This study describes a straightforward one-step protocol for differentiating human induced pluripotent stem cells (hiPSCs) into dopamine neurons based on the overexpression of the three cell lineage-specific transcription factors ASCL1, NURR1, and LMX1A. This protocol was applied to hiPSCs derived from fetal and Parkinson's disease patient adult fibroblasts, and both cases resulted in a high number of TH+ neurons, which showed dopaminergic and midbrain markers after 2 weeks and robust functional properties from 21 days.
Keywords: Reprogramming, Direct cell conversion, Pluripotent stem cells, Neuron, Dopamine
Abstract
Current protocols for in vitro differentiation of human induced pluripotent stem cells (hiPSCs) to generate dopamine (DA) neurons are laborious and time-expensive. In order to accelerate the overall process, we have established a fast protocol by expressing the developmental transcription factors ASCL1, NURR1, and LMX1A. With this method, we were able to generate mature and functional dopaminergic neurons in as few as 21 days, skipping all the intermediate steps for inducting and selecting embryoid bodies and rosette-neural precursors. Strikingly, the resulting neuronal conversion process was very proficient, with an overall efficiency that was more than 93% of all the coinfected cells. hiPSC-derived DA neurons expressed all the critical molecular markers of the DA molecular machinery and exhibited sophisticated functional features including spontaneous electrical activity and dopamine release. This one-step protocol holds important implications for in vitro disease modeling and is particularly amenable for exploitation in high-throughput screening protocols.
Introduction
Dopamine (DA) neurons are key regulators of emotional behavior and motor coordination in vertebrates. In humans, the degeneration of the DA neurons of the midbrain substantia nigra (A9) leads to Parkinson's disease (PD), which is the second most common neurodegenerative disease [1]. Great efforts are in place to understand the molecular mechanisms that trigger PD, taking advantage of human genetics [2] and animal modeling [3, 4]. However, a better comprehension of PD pathological mechanisms would certainly be accelerated if a renewable culture system of human DA neurons were accessible. With this aim, in the past decades immortalized cell lines were used [5], but they are far from replicating DA neurons' physiological properties. Primary cultures from aborted fetuses are not readily available, and the fraction of DA neurons in these cell preparations is very low [6]. In this scenario, human induced pluripotent stem cells (hiPSCs) [7, 8] provided an unprecedented tool for a straightforward and renewable source of human neurons from healthy and diseased donors [9]. Importantly, recent studies have demonstrated how hiPSC-derived DA neurons from PD patients could be advantageous in modeling disease-specific molecular mechanisms [10–12].
In order to obtain human embryonic stem cell (hESC)- and hiPSC-derived DA neurons, several differentiation protocols have been described that are based on embryoid body formation [13], coculture with feeder stromal cells [14], or neurosphere generation [15]. Moreover, genetic strategies, such as transcription factor (TF) overexpression, have been developed to further enhance the efficiency of hESC and hiPSC differentiation into DA neurons [16, 17]. However, all these protocols require several intermediate steps and many different cell culture conditions [10, 15, 18, 19]. Moreover, these protocols are extremely laborious and time-consuming, in particular when hiPSC-derived DA neurons are expected to acquire evident functional properties.
We previously reported that mouse and human fibroblasts can be directly reprogrammed into dopaminergic-like neuronal cells by the combined expression of the three cell lineage TFs ASCL1, NURR1 (NCBI: NR4A2), and LMX1A (ANL). Forced expression of these TFs is sufficient to convert fibroblasts into functional DA neurons without passing through a stage of pluripotent or neuronal stem cells [20, 21]. Although mouse fibroblasts can be reprogrammed at high efficiency (15% final yield) and in a short time window (2 weeks), human fibroblasts are significantly more resistant to undergoing neuronal reprogramming (<4% final yield).
