Abstract
A preparative regimen of reduced intensity that can reliably engraft cord blood (CB) and be used as an alternative to either high-dose myeloablative or non-myeloablative conditioning is needed. We evaluated double-unit CB transplantation (CBT) in 30 patients (median age 56 years range 18–69) with acute leukemia or myelodysplasia using a regimen of cyclophosphamide 50 mg/kg, fludarabine 150 mg/m2, thiotepa 10 mg/kg, and 400 cGy total body irradiation with cyclosporine-A/mycophenolate mofetil immunosuppression. Ninety-seven percent of patients engrafted at a median of 26 days (range 13–43), and 93% of patients had recovered platelets by day 180. Grade II–IV acute graft-versus-host disease (GVHD) incidence was 67% at day 180, and chronic GVHD was 10% at 1-year. Transplant-related mortality (TRM) was 20% at day 180, and relapse was 11% at 2-years. Overall, 2-year disease-free survival (DFS) is 60% at 2-years. There was a hierarchy in DFS according to the Sorror comorbidity score: the 11 patients (median age 55 years) with a score of 1 had a 2-year DFS of 82% compared with 62% in the 9 patients (median age 51 years) with a score of 2–3, and 40% in the 11 patients (median age 58 years) with a score of 4–5 (p = 0.13). This reduced intensity regimen combined with double-unit CBT reliably facilitates sustained donor engraftment without anti-thymocyte globulin. While other approaches are needed in patients with high comorbidity scores, this regimen is highly effective in patients ≥ 50 years who are otherwise reasonably fit. It also represents a promising alternative to high-dose conditioning in younger patients.
Introduction
Double-unit CB transplantation (CBT) has been effective at reducing transplant-related mortality (TRM) compared with single-unit CBT historical controls1. Improvement in high-dose myeloablative double-unit CBT is needed, however, due to the risk of lethal regimen-related organ toxicity2. Non-myeloablative (NMA) and reduced intensity conditioning have been investigated as strategies to reduce TRM and extend transplant access to older patients or those with significant comorbidities3–6. However, NMA conditioning is limited by the combined risks of graft rejection in patients without extensive prior chemotherapy3 and relapse7–9. While rejection may be reduced by adding anti-thymocyte globulin (ATG), this in vivo T-cell depletion increases the risk of viral infections and Epstein-Barr virus lymphoproliferative disease10,11, and has been associated with increased TRM4. ATG could also increase relapse risk12,13.
To address these limitations, we have investigated the safety and efficacy of a novel ATG-free reduced intensity regimen. We used the cyclophosphamide, fludarabine, total body irradiation (TBI) 200 cGy NMA platform originally reported by the University of Minnesota3,4, but intensified the regimen by adding thiotepa and increasing the TBI dose to 400 cGy. In addition, to augment engraftment and possibly the anti-leukemia potential14–17, we used double-unit grafts in all patients. We have investigated this double-unit CBT approach as an alternative to either high dose myeloablative or non-myeloablative conditioning in adult patients with the hypothesis that it would induce a high incidence of sustained donor engraftment without ATG and have a low incidence of relapse.
Methods
Patients Characteristics
Patients were transplanted at Memorial Sloan-Kettering Cancer Center between 10/1/2007-8/30/2011, and provided informed consent for transplantation and outcome analysis in accordance with the Declaration of Helsinki. The trial is registered on ClinicalTrials.gov (NCT00739141). All consecutive patients 18–69 years old who were recipients of first hematopoietic stem cell transplants and with diagnoses of acute myelogenous or lymphoblastic leukemia (AML/ALL) in complete morphologic remission (CR1-3) or myelodysplasia with ≤ 5% blasts are reported in this analysis. The indication for this reduced intensity regimen was a diagnosis of acute leukemia or MDS and one or more TRM risk factors of age ≥ 50 years, and/or extensive prior therapy, and/or significant co-morbidities making the patient ineligible or inappropriate for high dose myeloablative conditioning. Standard-risk disease for acute leukemia was defined as CR1 without high-risk cytogenetics/high-risk molecular abnormalities, or de novo myelodysplasia with an International Prognostic Scoring System score < 2. All remaining patients were considered high-risk2. The hematopoietic cell transplant co-morbidity index (HCT-CI) score of Sorror et al18 was retrospectively assigned for the purposes of this analysis.
