Cell transplantation strategies typically introduce a stress challenge at the time of transplantation as the cells are switched from 20% to 3% O2 (the average in adult organs). This study found that culture of neural precursor cells (NPCs) at 3% rather than 20% O2 approximately doubles survival in the immediate post-transplantation phase. Furthermore, NPC fate was affected by culture at low, physiological O2 tensions (3%), with particularly marked effects on the oligodendrocyte lineage, both in vitro and in vivo. It is proposed that careful consideration of physiological oxygen environments, and particularly changes in oxygen tension, has relevance for the practical approaches to cellular therapies.
Keywords: Hypoxia, Neural stem cell, Cell transplantation, Oligodendrocytes
Abstract
Traditionally, in vitro stem cell systems have used oxygen tensions that are far removed from the in vivo situation. This is particularly true for the central nervous system, where oxygen (O2) levels range from 8% at the pia to 0.5% in the midbrain, whereas cells are usually cultured in a 20% O2 environment. Cell transplantation strategies therefore typically introduce a stress challenge at the time of transplantation as the cells are switched from 20% to 3% O2 (the average in adult organs). We have modeled the oxygen stress that occurs during transplantation, demonstrating that in vitro transfer of neonatal rat cortical neural precursor cells (NPCs) from a 20% to a 3% O2 environment results in significant cell death, whereas maintenance at 3% O2 is protective. This survival benefit translates to the in vivo environment, where culture of NPCs at 3% rather than 20% O2 approximately doubles survival in the immediate post-transplantation phase. Furthermore, NPC fate is affected by culture at low, physiological O2 tensions (3%), with particularly marked effects on the oligodendrocyte lineage, both in vitro and in vivo. We propose that careful consideration of physiological oxygen environments, and particularly changes in oxygen tension, has relevance for the practical approaches to cellular therapies.
Introduction
The prospect of cell transplantation therapies is an exciting one, especially for the field of neuroscience, where they have the potential to treat conditions such as Parkinson's disease, multiple sclerosis, motor neuron disease, and stroke [1–4]. Currently, approaches are limited by a number of factors, such as the availability of material to transplant, cell survival following transplantation, and longer-term integration into the host nervous system [5]. Several studies have shown that the immediate post-transplantation phase has the most marked effect on survival, with many, or even most, transplanted cells dying during the first few days after transplantation [6–8]. This is thought to reflect a variety of environmental influences, including mechanical trauma and anoikis related to preparation of the cell suspension, inflammation, trophic factor depletion, hypoglycemia, hypoxia, and oxidative stress [5]. However, the effect of oxygen in the cell culture environment on cell survival following transplantation has been only minimally explored [9–14]. This is likely to be a highly relevant consideration when recalling that even in well-oxygenated arterial blood the partial pressure of oxygen (pO2) is only approximately 95 mmHg (13%), compared with 152 mmHg (20%) in typical cell culture incubators and a markedly lower average pO2 in the brain of 23 mmHg (3%; Table 1) [15]. Thus, routine transplantation studies present a stress challenge as a consequence of an oxygen switch from 20% in vitro to approximately 3% in vivo [16], which could potentially be reduced by maintenance at 3% O2 throughout.
Table 1.
Comparative oxygen tensions, based on an atmospheric pressure at sea level of 760 mmHg
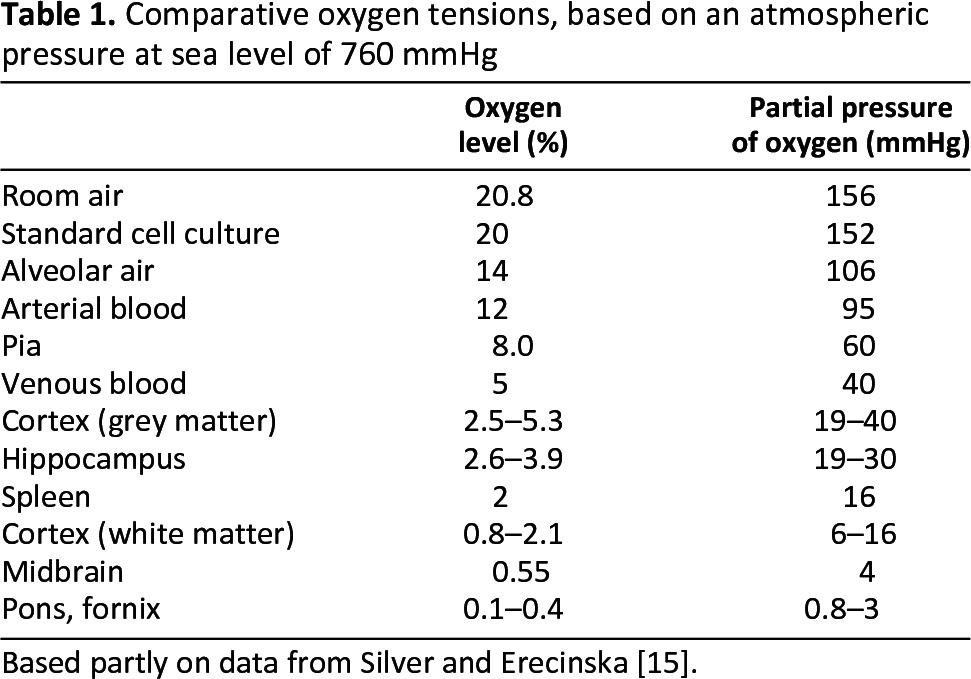
Based partly on data from Silver and Erecinska [15].
Several lines of evidence show that cell culture at physiologically normoxic conditions (1%–5% O2) is advantageous in terms of stem cell proliferation, survival, and fate, with particularly marked effects on dopaminergic neurons and oligodendrocytes [16–25]. One proposed mechanism for this is a reduction in the generation of reactive oxygen species (ROS) at low, physiological oxygen tensions compared with the more traditional, hyperoxic 20% O2 culture [19, 26, 27]. As yet, however, the effects of culture at low, physiological oxygen tensions prior to transplantation have been only minimally explored.
An emerging concept in the development of effective cell transplantation technologies is that of hypoxic or ischemic preconditioning. Here, the principle is that exposing cells that have been cultured in hyperoxic conditions at 20% O2 to a hypoxic insult, by transferring them to a low O2 (0.5%–3%) environment for up to 48 hours before transplantation (typically into models of ischemic disease), improves survival of transplanted cells and host tissue regeneration [9–11, 13, 14]. Proposed mechanisms include induction of hypoxia inducible factor-1α leading to a reduction in oxidative stress. Chemical approaches to reduce oxidative stress have also been trialled, with pretreatment of rat neural precursor cells (NPCs) with minocycline prior to transplantation into the middle cerebral artery occlusion model of stroke leading to upregulation of Nrf2 and the battery of antioxidant genes that it regulates, translating to enhanced survival of the grafted cells [28].