Here, we asked whether a similar strategy could be applied to hiPSCs and compared its outcome with that obtained with somatic cells. Therefore, we describe a straightforward one-step protocol for differentiating hiPSCs into DA neurons based on the overexpression of the three cell lineage-specific TFs ASCL1, NURR1, and LMX1A (ANL) [20]. We have applied this protocol to hiPSCs derived from fetal (IMR90) and PD patient adult fibroblasts, and in both cases we obtained a high number of tyrosine hydroxylase-positive (TH+) neurons, which showed dopaminergic and midbrain markers after 2 weeks and robust functional properties from 21 days.
Materials and Methods
See supplemental online data for details.
Results
Efficient Differentiation of hiPSCs Into DA Neurons by Forcing Expression of ASCL1, NURR1, and LMX1A
hiPSCs were generated (with 0.01% efficiency) by reprogramming IMR90 fetal fibroblasts by retroviral-mediated overexpression of SOX2, OCT4, and KLF4 according to the protocol developed by Yamanaka and colleagues [7, 8] (without using MYC) and tested for pluripotency by morphological analysis (supplemental online Fig. 1A, 1B) and pluripotency marker expression (alkaline phosphatase, NANOG, SOX-2, OCT 3/4, TRA-1–60, and SSEA-4) (supplemental online Fig. 1C–1H; supplemental online Tables 2, 3). To functionally test pluripotency in vitro, hiPSCs were validated for stemness markers and their multilineage differentiation ability (mesoderm, ectoderm, and endoderm) (supplemental online Fig. 1I–1L).
Thus, we optimized infections of hiPSCs with lentiviruses expressing the three reprogramming TFs and the doxycycline-inducible reverse tetracycline transactivator. hiPSCs were pretreated with ROCK inhibitor [22], selectively harvested from feeder cells by collagenase treatment, and dissociated to single cells by incubation with Accutase (Life Technologies, Rockville, MD, http://www.lifetech.com). hiPSCs were then infected in suspension with the lentiviral cocktail for 4 hours, washed, and plated on either Matrigel-coated glass or feeder layers for short- and long-term analysis, respectively (Fig. 1A). In these conditions, a single viral infection transduced approximately 85% of the entire cell population, whereas coinfection with all four viruses was estimated to be 55% (data not shown). Infected hiPSCs were then maintained in neuronal inducing medium for the subsequent differentiation process until the final analysis without any further manipulation. Control hiPSCs were disaggregated into single cells infected with either scrambled or green fluorescent protein (GFP)-expressing lentiviruses and then cultured in conditions identical to those of infected cells.
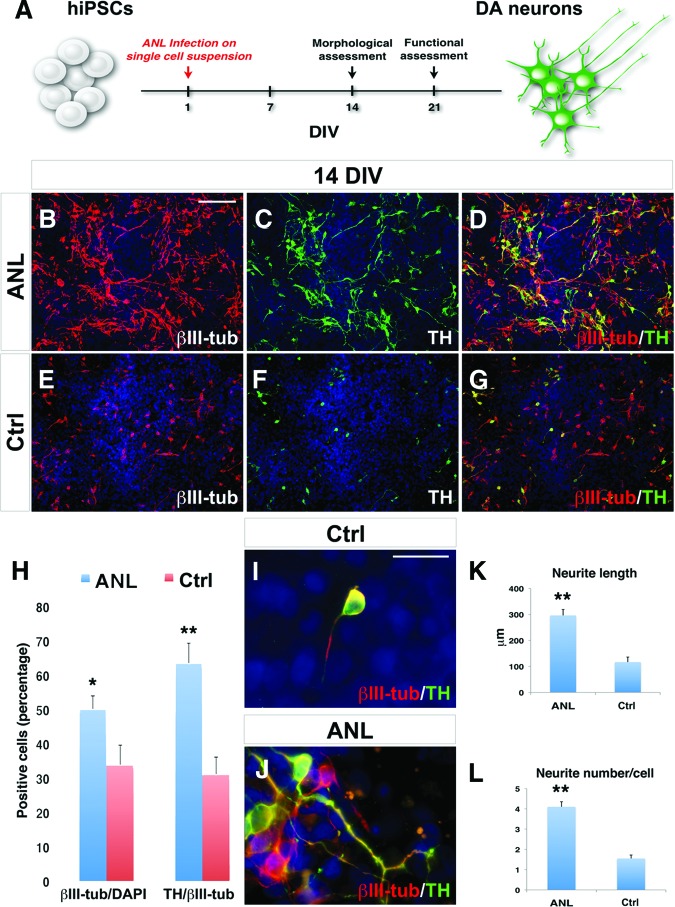
Figure 1.