Conditioning Regimen, GVHD Prophylaxis and Graft Characteristics
Conditioning consisted of cyclophosphamide 50 mg/kg (day -6), fludarabine 30 mg/m2/day × 5 (days -6 to -2), thiotepa 5 mg/kg/day × 2 (days -5, -4), and total body irradiation 200 cGy/day × 2 (days -2, -1) (Cy 50/ Flu 150/ Thio 10/ TBI 400). If the recipient was greater than 125% of ideal body weight the doses of cyclophosphamide, fludarabine, and thiotepa were calculated on adjusted body weight. Cyclosporine-A (CSA) and mycophenolate mofetil (MMF) were used as graft-versus-host disease (GVHD) prophylaxis starting on day -3 intravenously. CSA was dosed to achieve a trough level 200–400 ng/ml. MMF dose was 1 gram every 12 hours for the first 17 patients, and was increased to 1 gram every 8 hours for the subsequent 13 patients to augment GVHD prophylaxis19. Double-unit CB grafts were 4-6/6 HLA-A,-B antigen, -DRB1 allele matched to the recipient with a cryopreserved total nucleated cell (TNC) dose ≥ 1.5 × 107/kg/unit as previously described20. Unit-unit HLA-match was not considered in unit selection. Units were thawed with albumin-dextran dilution21 (n = 58) or were washed (n = 2). Granulocyte-colony-stimulating factor (5 mcg/kg/day rounded to vial size) was given from day 7 until neutrophil recovery.
Study Definitions
Time to neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) > 0.5 × 109/L. Time to platelet recovery was defined as the first of 3 consecutive days at > 50 × 109/L and at least 7 days without platelet transfusion support. Sustained donor engraftment was defined as sustained donor-derived count recovery with donor chimerism of at least 90% (both units combined). GVHD was diagnosed clinically with histologic confirmation when appropriate. Acute and chronic GVHD were graded according to International BM Transplant Registry22 and The National Institutes of Health consensus criteria23, respectively. The primary aim of this study was to obtain a preliminary estimate of disease-free survival (DFS) at 1-year after CBT. The target sample size was 30 patients. We proposed that the treatment would be considered efficacious if the DFS at 1-year is at least 50%. Using a single stage design, if at least 19/30 patients are disease-free at 1-year, there is a 90% confidence that the true 1-year DFS is > 50%. Overall survival (OS) and DFS were calculated using Kaplan-Meier methodology, and cumulative incidence was used to estimate all other outcomes. The permutation log-rank test was used to test for differences in DFS according to HCT-CI score.
Results
Patient and Graft Characteristics
Table 1 summarizes patient and graft characteristics. Thirty patients (median age 56 years) were transplanted. Twenty-six had acute leukemia (20 CR1, 5 CR2, 1 CR3), and 4 had MDS. All had high-risk disease (as described in Table 1) except a single patient who had refractory anemia with excess blasts-2 that achieved complete remission after treatment. All patients had been treated with either chemotherapy (n = 28) or hypo-methylating agents (n = 2). Twenty-seven had received therapy within the preceding 3 months, and 3 were treated > 3 months prior to transplantation.
Table 1.