Potentially greater benefits could be derived by culturing cells at low, physiological oxygen (1%–5%) from the point of derivation until the stage of transplantation. This approach has been tested once with cardiac stem cells derived from human heart biopsies and expanded at 5% versus 20% O2 [12]. Culture at 5% O2 led to a higher cell yield, decreased aneuploidy and senescence, and a greater resistance to oxidative stress in vitro. Transplantation of the 5% O2 maintained cardiac stem cells into infarcted hearts of mice resulted in improved cell engraftment and functional recovery. Furthermore, in what currently represents the only widely available “cell transplantation” treatment, in vitro fertilization, scientists have long since capitalized on the observation that the growth of preimplantation morulae is maximized at low (5%) O2 [29–31].
Collectively, these observations provide a strong rationale to test the effects of oxygen in the culture environment on NPC survival and fate following transplantation into nonischemic models. We report that (a) neonatal rat cortical NPC cultures can be established and more readily expanded at 3% compared with 20% O2; (b) these NPCs survive better in vitro when expanded and differentiated at 3% O2, compared with those expanded at 20% O2 and switched to 3% O2 for differentiation; (c) this survival benefit translates to the in vivo environment at 48 hours after transplantation into the adult rat hippocampus; and (d) stem cell fate is affected by the pO2 in the culture environment, with rat cortical NPCs showing a greater tendency to generate oligodendrocyte lineage cells at 3% than 20% O2, both in vitro and following transplantation into the hippocampus.
Materials and Methods
Reagents were obtained from Invitrogen (Carlsbad, CA, http://www.invitrogen.com) unless otherwise stated. All animal procedures were performed in compliance with national and institutional guidelines (U.K. Animals Scientific Procedures Act 1986 and the University of Cambridge Animal Care Committees).
Isolation and Culture of Rat Neonatal Cortical NPCs
Green fluorescent protein (GFP)-expressing rat NPCs were generated from postnatal day zero (P0) to P2 Sprague-Dawley pups expressing GFP under the control of the β-actin promoter. Pups were anesthetized with ice. Brains were removed from the skull and placed in Hanks' balanced saline solution. The cortex was separated and the meninges carefully removed using a dissecting microscope. A razor blade was used to generate small pieces of cortex, which were centrifuged at 132g for 2 minutes. The supernatant was exchanged for 0.05% trypsin, and the tissue was incubated at 37°C for 20 minutes. Five milliliters of fetal calf serum and 2 ml of DNase (4 mg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) were added to inactivate the trypsin and degrade any loose DNA, prior to centrifuging at 132g for 5 minutes. The supernatant was removed, the tissue resuspended in 2–5 ml of Dulbecco's modified Eagle's medium (DMEM), and a single-cell suspension was generated through gentle trituration. After centrifuging again at 132g for 5 minutes, the cells were resuspended in NPC expansion medium, consisting of 1:1 DMEM/Ham's F-12 medium, 2% B27, 1% N2, and 1% penicillin, streptomycin, amphotericin B (PSF) supplemented with 20 ng/ml epidermal growth factor (Sigma-Aldrich), 20 ng/ml fibroblast growth factor-2 (FGF-2) (Peprotech, Rocky Hill, NJ, http://www.peprotech.com), and 5 μg/ml heparin (Sigma-Aldrich). A cell count with trypan blue exclusion was performed prior to seeding at a density of 500,000 cells per milliliter. Flasks were placed either in a 20% O2 and 5% CO2 incubator or a 3% O2 and 5% CO2 incubator, with oxygen displaced by nitrogen.
Neurospheres started to form 3–4 days after seeding. By 7 days, multiple, large spheres were present, and these primary cultures (passage 0) were passaged. A single-cell suspension was generated by incubation with Accutase (PAA Laboratories, Linz, Austria, http://www.paa.at) for 10–20 minutes, and cells were reseeded at the same density as for primary cultures. Flasks were supplemented with an equal volume of fresh medium and growth factors after 3–4 days, and further passaging was performed at weekly intervals, by dissociation to single cells. A cell count including trypan blue exclusion was performed at each passage. At each passage, NPCs were plated for differentiation on poly-d-lysine (PDL)-laminin-coated 13-mm glass coverslips at a density of 40,000–100,000 cells per coverslip. Differentiation medium consisted of DMEM, 2% B27, and 1% PSF with or without 10 ng/ml platelet-derived growth factor (PDGF) (Peprotech), 10 ng/ml FGF-2, and 5 μg/ml heparin. After 2 days, 50% of the medium was exchanged.
In Vitro Model of the Oxygen Challenge Presented by Transplantation
Passage 2 NPCs (23 days in culture) were dissociated with Accutase and plated at 40,000 cells per coverslip in 30 μl of differentiation medium, to allow adherence. After 30 minutes, 500 μl of plating medium was added. Cells were either expanded and differentiated at 20% or 3% O2 throughout, or switched from expansion at 20% O2 to differentiation at 3% O2. For live-dead staining, NPCs differentiated for 48 hours were incubated for 10 minutes on ice with 4 μM calcein and 4 μM ethidium bromide in Dulbecco's phosphate-buffered saline. Four random fields from each of three coverslips in each group were counted (on an inverted microscope), from four different cell lines.
NPC Transplants
A single-cell suspension was generated from passage 2 GFP NPCs propagated at either 3% or 20% O2 for 24 days by incubating neurospheres with Accutase for 10 minutes followed by gentle trituration. NPCs were resuspended in culture medium (without growth factors) at a density of 20,000 cells per microliter and kept on ice prior to transplantation. One microliter was injected into the hippocampus of 2-month-old adult rats (non-GFP+), using coordinates anteroposterior −3.2, mediolateral −1.0, dorsoventral −4.0; n = 5 per group. Anesthesia was provided with isoflurane, and all animals received postoperative analgesia. Following completion of surgeries, the remaining NPCs were plated on PDL-laminin-coated coverslips in NPC expansion medium plus growth factors, for 24 hours prior to fixation, in order to confirm viability and NPC identity. Forty-eight hours after transplant, animals were perfused with 4% parafomaldehyde (PFA); brains were postfixed in 4% PFA overnight, rinsed with sucrose, and stored in 30% sucrose at 4°C for at least 7 days prior to sectioning.