Differentiation of IMR90-hiPSCs into DA neurons after ANL overexpression. (A): Schematic representation of the one-step differentiation protocol. DA neurons are characterized after 14 DIV from viral transduction. (B–G): Immunocytochemical analysis for TH and βIII-tubulin in ANL-infected (B–D) and Ctrl (E–G) IMR90-hiPSC-derived neurons. (H): Quantification of TH+/βIII-tubulin+ and βIII-tubulin+/4′,6-diamidino-2-phenylindole (DAPI) yield. (I, J): High magnification of βIII-tubulin/TH immunostaining highlights a more differentiated morphology in IMR90-hiPSC-derived neurons infected with ANL (J) when compared with control (I). (K, L): Comparison of neurite length and number between ANL and control conditions. The nuclei are stained with DAPI. Scale bars = 100 μm (B–G) and 20 μm (I, J). Student's t test was used. *, p < .05; **, p < .01. Abbreviations: ANL, ASCL1, NURR1, and LMX1A; Ctrl, control; DA, dopamine; DIV, days in vitro; hiPSCs, human induced pluripotent stem cells; TH, tyrosine hydroxylase; βIII-tub, βIII-tubulin.
After 2 weeks in neuronal inducing medium supplemented with doxycycline, 51 ± 4% of all the ANL-infected hiPSCs (ANL-hiPSCs) were differentiated into β-III-tubulin (βIII-tub)+ neurons, among which 65 ± 5% expressed the catecholaminergic marker TH (Fig. 1B–1D, 1H). In the control condition, the number of βIII-tub+ neurons was 32 ± 4%, and the βIII-tub+/TH+ ratio was 30 ± 3% (Fig. 1E–1H). In addition, ANL viral transduction elicited a robust effect on cell morphology. In fact, ANL-infected neurons developed multiple neurites with complex branched morphology, whereas the majority of control neurons displayed an unipolar morphology (Fig. 1I–1L). hiPSCs transduced with each single TF showed a reduced differentiation efficiency and limited mature morphology, indicating that the three factors have a strong synergic effect (supplemental online Fig. 2A–2F).
Molecular and Functional Characterization of IMR90-hiPSC-Derived DA Neurons
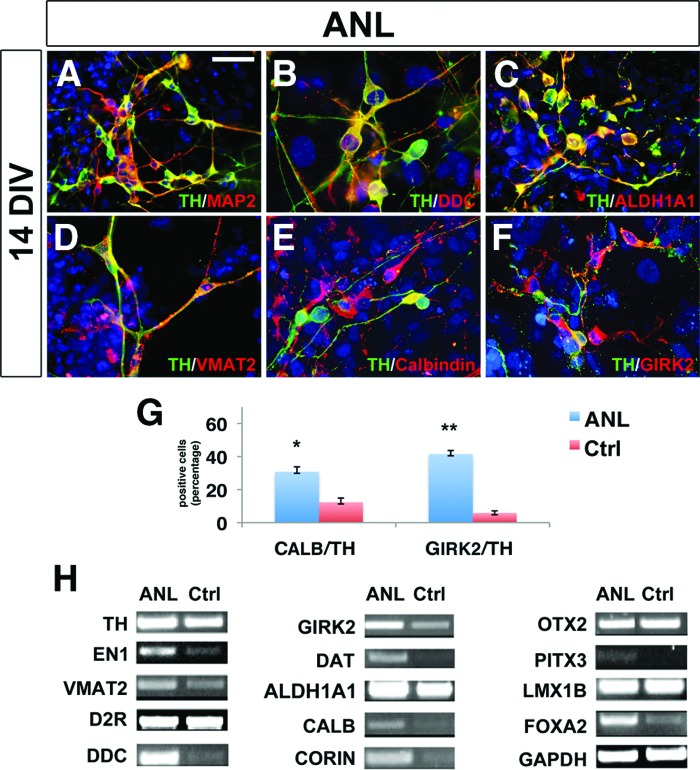
