Abstract
Both basic science and clinical studies support the concept that vitamin D deficiency is involved in the pathogenesis of cardiovascular and renal diseases through its association with diabetes, obesity, and hypertension. Understanding the underlying mechanisms may provide a rationale for advocating adequate intake of vitamin D and calcium in all populations, thereby preventing many chronic diseases. This review explores the effect of vitamin D deficiency in the development of cardiovascular and renal diseases, and the role of vitamin D supplementation on cardiovascular outcomes. In addition, it highlights the importance of vitamin D intake for the prevention of adverse long-term health consequences, and in ways to facilitate the management of cardiovascular disease. This is particularly true for African American and postmenopausal women, who are at added risk for cardiovascular disease. We suggest that the negative cardiovascular effects of low vitamin D in postmenopausal women could be improved by a combined treatment of vitamin D and sex steroids acting through endothelium-dependent and/or -independent mechanisms, resulting in the generation of nitric oxide and calcitonin gene-related peptide (CGRP).
Keywords: Vitamin D, Sex hormones, Health disparity, Nitric oxide, CGRP, Heart disease, Renal disease, Review
2. INTRODUCTION
In the United States and most other countries, the incidence of hypertension rises progressively with age and represents one of the most common and important risk factors leading to cardiovascular disease (1, 2). African Americans (AA) are at a higher risk for these conditions than any other age-matched race or ethnic group (3, 4), and AA women, in particular, are most vulnerable (5, 6). The mechanisms underlying these racial/sex disparities are multi-factorial and can involve: a family history of hypertension, an altered lipid and/or endocrine imbalance, socioeconomic status, psychosocial stressors/risks, environmental and other lifestyle factors, including a high sodium or low potassium intake, excessive consumption of calories and/or alcohol, physical inactivity, and insufficient vitamin D levels. Various combinations of these risk factors can lead to the metabolic syndrome in younger or midlife AAs, and thus provide an increased risk for hypertension, coronary heart disease (CHD), and associated renal disease.
3. VITAMIN D
3.1. Sources of vitamin D
The major source is through skin photosynthesis of 7-dehydrocholesterol to cholecalciferol. Healthy fair-skinned persons who receive 20–30 minutes of midday sun exposure on their face and forearms can generate up to 2000 IU of vitamin D. If this is done 2–3 times weekly, these individuals will get adequate levels of vitamin D. It is more challenging for the elderly and those with pigmented skin to generate adequate levels of vitamin D from sun exposure; the latter group may require 2–10 times more sun exposure compared to fair-skinned individuals (7,8). There are only a few foods, including oily fish and cod liver oil that are significant sources of vitamin D (9). Notably, farmed fish has less vitamin D than wild caught fish.
3.2. Metabolism of vitamin D
Ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) are inactive precursors of the metabolically active secosteroid 1, 25 dihydroxyvitamin D(1,25 OH2D3), also known as calcitriol (7, 10). Calcitriol levels are reduced if 25-hydroxyvitamin D (25-OHD) levels are reduced. Renal production of calcitriol is tightly regulated by plasma parathyroid hormone levels and serum calcium and phosphorus (10). Vitamin D from the diet is metabolized to 25-OHD, which is normally assayed to determine vitamin D status (11, 12). In a vitamin D deficient state, the parathyroid glands are maximally stimulated causing secondary hyperparathyroidism (13–16). Parathyroid hormone increases the metabolism of 25-OHD to calcitriol which further exacerbates the vitamin D deficiency. Calcitriol levels are also reduced if the number of functioning nephrons is reduced, or if there is a high concentration of fibroblast growth factor or inflammatory cytokines (11, 12). In the absence of vitamin D, only 10% of dietary calcium and 60% of phosphorus are intestinally absorbed (10). Excess parathyroid hormone also causes phosphaturia resulting in low-normal phosphorus. If there is an inadequate calcium-phosphorus product (the value for calcium times the value for serum phosphorus), mineralization of the collagen matrix of the bone is diminished causing rickets in children and osteomalacia in adults (9, 13, 17). Serum 25(OH) D concentrations have been used to assess vitamin D status. 25(OH)D concentrations may be assessed by using radioimmunoassay and ultra-high performance liquid chromatography (UHPLC) (18). UHPLC constitutes a reliable approach for nutrient/biomarker profiling allowing the rapid, simultaneous and low-cost determination of vitamins A, E, and D (including vitamers and ester forms) and the major carotenoids in clinical practice.