Tissue Processing and Immunolabeling
Tissue was processed on a cryostat. Brains were placed in OCT embedding matrix (Fisher Scientific, Loughborough, U.K., http://www.fisher.co.uk) and rapidly frozen on dry ice. Superfrost-plus charged slides (VWR International, Atlanta, GA, https://us.vwr.com) were used to collect coronal sections in a consecutive sequence at 20 μm intervals.
Slides were thawed at room temperature (RT) for 10 minutes, and a Pap pen (Dako, Glostrup, Denmark, http://www.dako.com) was used to draw a hydrophobic barrier around the sections. Several phosphate-buffered saline (PBS) rinses were performed to remove the OCT. Block (5% goat serum in 0.3% Triton-PBS) was applied for 2 hours at RT and primaries (Table 2) were added overnight at 4°C in 2% goat serum/0.3% Triton-PBS. Triton was omitted throughout for surface antigens (platelet-derived growth factor receptor-α [pdgf-rα], O4, myelin basic protein [mbp], and ng2). Following three PBS rinses, appropriate Alexa Fluor secondaries (488, 555, or 647) were applied at RT for 1.5 hours at 1:1,000 in 1% goat serum/0.3% Triton-PBS/1:4,000 Hoechst. After rinsing off the secondaries, if colabeling with intracellular antigens was required, cells were blocked in 5% horse serum/0.2% Triton and staining continued as above. Coverslips were placed with FluorSave mount (Calbiochem, Billerica, MA, http://www.merckmillipore.co.uk).
Table 2.
Details of primary antibodies used for immunocytochemistry and immunohistochemistry
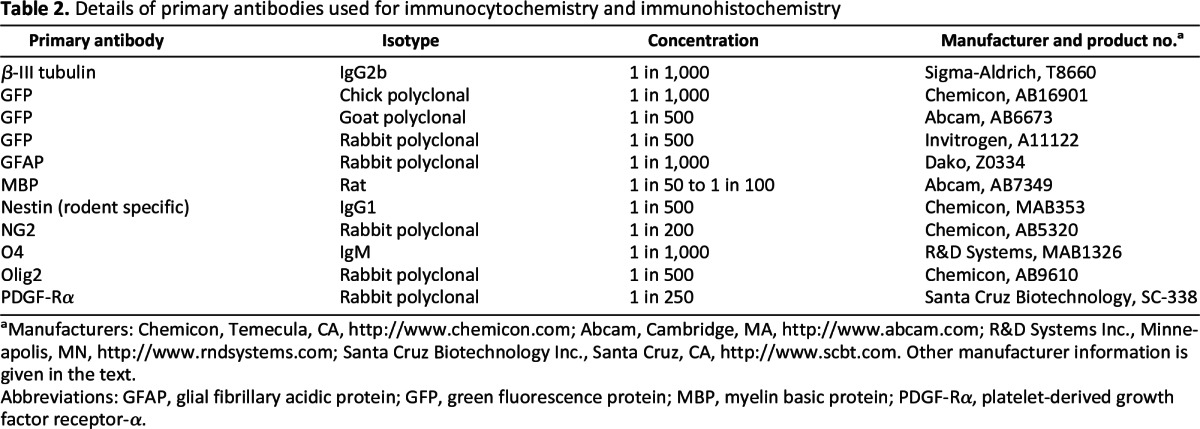
aManufacturers: Chemicon, Temecula, CA, http://www.chemicon.com; Abcam, Cambridge, MA, http://www.abcam.com; R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com. Other manufacturer information is given in the text.
Abbreviations: GFAP, glial fibrillary acidic protein; GFP, green fluorescence protein; MBP, myelin basic protein; PDGF-Rα, platelet-derived growth factor receptor-α.
Cells were fixed with 4% PFA for 15 minutes at RT and rinsed three times with PBS. Staining was performed as above, except that block, primaries, and secondaries were applied for 1 hour at RT in 0.2% Triton-PBS (Triton was again omitted for surface antigens). Negative controls were run in parallel with all immunostains, consisting of the same procedure but without the addition of primary antibody.
Microscopy
Cells and sections were viewed under a Leica (AF-6000; Heerbrugg, Switzerland, http://www.leica.com) microscope with appropriate filters for cell identification and counting. Confocal imaging, using a scanning laser confocal microscope (TCS-NT-UV; Leica), was performed to identify colabeled cells in sections. Typical stacks were composed of 10–20 optical sections of 1 μm thickness.
Quantification and Statistical Analysis
All experiments were performed a minimum of three times, unless otherwise stated. For in vitro cell counts, three coverslips (a total of 6–10 random fields at ×20 or 10–15 fields at ×40 magnification, selected with Hoechst) were analyzed for each experiment for each immunostain. Quantification of cell survival following transplantation was achieved through counting individual GFP+ cells; a one in two series of sections was analyzed for each animal, and the experimenter undertaking the cell counts was blinded to the experimental groups until all counts had been completed. Percent survival was calculated by dividing the total number of cells alive at 48 hours by the number initially transplanted (20,000 cells). The two data sets fell into a normal distribution, and therefore a Student's unpaired, two-tailed t test was used for statistical analysis, apart from the 48-hour transplant survival counts, which were analyzed using a paired, two-tailed t test. p values of ≤.05 were considered significant. Data are presented as mean ± SEM.