In order to characterize the subtype neuronal identity, ANL-hiPSC-derived neurons were analyzed for the expression of molecular markers, electrophysiological properties, and dopamine content. After 2 weeks of differentiation, ANL-hiPSC-derived neurons coexpressed TH together with other well-known dopaminergic markers, such as MAP2, DDC, ALDH1A1, VMAT2 (NCBI: SLC18A2), calbindin, and GIRK2 (Fig. 2A–2F), and expressed the DA transporter (DAT; NCBI: SLC6A3) and the DA receptor type 2 (D2R; NCBI: DRD2) (Fig. 2H). Moreover, expression of midbrain regional markers as FOXA2, PITX3, and CORIN was detected, suggesting that at least a fraction of ANL-hiPSC-derived neurons have acquired a specific midbrain-regional code (Fig. 2H). This was confirmed by neurons coexpressing TH/GIRK2 as shown by substantia nigra DA neurons (Fig. 2F, 2G). Interestingly, ANL-hiPSC-derived TH+ neurons presented synaptotagmin- and synapsin-positive puncta along the neurites, indicating the formation of bona fide DA presynaptic contacts (Fig. 3A–3C and data not shown). Moreover, we also evaluated the expression of the neural precursor marker nestin together with TH along the differentiation of ANL-hiPSC-derived neurons in order to assess the presence of neuronal precursors in our cultures. As shown by immunocytochemical analysis (supplemental online Fig. 3A, 3B), nestin and TH never colocalize, but nestin-positive precursors are still present after 21 days of differentiation (supplemental online Fig. 3C).
Figure 2.
IMR90-human induced pluripotent stem cell (hiPSC)-derived dopamine (DA) neurons express dopaminergic and midbrain markers after 14 days of differentiation. (A–F): Immunocytochemical analysis of IMR90-hiPSC-derived DA shows coexpression TH with MAP2 (A), DDC (B), ALDH1A1 (C), VMAT2 (D), calbindin (E), and GIRK2 (F). (G): Quantification of calbindin+/TH+ and GIRK2+/TH+ yield in ANL-infected and Ctrl cells. (H): Transcriptional characterization of dopaminergic and midbrain markers in ANL-infected and control IMR90 hiPSC-derived DA neurons. The nuclei are stained with 4′,6-diamidino-2-phenylindole. Scale bar = 40 μm. Student's t test was used. *, p < .05; **, p < .01. Abbreviations: ANL, ASCL1, NURR1, and LMX1A; Ctrl, control; DIV, days in vitro; TH, tyrosine hydroxylase.
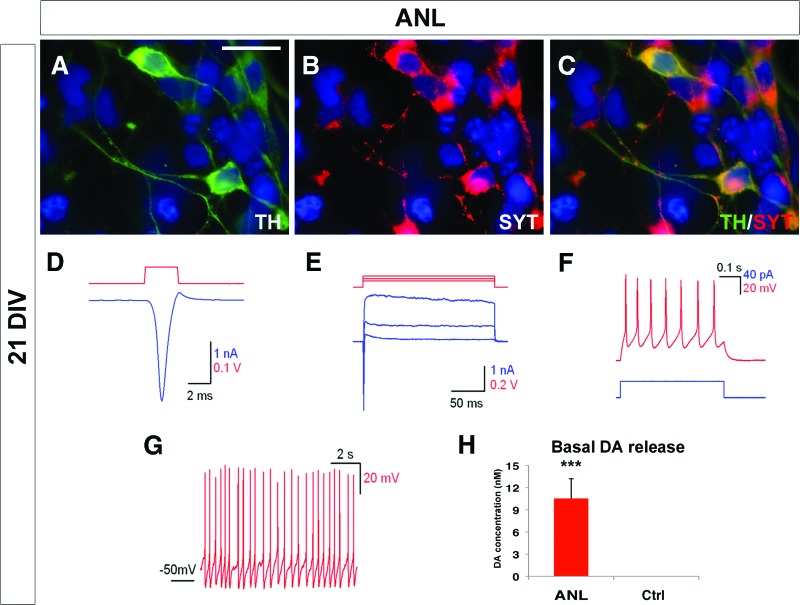
Figure 3.