Patient and graft characteristics (n = 30 patients and 60 units).
| Characteristics | |
|---|---|
| Median age (range) | 56 (18–69) |
| N (%) male | 15 (50%) |
| Median weight kg (range) | 69 (48–103) |
| N (%) recipient CMV seropositive | 21 (70%) |
| N (%) diagnosis/ disease status* | |
| AML | 21 (70%) |
| CR1 | 16 |
| 3 poor risk cytogenetics** | |
| 5 FLT3-ITD mutation | |
| 6 secondary to therapy or prior MDS/MPD | |
| 2 with three or more inductions | |
| CR2 | 5 |
| ALL | 5 (17%) |
| CR1 | 4 |
| 3 Philadelphia chromosome positive | |
| 1 refractory CNS disease that cleared prior to allograft. | |
| CR3 | 1 |
| MDS | 4 (13%) |
| CR1 | 1 |
| Stable disease | 1 |
| Hematologic improvement | 2 |
| N (%) disease risk (%) | |
| Standard | 1 (3%) |
| High | 29 (97%) |
| Donor-recipient HLA-match | |
| 6/6 | 4 (7%) |
| 5/6 | 32 (53%) |
| 4/6 | 24 (40%) |
| Median infused TNC × 107/kg (range) | |
| Larger unit | 2.6 (1.5–5.6) |
| Smaller unit | 1.9 (1.4–2.5) |
| Median infused CD34+ x 105/kg (range) | |
| Larger unit | 0.9 (0.4–3.3) |
| Smaller unit | 0.5 (0.2–1.5) |
Abbreviations: N, number; Kg, kilogram; CMV, cytomegalovirus; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MP, myeloproliferative disease; CR, complete remission; HLA, human leukocyte antigen; TNC, total nucleated cell.
2 patients had multiple hematologic malignancies: one with AML and chronic lymphocytic leukemia, and one with MDS and Non-Hodgkins lymphoma.
Included monosomy 7 (n = 1), 11q23 translocation (n = 1), and del 5q (n = 1).
The median HCT-CI score18 was 2.5 (range 1–5). The age distribution of patients with HCT-CI scores of 1 (n = 11, median 55 years, range 31–66), 2–3 (n = 9, median 51 years, range 35–61), and 4–5 (n = 10, median 58 years, range 18–69) was not different (p = 0.84). Co-morbidities as defined by Sorror et al18 included arrhythmia (n = 1), cardiovascular disease (n = 4), diabetes (n = 2), depression/ anxiety (n = 1), hepatic dysfunction (n = 7, all mild), infection requiring ongoing therapy (n = 5), pulmonary dysfunction (n = 15, 9 moderate, 6 severe), and prior solid tumor (n = 4).
Regimen-Related Toxicity, Engraftment, GVHD and Infections
The regimen was associated with delayed engraftment characteristic of myeloablative regimens. While it was better tolerated than high dose chemo-radiation, there were high rates of anorexia with 21/30 (70%) patients requiring total parenteral nutrition. However, mucositis was not severe and only 8 needed patient controlled analgesia with narcotics within the first 4 weeks post-transplant. Three patients suffered organ toxicity resulting in transplant-related mortality as described below. The only late toxicities were associated with GVHD and its treatment.