3.3. Vitamin D deficiency
Although there is no absolute consensus of opinion regarding an optimal level of 25-OHD, most experts agree that a vitamin D deficiency state exists when serum levels are <20ng/ml (9). Table 1 shows the values in humans that are frequently associated with healthy, at risk and disease states. Patients that have rickets and osteomalacia have serum 25-OHD levels of ≥10ng/ml, which represents a profound vitamin D deficiency. An adequate vitamin D status is ≥30ng/ml (1, 19). Vitamin D intoxication which does not occur from excessive sun exposure, but rather from overdosing on vitamin D supplements, happens when the serum level is >150ng/ml. Forty to 100% of elderly European men and women living in their communities (nursing home residents not included) were reported to be deficient in vitamin D (13–16, 19–22). In addition, 50% of postmenopausal women taking medications for osteoporosis had suboptimal levels below 30 ng/ml (16–22). Most commercial assays for 25-OHD can detect a vitamin D deficiency. Radioimmunoassays, on the other hand, measure 25-OHD, 25-OH D2 and 25-OH D3 concentrations. As long as the combined total is ≥30ng/ml the patient has sufficient stores. A 1, 25 OH2D3 assay should never be used to detect a vitamin D deficiency state because this compound can be either at a normal or an elevated level because of secondary hyperparathyroidism, in an otherwise vitamin D deficient patient (9). Since 25-OHD assays are costly, providing children and adults with 800–1000 IU of vitamin D/day should be sufficient to provide adequate serum levels unless there are other factors predisposing subjects to a deficiency (Table 2).
Table 1.
Serum 25-hydroxyvitamin D concentrations, health and disease
| Serum 25-OHD Concentrations |
Vitamin D status | Manifestations | Management |
|---|---|---|---|
| <10 ng/ml | Deficient | Rickets, osteomalacia | Treat with high dose cholecalciferol |
| 11–21 ng/ml | Deficient | Associated with disease risk | Vitamin D supplementation |
| 21–29 ng/ml | Adequate | Healthy | Lifestyle advice |
| >30 ng/ml | Optimal | Healthy | None |
Adapted with permission from (1)
Table 2.
Strategies to prevent and treat vitamin D (vit D) deficiency
| Cause of Deficiency | Preventive and Maintenance Measures to avoid deficiency | Treatment of Deficiency |
|---|---|---|
| Children | ||
| Breast-feeding without vitamin D supplementation | 400 IU of vitamin D/d, sensible sun exposure, 1000–2000 IU of vitamin D3/d is safe, maintenance 400–1000 IU of vitamin D3/d | 200,000 IU of vitamin D3 every 3 mo, 600,000 IU of vitamin D IM, repeat in 12 wk, 1000–2000 IU of vitamin D2 or vitamin D3/d with calcium supplementation |
| Inadequate sun exposure or supplementation, dark skin (age 1– 18 yr) | 400 IU of vitamin D/d, sensible sun exposure, 1000–2000 IU of vitamin D3/d is safe, maintenance 400–1000 IU of vitamin D3/d | 50,000 IU of vitamin D2 every wk for 8 wk |
| Adults | ||
| Inadequate sun exposure or supplementation, decreased 7-dehydrocholsterol in skin because of aging (over 50 yr) | 800–1000 IU of vitamin D3/d, 50,000 IU of vitamin D2 every 2 wk or every month, sensible sun exposure use of tanning bed or sensible UV device up to 10,000 IU of vitamin D3/d is safe for up to 5 months | 50,000 IU of vitamin D2 every wk for 8 wks, repeat for another 8 wks if 25-OHD <30 ng/ml |