Functional characterization of IMR90-human induced pluripotent stem cell (hiPSC)-derived DA neurons after 21 days of differentiation. (A–C): Immunocytochemical analysis shows that ANL-infected IMR90-hiPSC-derived DA neurons coexpress TH and SYT. (D–G): These cells share electrophysiological activity such as incoming Na+ (D) and outgoing K+ (E) ionic currents, multiple evoked potential (F), and spontaneous pace-making activity (G). (H): Dopamine content released in the cell culture medium in basal conditions. The nuclei are stained with 4′,6-diamidino-2-phenylindole. Scale bar = 20 μm. Student's t test was used. ***, p < .001. Abbreviations: ANL, ASCL1, NURR1, and LMX1A; Ctrl, control; DA, dopamine; DIV, days in vitro; SYT, synaptotagmin-I; TH, tyrosine hydroxylase.
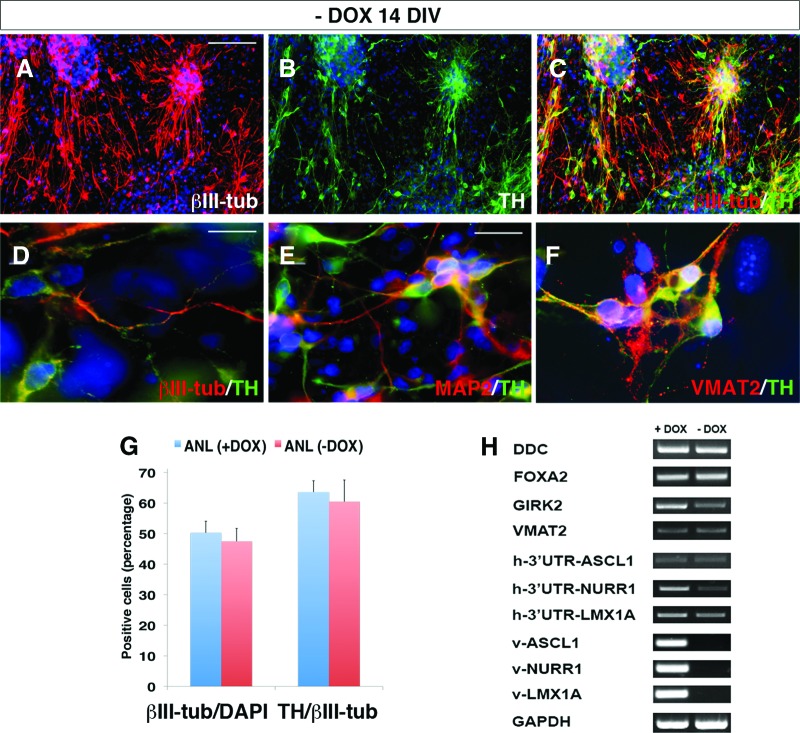
At the functional level, in voltage-clamp recordings ANL-hiPSC-derived DA neurons revealed prominent inward and outward currents, which according to their temporal profiles appeared as Na+ and K+ currents and were able to discharge a train of action potentials after current stimulation (Fig. 3D–3F; supplemental online Table 1). Importantly, approximately 50% of the hiPSC-derived neurons exhibited regular spontaneous discharges as typical for DA neurons (Fig. 3G; supplemental online Table 1). In addition, at the same differentiation stage, these neurons were able to produce and release DA in the culture medium even without any previous depolarizing treatment (Fig. 3H). Control hiPSC-derived neuron neither exhibited spontaneous neuronal firing nor released measurable DA levels in the culture medium (data not shown and Fig. 3H). To test the inherent stability of the reprogrammed neuronal state, ANL-hiPSC-derived neurons were studied after removal of doxycycline for 2 weeks. In these conditions, the rate of differentiated TH+/βIII-tub+ neurons remained unchanged, and ANL-hiPSC-derived neuronal progeny preserved the expression of MAP2 and VMAT2 (Fig. 4A–4G). Importantly, expression of the endogenous 3′-untranslated region of NURR1, LMX1A, and ASCL1 genes was maintained, whereas the exogenous viral genes were shut down after doxycycline withdrawal, as revealed by transcriptional analysis (Fig. 4H).