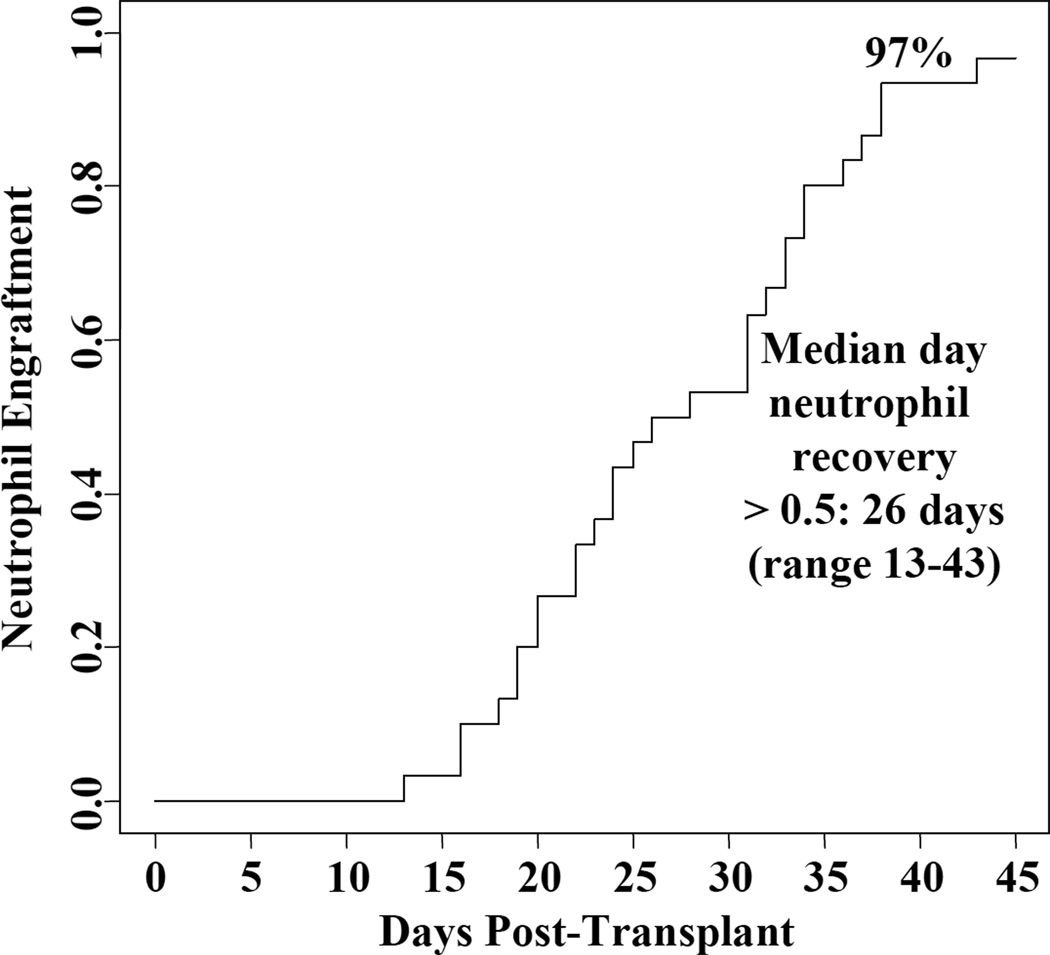
Ninety-seven percent (95%CI: 87–100) of patients had sustained donor-derived neutrophil engraftment (Figure 1). The median time to neutrophil recovery was 26 days (range 13–43). A single heavily pre-treated patient had graft failure in the context of early onset multi-organ failure. This patient was 100% donor in the day 21 bone marrow but died at day 35 without count recovery. The cumulative incidence of platelet recovery of 20 × 109/l by day 180 was 93% (95%CI: 83–100) and occurred at a median of 46 days (range 30–79). The median day 21 total donor bone marrow chimerism was 100% (range 71–100), and consisted of one unit in 25/30 (83%) of patients. All surviving patients were 100% donor by day 100, there were no late graft failures, and sustained hematopoiesis has been mediated by a single unit in all but one patient.
Figure 1.
The cumulative incidence of neutrophil engraftment.
Sixty-seven percent (95%CI: 49–84) of patients had grade II–IV and 7% (95%CI: 0–16) had grade III–IV acute GVHD by day 180 (17 grade II, 2 grade III, and 1 grade IV). Of the 27 patients who engrafted and were alive at day 100, 3 had chronic GVHD (2 overlap and 1 classical; 2 mild and 1 moderate severity). The 1-year cumulative incidence of chronic GVHD was 10% (95%CI: 0–21). The pattern of infectious complications was similar to that previously described in recipients of double-unit CBT transplanted without ATG24. Eighteen of the 21 (86%) patients who were CMV seropositive reactivated CMV at a median of 43 days (range 14–111) post-transplant. However, only 2 had end-organ disease (both with gastro-intestinal involvement, one in the setting of GVHD). Two patients had EBV viremia at +5 and + 21 months post-transplant, both in the setting of treatment for GVHD. Both responded to Rituximab therapy and no patient had EBV lymphoma.