Results
Rat Cortical NPC Cultures Can Be Established at Both 3% and 20% O2
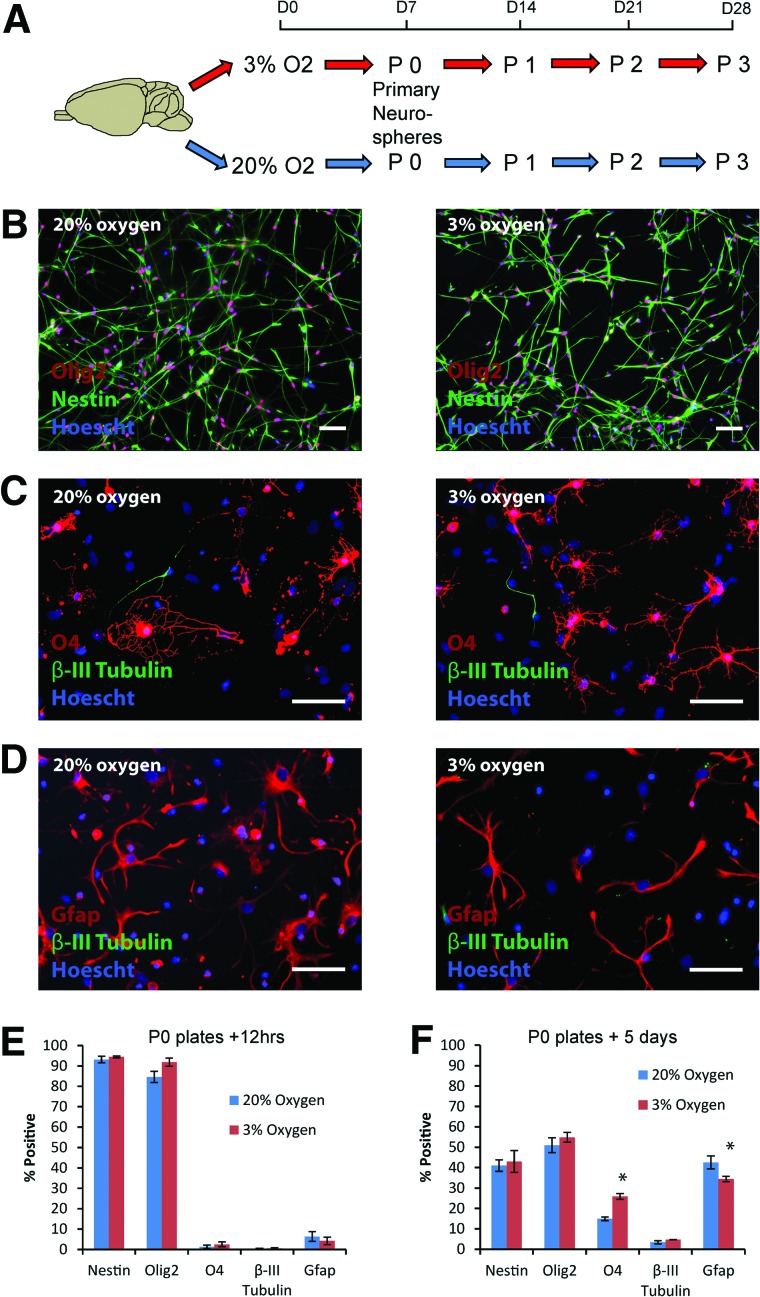
Cortical NPC cultures were obtained from P0–P2 neonatal GFP-transgenic rat pups by splitting the cells obtained from cortical dissections into two halves and establishing neurosphere cultures at 3% and 20% O2, with a seeding density of 500,000/ml (Fig. 1A). After 7 days in culture, the primary neurospheres generated in each condition were characterized. The majority of cells expressed both nestin and olig2, consistent with the reported role of olig2 in driving self-renewal in cortical NPC cultures [32], with no significant difference between the two conditions (Fig. 1B, 1E). Following differentiation for 5 days on PDL/laminin-coated glass coverslips, tripotential lineage commitment was evident in both conditions, consistent with an initial NPC identity (Fig. 1C, 1D, 1F). However, significantly more O4-positive oligodendrocytes (25.9 ± 1.4% vs. 14.9 ± 0.9%; p = .001), at the expense of glial fibrillary acidic protein (gfap)-positive astrocytes (34.4 ± 1.3% vs. 42.5 ± 3.2%; p = .048), were generated following differentiation at 3% versus 20% O2 respectively (Fig. 1F). The persistent nestin expression after 5 days of differentiation overlapped with gfap, consistent with its recognized expression by astrocytes as well as undifferentiated NPCs [33, 34].
Figure 1.
Characterization of rat cortical neural precursor cell (NPC) cultures established at 3% versus 20% oxygen. (A): Primary rat neonatal cortical neurospheres were established after 7 days in culture at either 20% or 3% O2. (B, E): Following dissociation of these passage 0 primary neurospheres to single cells, the majority of NPCs labeled with nestin and olig2, with no significant difference between the two culture conditions (n = 4–5 per group). (C, D, F): After 5 days of differentiation at either 20% or 3% O2, all three neural lineages were generated, with a significant increase in O4-positive oligodendrocytes (p = .001) at 3% compared with 20% O2, along with a concomitant decrease in gfap-positive astrocytes (p = .048). Scale bars = 50 μm. Pictures shown are from NPCs derived from green fluorescence protein (GFP)-negative littermates for ease of representation (no significant differences were observed between GFP+ and GFP− NPC cultures). Abbreviations: Gfap, glial fibrillary acidic protein; P, passage.
Longer Term Culture of Rat Cortical NPCs at 3% O2 Skews Differentiation Towards Oligodendrocytes
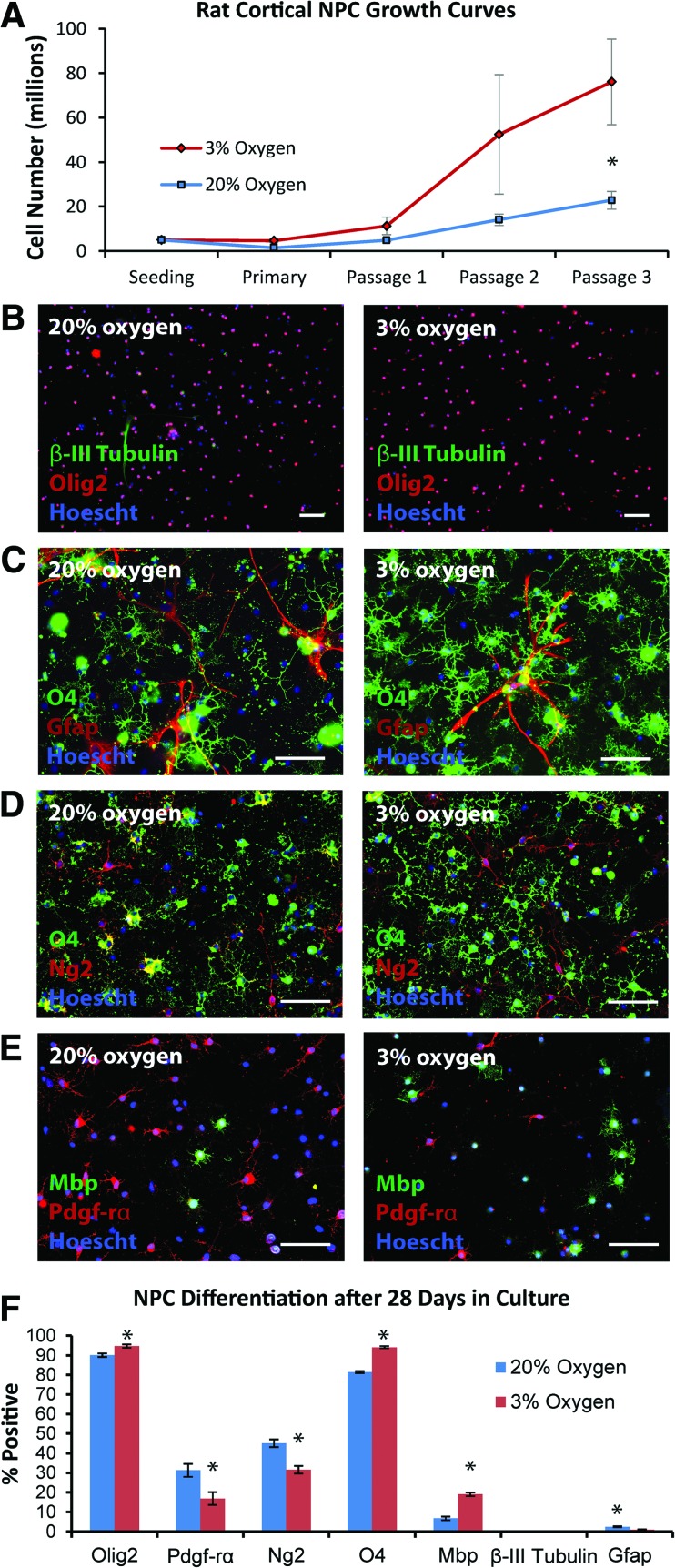
Given that rat cortical NPCs maintained at 3% O2 for 7 days had a greater propensity to differentiate into oligodendrocytes than their 20% O2 counterparts (Fig. 1F), we next investigated whether this fate difference was maintained on prolonged culture (28 days), followed by 48 hours of terminal differentiation, remaining at either 20% or 3% O2 throughout (Fig. 1A). Growth curves showed greater numbers of NPCs at 3% O2 by passage 3, on the border of statistical significance (Fig. 2A; p = .050). The vast majority of cells generated after differentiation in either condition were oligodendrocyte lineage