| Pregnant or lactating (fetal utilization, inadequate sun exposure or supplementation) | 1000–2000 IU of vit D3, 50,000 IU of vit D2 every 2 wk, up to 4000 IU of vit D3/d is safe for 5 month, maintenance dose is 50,000 IU of D2 every 2–4 wks | 50,000 IU vit D2 q wk for 8 wks, repeat if 25 –OHD ≤30 ng/ml |
| Malabsorption syndromes (malabsorption of vitamin D, inadequate sun exposure or supplementation) | Adequate sun exposure or UV radiation, 50,000 IU of vitamin D2 every day, every other day, or every wk, up to 10,000 IU of vitamin D3/d is safe for 5 month, maintenance dose is 50,000 IU vitamin D2 every wk | UVB irradiation (tanning bed or portable UVB device e.g. Sperti lamp), 50,000 IU of vitamin D2 every day or every other day |
| Drugs that activate steroid and xenobiotic receptors, and drugs used in transplantation | 50,000 IU of vitamin D2 every other day or every wk, maintenance dose is 50,000 IU of vitamin D2 every 1,2, or 4 wk | 50,000 IU of vitamin D2 every 2 wk for 8–10 wk, or every wk if 25-OHD <30 ng/ml |
| Obesity | 1000 IU-2000 IU of vit D3/d, 50,000 IU of vit D2 every 1–2 wk, maintenance dose is 50,000 IU of vitamin D2 every 1, 2 or 4 wk | 50,000 IU of vit D2 every wk for 8–12 wk, repeat for 8–12 wk if 25-OHD <30 ng/ml |
| Nephrotic syndrome | 1000–2000 IU of vitamin D3/d, 50,000 IU of vitamin D2 every 1 or 2 wk, maintenance dose is 50,000 IU of vitamin D2 every 1,2 or 4 wk | 50,000 IU vitamin D2 twice/wk for 8–12 wk, repeat for another 8–12 wk if 25-OHD <30 ng/ml |
| Chronic kidney disease Stages 2 and 3 | Control serum phosphate, 1000 IU of vitamin D3/d, 50,000 IU of vitamin D2 every 2 wks, maintenance dose of 50,000 IU of vitamin D2 every 2 or 4 wk; may also need to treat with an active vitamin D analog when vitamin D sufficiency obtained | 50,000 IU of vitamin D2 once/wk for 8 wk, repeat for another 8 wk if 25-OHD <30ng/ml |
| Chronic kidney disease Stages 4 and 5 | 1000 IU of vitamin D3/d, 50,000 IU of vitamin D2 every 2 wk, need to treat with 1,25 OH2D3 or active analog | 0.25–1.0 µg of 1,25 OH2D3 (calcitriol) by mouth twice a day or one of the following: 1–2 µg of paricalcitol IV every 3 d, 0.04–1.0 ug/kg IV every other day initially and can increase to 0.24 µg/kg, 2– 4µg by mouth three times/wk, or doxercalciferol 10– 20 µg by mouth three times/wk or 2–6 µg IV three times/wk |
Adapted with permission from (3). Abbreviations: IU: International Units; /d: per day; mo: month; wk: week; yr: year; IV: Intravenous, IM: intramuscular
3.4. Risk factors for developing vitamin D deficiency
The most significant risk factor for developing vitamin D deficiency is pigmented skin, even in individuals who live in sunny climates. Sunscreens with a sun protection factor (SPF) ≥15 block 99% of vitamin D photosynthesis, and thus they represent another factor that could lead to a vitamin D deficiency state. The causes, prevention and treatment of vitamin D deficiency are listed in Table 2 (7).
3.5. Daily requirements
The recommended daily intake of vitamin D of 400 IU is sufficient only to prevent osteomalacia and rickets, but will not provide optimal vitamin D levels to prevent other diseases (7). Supplementation of 800 – 1000 IU/day should be given to children and adults who do not receive adequate sun exposure (10, 18–21, 23–25). If rickets is present, 50,000 IU vitamin D2 each week for 8 weeks, then every 2–4 weeks; or 1000 IU D3 each day should be given to achieve adequate serum levels of vitamin D. According to Institute of Medicine, Food and Nutrition Board (26), the Recommended Dietary Allowances (RDAs) for Vitamin D are: 0–12 months: 400 IU; 1–70 years: 600 IU; > 70 years: 800 IU.
