;264:171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 33.Torensma R, Prins HJ, Schrama E, et al. The impact of cell source, culture methodology, culture location and individual donors on gene expression profiles of bone marrow-derived and adipose-derived stromal cells. Stem Cells Dev. 2013;22:1086–1096. doi: 10.1089/scd.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Niemeyer P, Kornacker M, Mehlhorn A, et al. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111–121. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- 36.Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–156. doi: 10.1016/j.cellimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 37.D'Ippolito G, Schiller PC, Ricordi C, et al. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 38.Maijenburg MW, Kleijer M, Vermeul K, et al. The composition of the mesenchymal stromal cell compartment in human bone marrow changes during development and aging. Haematologica. 2012;97:179–183. doi: 10.3324/haematol.2011.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dicker A, Le Blanc K, Astrom G, van H V, Gotherstrom C, Blomqvist L, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 42.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 43.Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 44.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 45.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 46.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 47.Melief SM, Geutskens SB, Fibbe W, et al. Multipotent stromal cells skew monocytes towards an anti-inflammatory IL-10 producing phenotype by production of IL-6. Haematologica. 2013 doi: 10.3324/haematol.2012.078055. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chomarat P, Banchereau J, Davoust J, et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 50.Patel SA, Meyer JR, Greco SJ, et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: Role of mesenchymal stem cell-derived TGF-β. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 51.Yañez R, Oviedo A, Aldea M, et al. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316:3109–3123. doi: 10.1016/j.yexcr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Gieseke F, Bohringer J, Bussolari R, et al. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 53.Najar M, Raicevic G, Id BH, et al. Modulated expression of adhesion molecules and galectin-1: Role during mesenchymal stromal cell immunoregulatory functions. Exp Hematol. 2010;38:922–932. doi: 10.1016/j.exphem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]