cells, although a slightly greater percentage were olig2-positive at 3% O2 (94.7 ± 0.9%) compared with 20% O2 (90.0 ± 1.6%; p = .04) (Fig. 2B–2F). Notably, the oligodendrocyte lineage cells generated at 3% O2 were skewed towards more differentiated stages of the lineage compared with their 20% O2 counterparts, with a shift from pdgf-rα-positive precursors (16.9 ± 3.3% at low O2 vs. 31.3 ± 3.7% at high O2; p = .03) towards more mature O4 (94.1 ± 0.5% at low O2 vs. 81.4 ± 1.8% at high O2; p < .0001) and subsequently mbp-positive (19.0 ± 0.9% at low O2 vs. 6.7 ± 1.0% at high O2; p < .0001) oligodendrocytes (Fig. 2D–2F). As expected, there was some overlap between pdgf-rα/ng2 and O4 expression, but not between pdgf-rα and mbp expression (Fig. 2D, 2E). Very few β-III tubulin-expressing neurons (< 0.2%) were generated in either condition (Fig. 2B, 2F). Although only occasional gfap-positive astrocytes were observed, typically occurring in small clusters, significantly fewer were generated at 3% O2 (1.0 ± 0.2%) than at 20% O2 (2.5 ± 0.3%; p = .003) (Fig. 2C, 2F).
Figure 2.
Longer term expansion of rat cortical NPCs at 3% versus 20% O2 demonstrates increased generation of oligodendrocytes at 3% O2. (A): Rat cortical NPCs were expanded at either 3% or 20% O2, with greater NPC numbers observed by passage 3 at 3% O2 (p = .05; n = 3–4 per group). (B, C): NPCs plated for differentiation for 48 hours after 28 days in culture tended to generate oligodendrocyte lineage cells, rather than neurons or astrocytes, although significantly more O4-positive oligodendrocytes were generated at 3% O2. (D, E): These oligodendrocyte lineage cells matured more rapidly when expanded and differentiated at 3% versus 20% O2, as indicated through ng2, pdgf-rα, and mbp staining. (F): Quantification of day 28 NPC fate after 48 hours of differentiation; p values are .04 (olig2), .03 (pdgf-rα), .04 (ng2), <.0001 (O4), <.0001 (mbp), .45 (β-III tubulin), and .003 (gfap); n = 4–8 per group. Scale bars = 100 μm (B) and 50 μm (C–E). Abbreviations: Gfap, glial fibrillary acidic protein; Mbp, myelin basic protein; NPC, neural precursor cell; Pdgf-rα, platelet-derived growth factor receptor-α.
In Vitro Switch From 20% to 3% O2, Simulating Transplantation, Leads to NPC Death
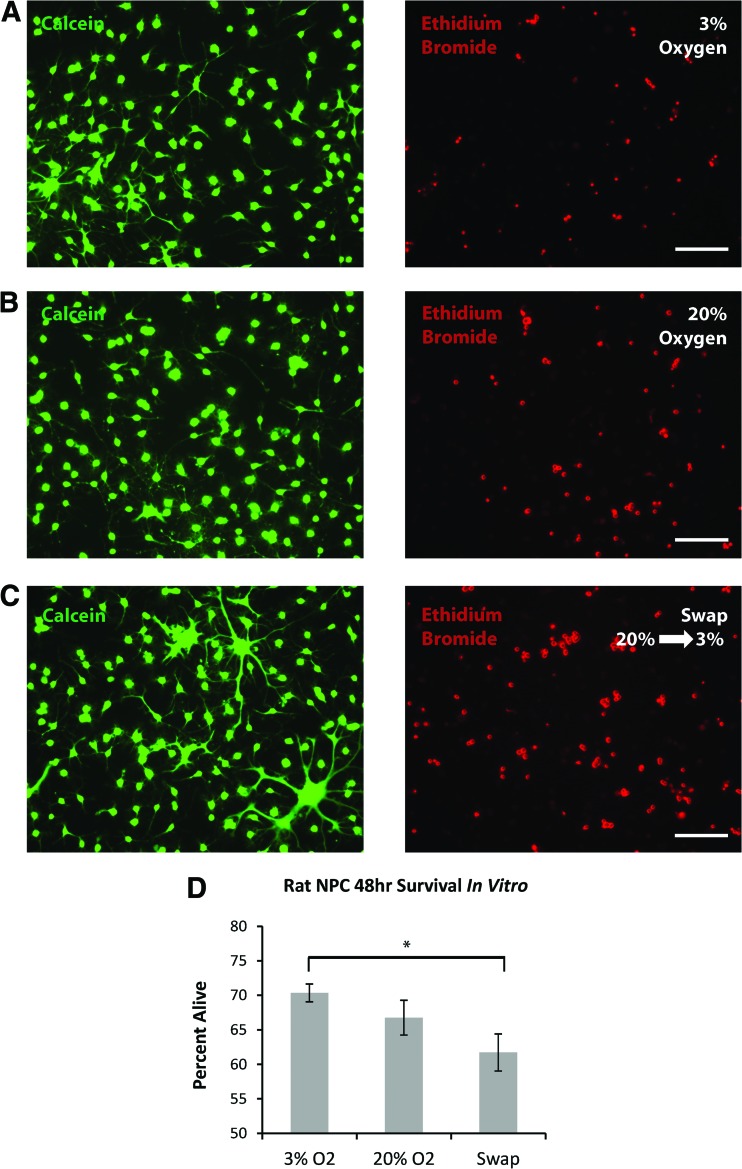
Having established that rat neonatal cortical NPCs can be propagated at either 3% or 20% O2, we investigated the effects of the oxygen stress presented by transplantation in our previously established in vitro model [16]. Passage 2 NPCs (23 days in culture) were plated for differentiation as dissociated single cells in the absence of growth factors and maintained at either 20% or 3% O2, or switched from 20% to 3% O2. Ethidium bromide (dead cell) and calcein (live cell) staining at 48 hours showed a significant survival advantage for NPCs maintained and differentiated at 3% O2 (70.3 ± 1.3% alive) compared with those expanded at 20% O2 and switched to 3% O2 (61.7 ± 2.7%) for differentiation (Fig. 3A–3D; p = .007). There was no significant difference in survival between cells expanded and differentiated at 3% versus 20% O2 throughout (p = .13).