Figure 4.
IMR90-human induced pluripotent stem cell (hiPSC)-derived DA neurons show a stable phenotype after doxycycline withdrawal. IMR90-hiPSCs were infected with ANL viral cocktail, and then DOX was added for the first 6 days of differentiation and withdrawn for other 14 DIV. (A–D): Immunocytochemical analysis shows that 14 days after DOX withdrawal, IMR90-hiPSC-derived DA neurons stably coexpress βIII-tubulin and TH (A–C), sharing differentiated morphology (D). (E, F): DA neurons obtained after 2 weeks of DOX withdrawal also express MAP2 (E) and VMAT2 (F). (G): Quantification of TH/βIII-tubulin and βIII-tubulin/DAPI yield in cells kept with DOX (ANL (+DOX)) and 14 DIV after DOX withdrawal (ANL (−DOX)). (H): Transcriptional characterization of DA neurons kept with DOX (+DOX) or after 14 DIV of DOX withdrawal (−DOX). h-3′UTR-ASCL1, h-3′UTR-NURR1, and h-3′UTR-LMX1A indicate the human endogenous gene expression, whereas v-ASCL1, v-NURR1, and v-LMX1A indicate the expression of the lentiviral vectors. The cell nuclei are stained with DAPI. Scale bars = 100 μm (A–C), 40 μm (E), and 20 μm (D, F). Abbreviations: ANL, ASCL1, NURR1, and LMX1A; DAPI, 4′,6-diamidino-2-phenylindole; DA, dopamine; DIV, days in vitro; DOX, doxycycline; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TH, tyrosine hydroxylase; βIII-tub, βIII-tubulin; UTR, untranslated region.
We next decided to test the in vivo integration ability of these cells. With this aim, we transplanted GFP+ ANL-hiPSC-derived DA neurons into P1 mice brain (n = 6). Interestingly, 12 days after transplantation, ANL-hiPSC-derived DA neurons were found integrated into four out of six mice brains, and a fraction of them displayed a neuronal-like morphology and TH expression (supplemental online Fig. 4A–4F).
Efficient Differentiation of hiPSCs Derived From Adult PD Patient Fibroblasts Into DA Neurons Through Overexpression of ANL
To expand the significance of this protocol, we applied it to differentiate hiPSCs derived (efficiency 0.008%) from fibroblasts of a PD patient with α-synuclein (SNCA) gene duplication (αSYN-dup-hiPSCs). After ascertainment of the pluripotent state (supplemental online Fig. 5A–5I), αSYN-dup-hiPSCs were differentiated with or without ANL transduction, and neuronal progeny were analyzed 14 and 21 days later. Similar to previous results, a high fraction of ANL-hiPSC-derived neurons were βIII-tub/TH double-positive (48 ± 4% βIII-tub+/4′,6-diamidino-2-phenylindole-positive [DAPI+] neurons; 26 ± 3% TH+/ βIII-tub+/DAPI+; supplemental online Fig. 6A–6G), showing a differentiated morphology as compared with control cultures (supplemental online Fig. 6H, 6I). In addition, most of the TH+ neuronal progeny also coexpressed dopaminergic markers such as DDC, calbindin, and GIRK2 (supplemental online Fig. 7A–7D). Finally, αSYN-dup-hiPSC-derived DA neurons showed Na+ and K+ currents, spontaneous and evoked firing of action potentials, and basal release of DA (supplemental online Fig. 7E–7I). In contrast, control hiPSC-derived neurons underwent a limited differentiation and failed to display active membrane properties and DA production and release (supplemental online Fig. 7I and data not shown).