Vitamin D deficiency is also linked to mortality. An inadequate supply of vitamin D may have negative health effects in almost 50% of the population worldwide (27). The role of vitamin D in regulating calcium and phosphorus and in maintaining bone structure has been known for some time. In particular, vitamin D deficiency has been linked to obesity, nonspecific musculoskeletal pain, decreased insulin sensitivity, and an increased risk for developing the metabolic syndrome, autoimmune diseases such as multiple sclerosis, cancer, cardiovascular disease and more (28). It is postulated that decreased levels of vitamin D may be one of the causative factors leading to health disparities in AA compared to non-AA groups (29). Further, clinical studies largely, but not consistently, favor the hypothesis that vitamin D is involved in the regulation of blood pressure (30, 31). More basic research as well as clinical research needs to be done to elucidate the exact role of vitamin D metabolites in promoting CHD in AA as well as in others who have low circulating vitamin D in general.
3.6. Vitamin D deficiency and cardiovascular disease
Vitamin D deficiency (VDD) is associated with an increased cardiovascular risk. VDD in early life increases arterial blood pressure, promotes vascular oxidative stress, and induces changes in cardiac gene expression (32). DNA sequence variation in genes regulating vitamin D metabolism and signaling pathways also influence variation in coronary artery calcification (CAC) (33).
The relationship between vitamin D and cardiac function has been investigated for several years. The biological actions of vitamin D on the cardiovascular system are expected to encompass a wide spectrum of molecular mechanisms that are not limited to the regulation of calcium homeostasis. Data from the Framingham Offspring Study suggested that a first cardiovascular event is correlated with a low serum level of the metabolically active form, 1, 25-OH2D3 (34). A low serum level of 1, 25 OH2D3 that is associated with vitamin D receptor genotypes producing a deficiency in the conversion of 25-OHD to 1, 25-OH2D3, was reported to correlate with the severity of coronary artery disease (35) and left ventricular hypertrophy. In addition, a deficiency in 1, 25 OH2D3 is associated with expression of the B-allele of the BsmI restriction fragment of the vitamin D receptor gene (36). A polymorphism of the B-allele of this BsmI restriction fragment is also associated with Graves disease in the Japanese population (37). Graves’ disease is an autoimmune disorder affecting the beta-1 adrenergic receptor that causes idiopathic dilated cardiomyopathy (38). Importantly, dilated cardiomyopathy is also the leading cause of heart failure in young adults. Early studies indicated that rats maintained on a vitamin D-3 deficient diet showed a transient elevation of blood pressure accompanied by a reduction in serum calcium (39). Vitamin D receptor-null mice displayed hypertension and cardiac hypertrophy, resulting from an underlying activation of the systemic and cardiac renin-angiotensin-aldosterone system (RAAS) (40, 41). Emerging evidence suggests that VDR plays important roles in modulating cardiovascular, immunological, metabolic and other functions. In patients with congestive heart failure, vitamin D supplementation improved the cytokine profiles by modulating TNF-alpha and IL-10 levels (42). It was recently found that vitamin D plays a cellular protective and anti-proliferative role via the PI3-kinase/Akt signaling pathway (43–46). Epigenetic mechanisms for activation and silencing of the vitamin D receptor gene by histone modifications are thought to play critical roles in the differentiation of mesenchymal stem cells which also transform and repair cardiomyocytes (47).
Vitamin D receptors are expressed in vascular smooth and cardiac muscles, as well as in endothelial cells where they appear to provide a complex mechanism for protecting against various forms of cardiovascular failure. The vitamin D receptor activator paricalcitol has been shown to attenuate left ventricular hypertrophy by mechanisms involving a reduction in oxidative stress (48). Vitamin D receptors in cardiac myocytes and fibroblasts are colocalized with 1- and 25-vitamin D hydroxylases and these vitamin D receptors are shown to bind to the human B-type natriuretic peptide gene promoter, a marker for increased mRNA transcription during cardiomyocyte hypertrophy (49). The cardiac hypertrophy and hypertension observed in vitamin D receptor-null mice are associated with marked increases in expression of renin and production of angiotensin II (40), suggesting several genomic and proteomic mechanisms by which vitamin D plays an important role in the etiology of heart failure. Despite these reports, our understanding of the molecular pathways involved in the effects of vitamin D on cardiomyocytes and cardiovascular disease remains limited.