Figure 3.
In vitro modeling of the oxygen switch that occurs during transplantation shows that switching neural precursor cells (NPCs) from 20% to 3% O2 causes significant cell death. (A–C): Representative imaging of calcein and ethidium bromide labeling of the same field of view, after 48 hours of differentiation of day 23 NPCs at 3% O2, at 20% O2, or swapped from 20% to 3% O2. (D): Quantification demonstrated a significant increase in cell death in differentiating NPCs switched from 20% to 3% O2 compared with those maintained at 3% O2 throughout (p = .007; n = 4 per group). Scale bars = 50 μm.
NPCs Cultured at 3% O2 Survive Better Than Those Cultured at 20% O2, 48 Hours After Transplantation Into the Adult Rat Hippocampus
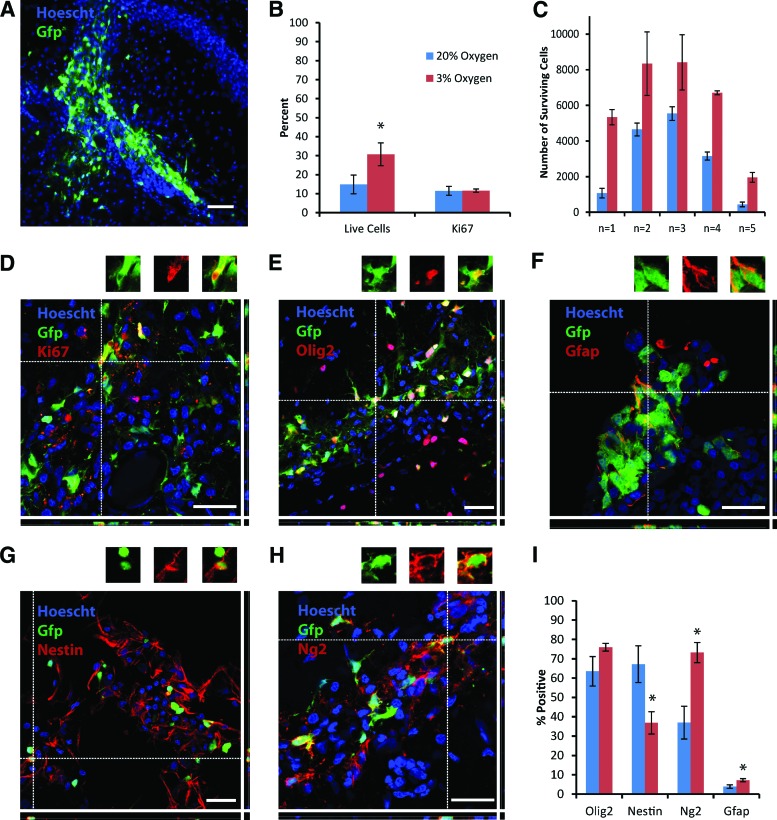
The next step was to investigate whether the beneficial effects of continuous culture at 3% O2 on NPC survival and oligodendrocyte fate in vitro would translate to the in vivo environment. Transplantation of 20,000 GFP+ passage 2 rat cortical NPCs, expanded for 24 days at either 20% or 3% O2, into the adult rat hippocampus, yielded surviving grafts in five out of five animals in each group at 48 hours (Fig. 4A). Quantification of the numbers of GFP+ cells identified a significant survival advantage in favor of the 3% O2 cells (Fig. 4B, 4C; p = .003). Overall, survival was roughly doubled from 14.8 ± 4.9% to 30.7 ± 6.0% by culturing NPCs at 3% O2. There was no difference in proliferation between the two groups, as assessed by Ki67 immunolabeling (Fig. 4B, 4D; p = .95).
Figure 4.
Transplantation of rat cortical neural precursor cells (NPCs) into the adult rat hippocampus shows a survival advantage and increased differentiation towards the oligodendrocyte lineage for NPCs expanded at 3% compared with 20% O2. (A): A typical graft of green fluorescence protein (GFP)+ rat cortical NPCs is shown at low magnification, 48 hours after transplantation. (B): A significantly greater percentage of GFP+ cells survived from the rat cortical NPCs maintained at 3% compared with 20% O2; there was no difference in proliferation between the two groups. (C): Total cell numbers from each individual animal further demonstrate the enhanced survival seen with the NPCs expanded at 3% O2. (D–H): Representative images demonstrating colabeling between GFP and either Ki67, olig2, gfap, nestin, and ng2 are shown. All images are from animals that received NPCs cultured at 3% O2; individual colabeled cells are shown above the main images. (I): Quantification of expression of olig2, nestin, ng2, and gfap by grafted GFP+ cells derived from NPCs expanded at 3% versus 20% O2. p values are .003 (live cells), .95 (Ki67), .12 (olig2), .02 (nestin), .03 (ng2), and 0.04 (gfap). Scale bars = 50 μm (A) and 25 μm (D–H). Abbreviations: Gfap, glial fibrillary acidic protein; Gfp, green fluorescent protein.