TRM, Relapse and Survival
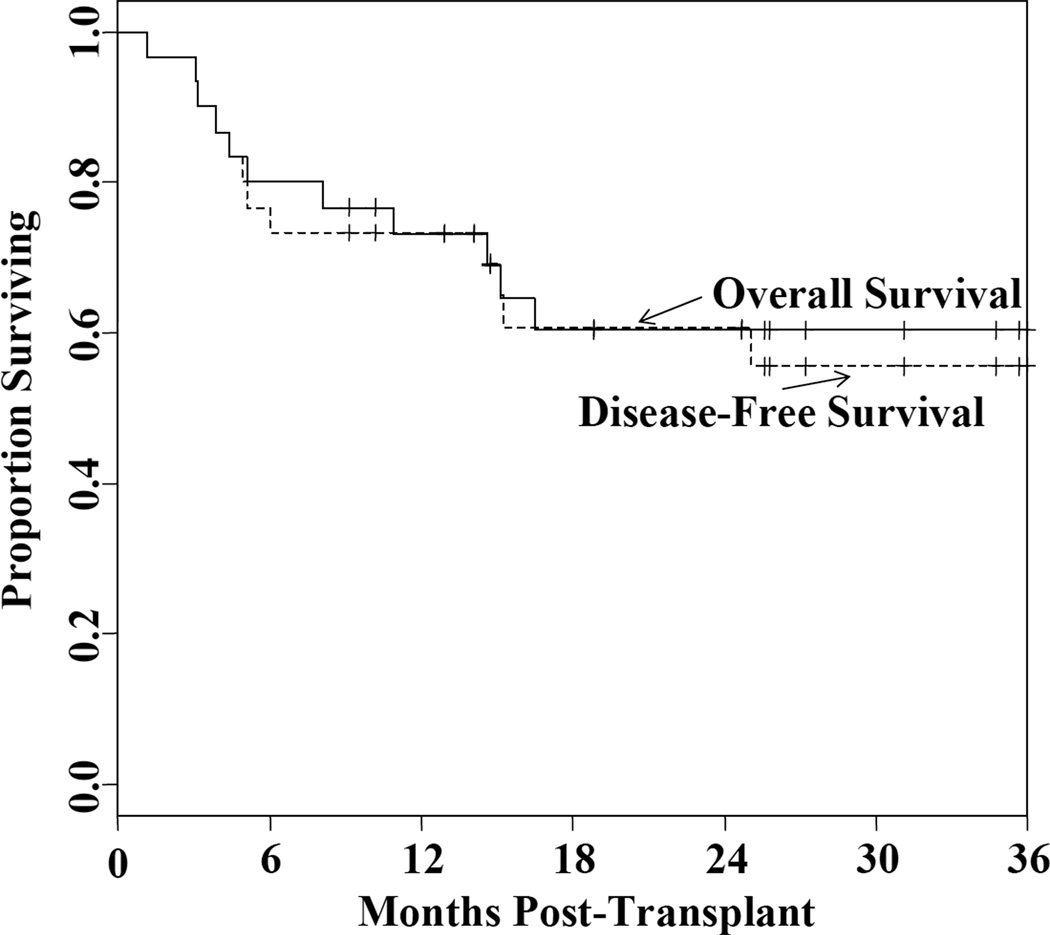
TRM was 20% (95%CI: 5–35) at day 180, and 28% (95%CI: 11–46) at 2 years. Of the 8 patients who died of transplant-related causes, one died of graft failure, 4 of GVHD, and 3 of organ failure (2 pulmonary, 1 central nervous system). No patient died of infection as the primary cause of death. Relapse was 11% (95%CI: 3–26) at 2 years. With a median 26.5 month (range 9–53) follow-up of survivors, the 2-year OS and DFS are both 60% (95%CI: 44–82) (Figure 2).
Figure 2.
The Kaplan-Meier estimate of OS and DFS.