Discussion
The aim of this study was to develop a fast and straightforward protocol for generating a large number of functional dopaminergic neurons starting from any hiPSC line. In fact, several protocols have been already reported for generating such a neuronal subtype, but all these methods necessitate several laborious and inefficient differentiation steps requiring numerous weeks of in vitro culturing before differentiated dopaminergic neurons might exhibit functional properties. With this aim, we have here reported an extremely efficient procedure for the in vitro generation of hiPSC-derived DA neurons that relies on a unique technical step consisting of the infection of naïve hiPSCs with the ANL-expressing viral cocktail. Through the overexpression of the three developmental TFs, approximately 93% of all the coinfected hiPSC population is forced to differentiate into postmitotic DA neurons.
The easiness and reliability of this method makes it particularly amenable for applications in high-throughput screening mode. Indeed, with this protocol it would be feasible to produce a large number of functional human DA neurons suitable for rapid pharmacological and toxicity tests.
The procedure herein described allows for detecting dopaminergic functional activity after only 3 weeks of in vitro differentiation, and this was possible only when all three (ANL) TFs were equally expressed. Indeed, control cells or cells infected with a single or any pairwise gene-viral combination never acquired electrical activity and DA release capability at the same differentiation time point (data not shown).
In this study, we proved that this protocol is robust enough to be applied to different hiPSC lines producing similar levels of DA neuronal production. We initially used hiPSCs derived from IMR90, fetal fibroblasts that differentiated into DA neurons after ANL overexpression in high amounts. Indeed, after 2 and 3 weeks of in vitro differentiation, more than 60% of the neurons showed, respectively, dopaminergic marker expression and functional properties. These hiPSC-derived DA neurons were able to release DA in the medium without requiring any deporalizing stimulus and were able to integrate in vivo after grafting into newborn mouse brains.
Subsequently, in order to prove this protocol suitable for adult fibroblast-derived hiPSCs, we overexpressed ANL in hiPSCs derived from PD patient adult fibroblasts harboring a SNCA gene duplication. These experiments proved this protocol to be able also in this setting to generate a high number of dopaminergic neurons that become functional after 21 days of in vitro cell differentiation. These results provide clear evidence that this protocol represents a valuable method even for in vitro modeling of late-onset neuropathologies such as PD.
Our results show that the ANL combination is the minimal set of TFs able to give rise to functional DA neurons in a short time frame. Indeed, as we previously described [20], the ANL factor combination was able to directly convert mouse fibroblasts into functional dopaminergic neurons in an efficient way. However, human adult fibroblasts are less prone to transdifferentiate into functional DA neurons, with only a minority of them (<5%) successfully completing neuronal conversion [20]. Interestingly, in stark contrast with adult human fibroblasts, hiPSCs proved extremely responsive to ANL forced expression; indeed, the ratio between neuronal-reprogrammed and ANL-infected cells is approximately 93% (51%:55%) in fetal fibroblast-derived hiPSCs and 87% (48%:55%) in adult fibroblast-derived hiPSCs. This significant difference between human differentiated somatic cells (fibroblasts) and iPSCs likely lies in the different epigenomic profile and the relative chromatin state, which strongly influence the cell differentiation plasticity of the two different cell types. It would be valuable to identify those particular epigenetic determinants that strongly influence this different response to neuronal differentiation stimuli.
We anticipate that this procedure will be particularly useful for drug-screening and disease-modeling experimental purposes. In contrast, it should be emphasized that our protocol at this stage is not suitable for direct applications of cell replacement therapy. Indeed, this approach uses integrating lentiviral vectors that bear a considerable risk of genotoxicity caused by their almost random insertion in the host cell genome. Moreover, we found that even after weeks of differentiation, hiPSC cultures included a fraction of proliferating nestin-positive precursors. Thus, it is conceivable that after transplantation in vivo, these cells might generate neural outgrowth similar to what was previously reported by using other neuronal differentiation procedures [23, 24], although at reduced levels. The presence of nestin-positive precursors in the neural progenitors of the hiPSC-differentiated progeny is likely the consequence of a lack or insufficient overexpression of any of the three TFs. This shortcoming might be overcome by generating a single multicistronic viral vector, which would guarantee the expression of nearly equal amounts of the three programming TFs in infected cells. With this and possible other future implementations, we anticipate that this hiPSC differentiation method assisted by neuronal cell lineage TF overexpression might become the system of choice for multiple applications in hiPSC-based high-throughput screenings, systematic in vitro disease modeling, and experimental cell therapy approaches.