Culture at 3% O2 Affects NPC Fate Following Transplantation
Beyond effects on cell survival, and in line with the in vitro differentiation findings, rat cortical NPCs expanded at 3% O2 prior to transplantation had a greater propensity (than their 20% O2 counterparts) to differentiate into oligodendrocyte lineage cells at just 48 hours following transplantation. This is shown by their equivalent and high levels of olig2 expression (75.9 ± 2.0% at low O2 vs. 63.4 ± 7.6% at high O2; p = .12) alongside a significant decrease in nestin (36.9 ± 5.8% at low O2 vs. 67.2 ± 9.5% at high O2; p = .02) and a corresponding increase in ng2 expression (73.2 ± 5.2% at low O2 vs. 36.9 ± 8.5% at high O2; p = .03) (Fig. 4E, 4G–4I), which, taken together, indicate a shift from a neural precursor cell identity towards that of oligodendrocyte precursor cells (OPCs). Few transplanted NPCs generated gfap-positive astrocytes at this early time point, but this was more frequent in the 3% O2 group (Fig. 4F, 4I; p = .04). No β-III tubulin-positive neurons were observed in either group.
Discussion
The results presented here show that culture of rat cortical NPCs in a more physiologically relevant, 3% O2 environment affects cell survival and fate, both in vitro and following transplantation into the adult rat hippocampus, with particular effects on the oligodendrocyte lineage.
Using an in vitro model of the oxygen challenge that occurs in routine transplantation studies, we found that switching differentiating NPCs from 20% to 3% O2 caused significant cell death that was reduced by culture at 3% O2 throughout. This is consistent with our previous report of the protective effect of continuous culture at 3% O2, using the same in vitro model but human embryonic stem (ES)-derived NPCs [16]. The significant stress caused by such changes in pO2 has previously been used in in vitro systems designed to model stroke and oxidative stress, misleadingly drawing comparisons to a base line of 20% O2, representing hyperoxic conditions. Thus, oxygen-glucose deprivation models involve the transfer of cultures from a 20% to 0.5%–3% O2 environment [35–37], whereas other systems rely on the generation of ROS through the addition of agents such as hydrogen peroxide to cells cultured in a hyperoxic, 20% O2 environment [38–40]. Overall, the impact of physiological normoxia (1%–5% O2) in neuronal stress assays has been largely overlooked [19, 39], which is likely to be a particularly relevant consideration when modeling conditions such as Alzheimer's, motor neuron, and Parkinson's diseases, where oxidative stress has been implicated in neuronal injury [41, 42]. This may be particularly pertinent to the growing use of ES cells and induced pluripotent stem cells carrying disease-specific mutations to model these conditions [43].
Translating this observation to the in vivo environment, our data show that expansion of rat cortical NPCs at 3% O2 rather than standard 20% O2 culture conditions actually doubles survival in the immediate post-transplantation period, from 14.8 ± 4.9% to 30.7 ± 6.0%. At 48 hours after transplantation, proliferation would not be expected to account for this difference, and the lack of a significant difference in Ki67 labeling between the two transplanted populations indicates that the greater cell numbers found in the immediate post-transplantation phase of NPCs cultured at 3% O2 compared with 20% O2 reflects improved survival rather than increased proliferation. These advantageous effects of culture at low oxygen prior to transplantation into a nonischemic lesion are in agreement with a study based on human cardiac stem cells [12], and other reports of the beneficial effects of “hypoxic-ischemic preconditioning” on human bone marrow and adipose tissue-derived mesenchymal stem cells (MSCs) [11, 13], mouse bone marrow-derived MSCs [9, 14], and mouse ES-derived NPCs [10], prior to transplantation into ischemic models.
In addition to effects on survival, culture of rat cortical NPCs at 3% O2 affects cell fate, both in vitro and following transplantation into the adult rat brain, with more oligodendrocytes generated in vitro and OPCs in vivo from NPCs cultured at 3% rather than 20% O2. The particular effect of low, physiological oxygen in promoting the oligodendrocyte fate from NPCs is supported by previous in vitro studies in humans and mice. Expansion of human neonatal NPCs (one line) at 5% rather than 20% O2 resulted in a 17-fold increase in the numbers of oligodendrocytes generated, alongside a significant decrease in production of gfap-expressing astrocytes at 5% O2 [21]. Furthermore, mouse cortical NPCs from embryonic day 13.5 embryos expanded at 2%, 5%, or 20% O2 generated virtually no O4+ oligodendrocytes when expanded and differentiated at 20% O2, compared with approximately 5% at 5% O2 and 18% at 2% O2 [20]. The proposed mechanism for this difference was a particular susceptibility of multipotent stem cells, including OPCs, to apoptosis at 20% O2, compared with committed neuronal progenitors.
The shift from pdgf-rα+ OPCs towards more mature mbp+ oligodendrocytes following in vitro differentiation at 3% O2 is consistent with the single report that has looked at the effects of low (1%) versus high (20%) O2 on the behavior of rat OPCs in vitro [22]. That study found that maturation was significantly more advanced in OPCs differentiated at 1% O2, as indicated by relative expressions of mbp and pdgf-rα after 7 days of differentiation (although there was no significant difference after 48 hours). It should be noted that in those experiments, OPCs were cultured up until the point of shake-off at 20% O2, thereby introducing two O2 shifts: first from the brain into the 20% O2 culture environment, and, after adapting to those conditions for 8–9 days, second from the 20% O2 to 1% O2 culture environment. This contrasts with the rat cortical NPC cultures described here, where NPCs were expanded at either 3% or 20% O2 immediately following preparation of the initial cell suspension. Despite these differences, the results of the two studies are in good agreement and suggest that further experiments investigating the effects of culture at low, physiologically relevant oxygen tensions prior to transplantation of OPCs into rodent models of demyelinating disease are warranted.
Conclusion
Overall, it is clear that oxygen in the cell culture environment is an important consideration when preparing cells for transplantation, both in terms of survival and fate, and represents one of several variables that should be considered when optimizing cell transplantation techniques. This finding is likely to be broadly relevant across the field of cell-based therapies.
Acknowledgments
We are grateful to Xia Chen and Joe Herbert for providing the GFP+ rats. This work was supported by the Multiple Sclerosis Society UK, the Evelyn Trust, the Medical Research Council, and the National Institute for Health Research (Cambridge Biomedical Research Centre). S.R.L.S. was supported by a Sir David Walker Fellowship, a joint Medical Research Council and Multiple Sclerosis Society Clinical Research Training Fellowship (no. G0800487), and a Raymond and Beverly Sackler Studentship.