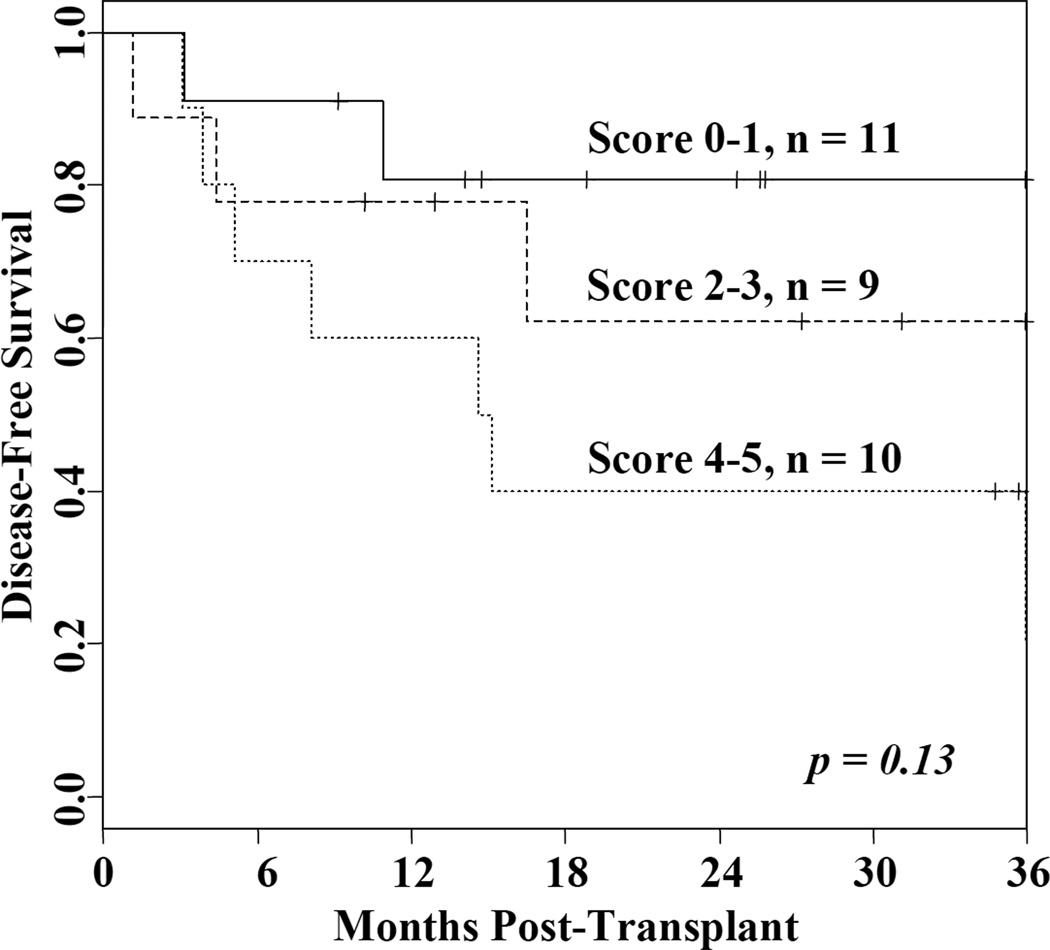
The comparison of DFS according to pre-transplant HCT-CI is shown in Figure 3. In the 11 patients (median age 55 years) with a HCT-CI score of 1, one has died of transplant-related causes and one has relapsed resulting in a 2-year DFS of 82% (95%CI: 62–100) in this group. This compares favorably with the 2-year DFS of 62% (95%CI: 36–100) in the 9 patients (median age 51 years) with a score of 2–3 (2 cases of TRM and 1 relapse), and the 2-year DFS of 40% (95%CI: 44–82) in the 10 patients (median age 58 years) with a score of 4–5 (5 cases of TRM and 2 relapses) (permutation log-rank p = 0.13).
Figure 3.