Conclusion
Herein, we describe a protocol for differentiating hiPSCs into mature and functional dopaminergic neurons based on the simple viral transduction of three cell lineage TFs. This single manipulation is sufficient for a fast and efficient DA neuronal differentiation of naïve hiPSCs skipping any intermediate differentiation step procedure. ANL-hiPSC-derived DA neurons exhibited sophisticated functional properties like spontaneous regular action potentials and DA release in as few as 21 days from transduction. This one-step protocol is extremely attractive for high-throughput screening applications where a large quantity of neurons needs to be generated in a manner that is reproducible and amenable to automation.
Supplementary Material
Acknowledgments
We acknowledge the Cell Line and DNA Biobank (G. Gaslini Institute) and Human Genetic Bank of Patients Affected by Parkinson Disease and Parkinsonism (Parkinson Institute of Milan) of the Telethon Genetic Biobank Network for human fibroblast samples. We thank Stefano Goldwurm for helpful discussion. This work was supported by grants from Italian Ministry of Health, ERANET Neuron, Cariplo Foundation, Fondazione Grigioni per il Morbo di Parkinson, IIT-SEED project, Michael J. Fox Foundation (to V.B.), Telethon-GGP11095 (to V.B and A.D.), and Fondazione Istituto Italiano di Tecnologia (to R.R.G. and A.D.).
Author Contributions
I.T.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; M.C. conception and design, collection and assembly of data, data analysis and interpretation; E.D., D.L., S.C., and F.M.: collection and assembly of data; F.U. and M.T.D.: contribution to hiPSC derivation; G.P.: provision of study material or patients; R.R.G. and A.D.: conception and design; V.B.: conception and design, data analysis and interpretation, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Lewis P, Revesz T, et al. The genetics of Parkinson's disease syndromes: A critical review. Curr Opin Genet Dev. 2009;19:254–265. doi: 10.1016/j.gde.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 4.Dawson TM, Kho HS, Dawson VL, et al. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Santos C, Ferrer I, Santidrian AF, et al. Dopamine induces autophagic cell death and alpha-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res. 2003;73:341–350. doi: 10.1002/jnr.10663. [DOI] [PubMed] [Google Scholar]
- 6.Björklund A, Dunnet SB. Dopamine neuron system in the brain: An update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 9.Marchetto MC, Carromeu C, Acab A, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper O, Hargus G, Deleidi M, et al. Differentiation of human ES and Parkinson's disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen HN, Byers B, Cord B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devine MJ, Ryten M, Vodicka P, et al. Parkinson's disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Lumelsky N, Studer L, et al. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 14.Perrier AL, Tabar V, Barberi T, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho MS, Hwang DY, Kim DW, et al. Efficient derivation of functional dopaminergic neurons from human embryonic stem cells on a large scale. Nat Protoc. 2008;3:1888–1894. doi: 10.1038/nprot.2008.188. [DOI] [PubMed] [Google Scholar]
- 16.Martinat C, Bacci JJ, Leete T, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci USA. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Danés A, Consiglio A, Richaud Y, et al. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of Lmx1a in human embryonic stem cells and iPS cells. Hum Gene Ther. 2012;23:56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J, Yang M, Poremsky E, et al. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19:1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caiazzo M, Dell'Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Su SC, Wang H, et al. Functional integration of dopaminergic neurons directly converted form mouse fibroblasts. Cell Stem Cell. 2011;9:413–419. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 23.Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 24.Sonntag KC, Pruszak J, Yoshizaki T, et al. Enhanced yield of neuroepitelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.