Author Contributions
S.R.L.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; D.J.W.: collection and assembly of data; data analysis and interpretation; B.B.: conception and design; D.A.S.C. (A.C.): manuscript writing; S.C. and R.J.M.F.: conception and design, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Smith HK, Gavins FN. The potential of stem cell therapy for stroke: Is PISCES the sign? FASEB J. 2012;26:2239–2252. doi: 10.1096/fj.11-195719. [DOI] [PubMed] [Google Scholar]
- 2.Martino G, Franklin RJM, Baron Van Evercooren A, et al. Stem cell transplantation in multiple sclerosis: Current status and future prospects. Nat Rev Neurol. 2010;6:247–255. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 3.Cova L, Ratti A, Volta M, et al. Stem cell therapy for neurodegenerative diseases: The issue of transdifferentiation. Stem Cells Dev. 2004;13:121–131. doi: 10.1089/154732804773099326. [DOI] [PubMed] [Google Scholar]
- 4.Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson's disease: Problems and possibilities. Prog Brain Res. 2010;184:265–294. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- 5.Brundin P, Karlsson J, Emgard M, et al. Improving the survival of grafted dopaminergic neurons: A review over current approaches. Cell Transplant. 2000;9:179–195. doi: 10.1177/096368970000900205. [DOI] [PubMed] [Google Scholar]
- 6.Zawada WM, Zastrow DJ, Clarkson ED, et al. Growth factors improve immediate survival of embryonic dopamine neurons after transplantation into rats. Brain Res. 1998;786:96–103. doi: 10.1016/s0006-8993(97)01408-x. [DOI] [PubMed] [Google Scholar]
- 7.Barker RA, Dunnett SB, Faissner A, et al. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 8.Schierle GS, Karlsson J, Brundin P. MK-801 does not enhance dopaminergic cell survival in embryonic nigral grafts. Neuroreport. 1998;9:1313–1316. doi: 10.1097/00001756-199805110-00011. [DOI] [PubMed] [Google Scholar]
- 9.Leroux L, Descamps B, Tojais NF, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther. 2010;18:1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 11.Oh JS, Ha Y, An SS, et al. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010;472:215–219. doi: 10.1016/j.neulet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Li TS, Cheng K, Malliaras K, et al. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc Res. 2011;89:157–165. doi: 10.1093/cvr/cvq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Yu SP, Fraser JL, et al. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 15.Silver I, Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv Exp Med Biol. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- 16.Stacpoole SR, Bilican B, Webber DJ, et al. Derivation of neural precursor cells from human ES cells at 3% O(2) is efficient, enhances survival and presents no barrier to regional specification and functional differentiation. Cell Death Differ. 2011;18:1016–1023. doi: 10.1038/cdd.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studer L, Csete M, Lee SH, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Csete M, Groves AK, et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csete M. Oxygen in the cultivation of stem cells. Ann NY Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 20.Chen HL, Pistollato F, Hoeppner DJ, et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- 21.Pistollato F, Chen HL, Schwartz PH, et al. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci. 2007;35:424–435. doi: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Akundi RS, Rivkees SA. Hypoxia alters cell cycle regulatory protein expression and induces premature maturation of oligodendrocyte precursor cells. PLoS One. 2009;4:e4739. doi: 10.1371/journal.pone.0004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida Y, Takahashi K, Okita K, et al. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Li TS, Marbán E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28:1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santilli G, Lamorte G, Carlessi L, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimadamore F, Curchoe CL, Alderson N, et al. Nicotinamide rescues human embryonic stem cell-derived neuroectoderm from parthanatic cell death. Stem Cells. 2009;27:1772–1781. doi: 10.1002/stem.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke L, van der Kooy D. Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells. 2009;27:1879–1886. doi: 10.1002/stem.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakata H, Niizuma K, Yoshioka H, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adam AA, Takahashi Y, Katagiri S, et al. Effects of oxygen tension in the gas atmosphere during in vitro maturation, in vitro fertilization and in vitro culture on the efficiency of in vitro production of mouse embryos. Jpn J Vet Res. 2004;52:77–84. [PubMed] [Google Scholar]
- 30.Meintjes M, Chantilis SJ, Douglas JD, et al. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum Reprod. 2009;24:300–307. doi: 10.1093/humrep/den368. [DOI] [PubMed] [Google Scholar]
- 31.Bontekoe S, Mantikou E, van Wely M, et al. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst Rev. 2012;7:CD008950. doi: 10.1002/14651858.CD008950.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Hack MA, Sugimori M, Lundberg C, et al. Regionalization and fate specification in neurospheres: The role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 34.Eliasson C, Sahlgren C, Berthold CH, et al. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999;274:23996–24006. doi: 10.1074/jbc.274.34.23996. [DOI] [PubMed] [Google Scholar]
- 35.Cimarosti H, Henley JM. Investigating the mechanisms underlying neuronal death in ischemia using in vitro oxygen-glucose deprivation: Potential involvement of protein SUMOylation. Neuroscientist. 2008;14:626–636. doi: 10.1177/1073858408322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mei JM, Chi WM, Trump BF, et al. Involvement of nitric oxide in the deregulation of cytosolic calcium in cerebellar neurons during combined glucose-oxygen deprivation. Mol Chem Neuropathol. 1996;27:155–166. doi: 10.1007/BF02815091. [DOI] [PubMed] [Google Scholar]
- 38.Bell KF, Al-Mubarak B, Fowler JH, et al. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci USA. 2011;108:E1–E2. doi: 10.1073/pnas.1015229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: A new paradigm for oxidative stress. IUBMB Life. 1999;48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 40.Oliver PL, Finelli MJ, Edwards B, et al. Oxr1 is essential for protection against oxidative stress-induced neurodegeneration. PLoS Genet. 2011;7:e1002338. doi: 10.1371/journal.pgen.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behl C, Moosmann B. Oxidative nerve cell death in Alzheimer's disease and stroke: Antioxidants as neuroprotective compounds. Biol Chem. 2002;383:521–536. doi: 10.1515/BC.2002.053. [DOI] [PubMed] [Google Scholar]
- 42.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: A molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 43.Stacpoole SR, Bilican B, Webber DJ, et al. Efficient derivation of NPCs, spinal motor neurons and midbrain dopaminergic neurons from hESCs at 3% oxygen. Nat Protoc. 2011;6:1229–1240. doi: 10.1038/nprot.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]