DFS according to the HCT-CI after double-unit CBT using Cy 50/ Flu 150/ Thio 10/ TBI 400 conditioning.
Discussion
We demonstrate that Cy 50/ Flu 150/ Thio 10/ TBI 400 conditioning supports a high incidence of sustained neutrophil and platelet engraftment after double-unit CBT. This regimen is reduced intensity based on the lower doses of chemotherapy and TBI than are delivered in high-dose regimens and as defined by the criteria of Bacigalupo et al25. However, it remains functionally myeloablative as illustrated by the delayed neutrophil recovery characteristic of adult CBT and the median day 21 total donor chimerism of 100%. This is in contrast to the transient autologous recovery and initial mixed chimerism characteristic of non-myeloablative double-unit CBT3. Other centers have investigated CBT with reduced intensity regimens in an effort to avoid the toxicity of the most intense regimens. These include Melphalan/Fludarabine5, Melphalan/Thiotepa/Fludarabine26 and Treosulfan,/Fludarabine/TBI27.
The Cy 50/ Flu 150/ Thio 10/ TBI 400 regimen induces sufficient immunosuppression to facilitate sustained donor engraftment when combined with a double-unit CB graft, and this is achieved without the profound post-transplant immunosuppression associated with ATG. It should be acknowledged, however, that our series did not contain any patient without prior therapy, although 2 patients had had only hypomethylating agents. Patients without prior combination chemotherapy (who are at an increased risk of graft rejection after non-myeloablative CBT3) require further investigation. Furthermore, while the incidences of both neutrophil and platelet engraftment were high, the rate of recovery is quite delayed relative to the transplantation of adult donor peripheral blood stem cells. New strategies such as a larger CB inventory to enable transplantation of larger and better matched units, ex vivo expansion28,29 or the addition of a T-cell depleted haplo-identical graft30,31 could further improve the rate of count recovery after this regimen. In addition, while we found a high incidence of acute GVHD, most cases were not severe. Whether the increase in MMF dosing will afford more effective GVHD prophylaxis will require analysis of larger patient numbers. It is likely, however, that new agents will be needed to augment GVHD prevention without the disadvantages of ATG. As previously described by our group, in the absence of ATG late infection risk was low in the absence of moderate to severe GVHD24.
The pre-transplant HCT-CI of Sorror et al has been validated as a reliable tool to predict allograft TRM and survival based on recipient pre-transplant co-morbidities (significant prior illnesses and organ dysfunction)24. The utility of the HCT-CI has recently been demonstrated in a large prospective study32. Notably, in our much smaller double-unit CBT series, an association between HCT-CI and DFS is also suggested. The low TRM affecting only one of 11 patients in those with a score of 1 is notable given their relatively advanced median age of 55 years by CBT standards. Thus, Cy 50/ Flu 150/ Thio 10/ TBI 400 double-unit CBT can be safely used in patients ≥ 50 years without other major TRM risks. While the upper age limit for safe administration of this regimen is not known, our findings are highly significant for middle-aged patients with high-risk acute leukemias who are otherwise fit, and suggests that double-unit CBT with Cy 50/ Flu 150/ Thio 10/ TBI 400 could be an immediate alternative if an unrelated donor is not identified within the first few weeks of a search. In contrast, TRM risk for patients with a high HCT-CI score (≥ 4) remains substantial with this regimen. Unfortunately, high co-morbidity scores cannot be modified. While earlier referral of patients with high risk disease could reduce the impact of prior therapy, our data suggest this regimen remains too intense for such patients and other treatment approaches are needed. These could include non-transplant therapies, or the investigation of non-myeloablative allografts with post-transplant maintenance to reduce relapse risk as has been investigated for patients with AML and MDS33.
Unlike NMA conditioning, which has been associated with a 31% relapse incidence at 1 year follow-up8, the incidence of leukemic relapse in this high-risk population has been low (11% at 2-years with a median follow-up of 26.5 months). The combined effects of this conditioning and the graft-versus-leukemia potential of double-unit grafts14–17 may well account for this outcome. It should be acknowledged that this series is relatively small. Nonetheless, our promising preliminary results warrant further investigation in patients ≥ 50 years with high-risk hematologic malignancies, who have high relapse risks after non-myeloablative conditioning and acceptable co-morbidity scores. It may also be a promising alternative to high-dose conditioning in younger patients with hematologic malignancies, due to the reduced organ toxicity and preservation of the protection against relapse. As a result, we are now prioritizing this approach for the majority of our adult patients undergoing double-unit CBT, even those previously considered eligible for high-dose conditioning.
Acknowledgements
This work was supported in part by the Gabrielle’s Angel Foundation for Cancer Research (J.N.B.), the Society of Memorial Sloan-Kettering Cancer Center (J.N.B. and S.G.), the Memorial Sloan-Kettering Cancer Center Translational and Integrative Medicine Research Program (J.N.B.), the American Society of Clinical Oncology Young Investigator Award (C.S.), and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: D.M.P. interpreted the data and wrote the manuscript. M.L., A.M.G., S.D., analyzed the data and wrote the manuscript. C.S., N.A.K, A.S., S.G., J.G., G.K, M.A.P., J.W.Y., H.C.M., A.J., and E.P. wrote the manuscript. J.N.B. designed the study, interpreted the data, and wrote the manuscript.
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
References
- 1.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of two partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 2.Ponce DM, Zheng J, Gonzales AM, et al. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1316–1326. doi: 10.1016/j.bbmt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, Miller JS, Wagner JE. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 4.Brunstein C, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after non-myeloablative conditioning: impact on transplant outcomes in 110 adults with hematological disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida N, Wake A, Takagi S, et al. Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biol Blood Marrow Transplant. 2008;14:583–590. doi: 10.1016/j.bbmt.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17:1327–1334. doi: 10.1016/j.bbmt.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Brunstein CG, Shanley R, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012;47:494–498. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Weisdorf DJ, Defor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of anti-thymocyte globulin to a non-myeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J, Gandhi M, Naik P, et al. Increased incidence of EBV-related disease following paediatric stem cell transplantation with reduced-intensity conditioning. Br J Haematol. 2005;129:229–239. doi: 10.1111/j.1365-2141.2005.05439.x. [DOI] [PubMed] [Google Scholar]
- 12.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 16.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson PA, Huang J, Wu J, et al. Mycophenolate pharmacokinetics and association with response to acute graft-versus-host disease treatment from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2010;16:421–429. doi: 10.1016/j.bbmt.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117:2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker JN, Abboud M, Rice RD, et al. A "no-wash" albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15:1596–1602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British Journal of Haematology. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17:1460–1471. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciurea S, Saliba R, Hamerschlak N. Fludarabine, melphalan, thiotepa and anti-thymocyte globulin conditioning for unrelated cord blood transplant. Leukemia & Lymphoma. 2012;53:901–906. doi: 10.3109/10428194.2011.631159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaney C, Gutman JA, Appelbaum FR. Cord blood transplantation for haematological malignancies: conditioning regimens, double cord transplant and infectious complications. Br J Haematol. 2009;147:207–216. doi: 10.1111/j.1365-2141.2009.07782.x. [DOI] [PubMed] [Google Scholar]
- 28.de Lima M, McMannis J, Hosing C, et al. Rapid engraftment of neutrophils and platelets with mesenchymal stem cell based cord blood expansion. Blood. 2009;114 Abstract 3394. [Google Scholar]
- 29.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43:365–373. doi: 10.1038/bmt.2008.329. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118:6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raimondi R, Tosetto A, Oneto R, et al. Validation of the Hematopoietic Cell Transplantation-Specific Comorbidity Index: a prospective, multicenter GITMO study. Blood. 2012;120:1327–1333. doi: 10.1182/blood-2012-03-414573. [DOI] [PubMed] [Google Scholar]
- 33.de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]