3.7. Vitamin D deficiency and renal disease
In patients with any stage of chronic kidney disease (CKD), 25-OHD should be measured annually and the level should be maintained at ≥30 ng/ml, as recommended by the Kidney Disease Outcomes Quality Initiative (K/DOQ1) guidelines from the National Kidney Foundation (50–52). It is a misconception to assume that a patient with CKD who is taking vitamin D will have sufficient vitamin D stores, since vitamin D levels are inversely associated with parathyroid hormone levels regardless of the degree of CKD. As stated previously, the parathyroid glands convert 25-OHD to calcitriol, which then directly inhibits the expression of parathyroid hormone (9). Patients at CKD stages 4 and 5 are unable to generate enough 1, 25 dihydroxyvitamin OH2D3 and may need to be supplemented with calcitriol or another analogs to maintain adequate calcium metabolism and prevent metabolic bone disease (Table 2) (9, 14, 51,52).
4. SEX HORMONES AND CARDIOVASCULAR DISEASE
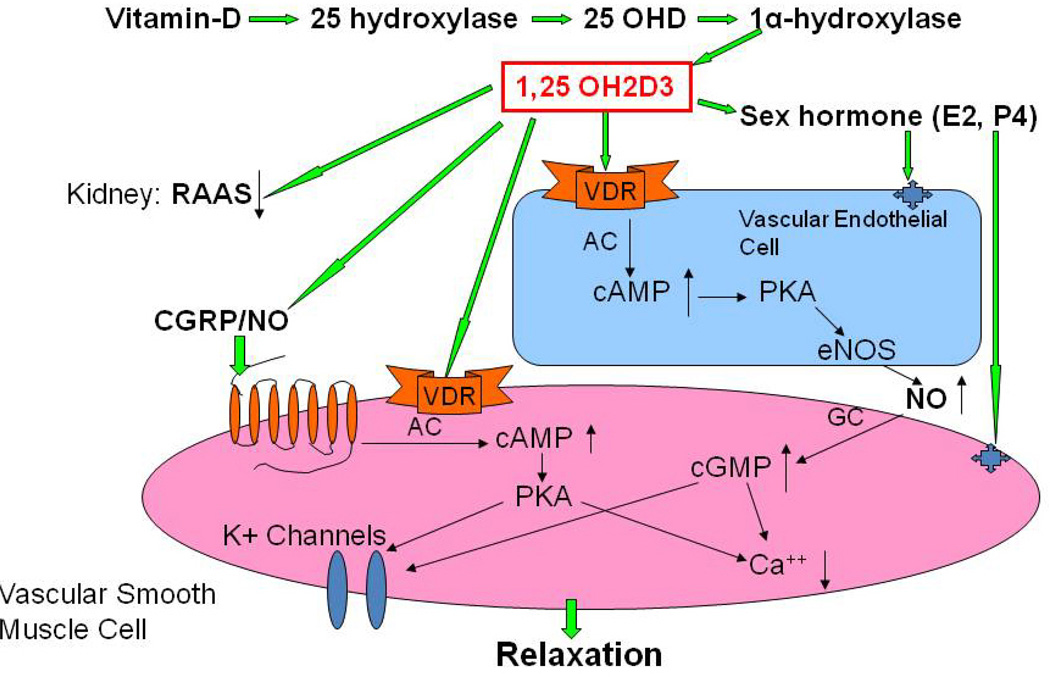
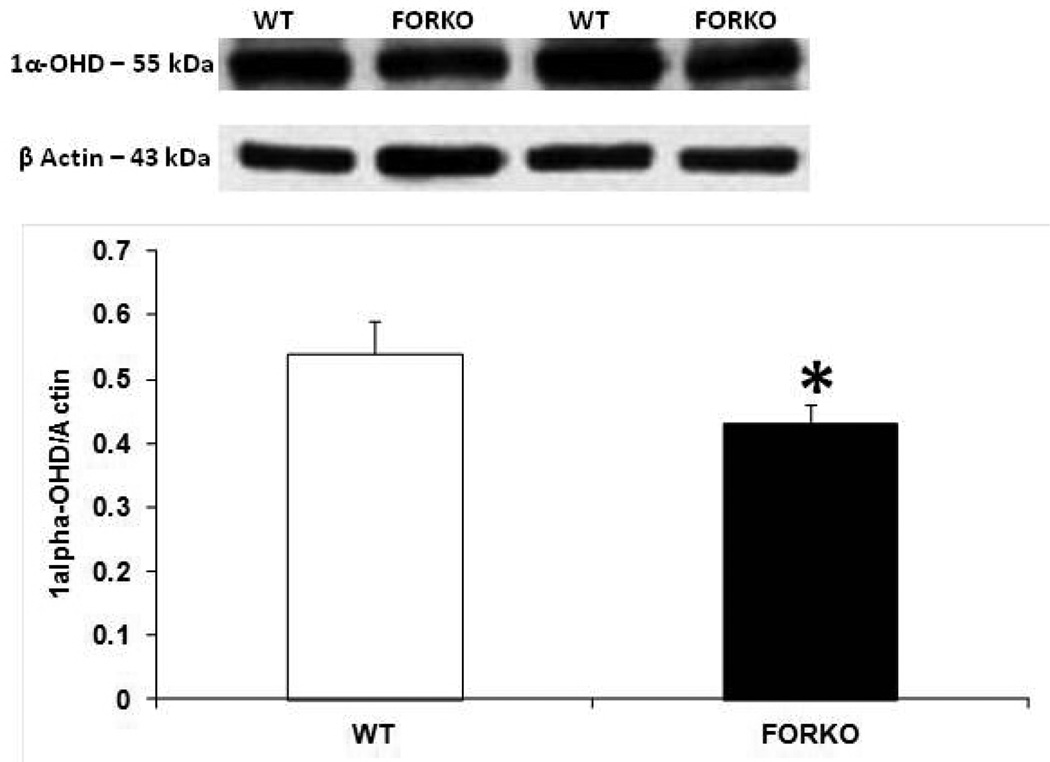
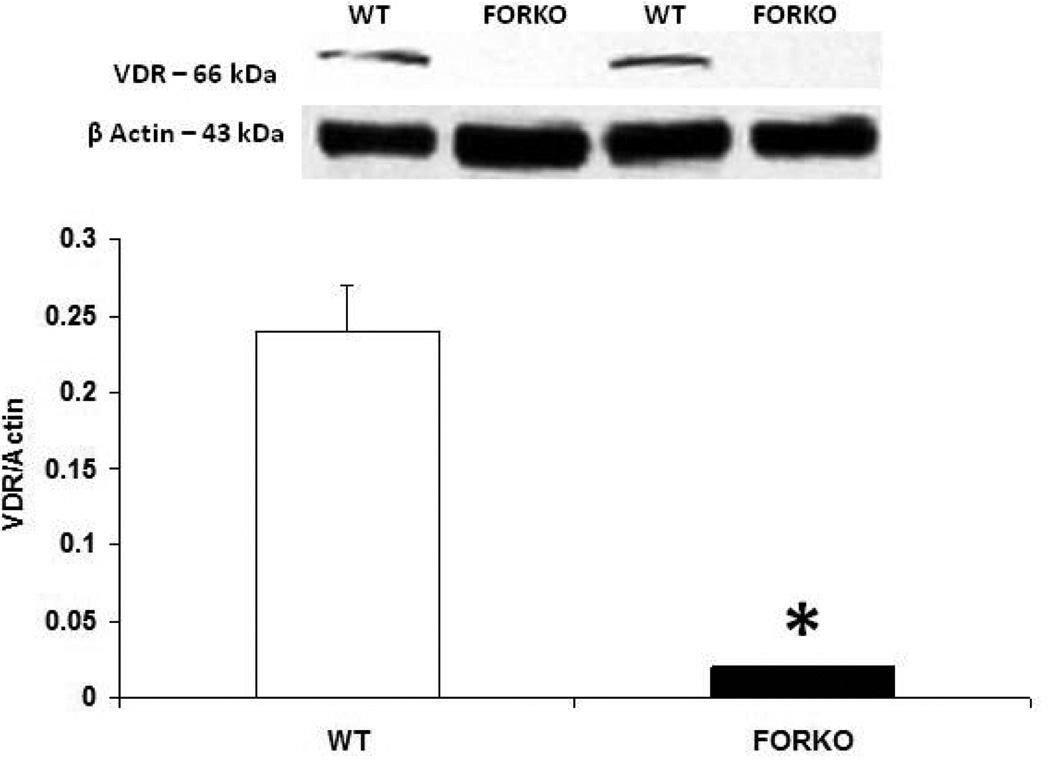
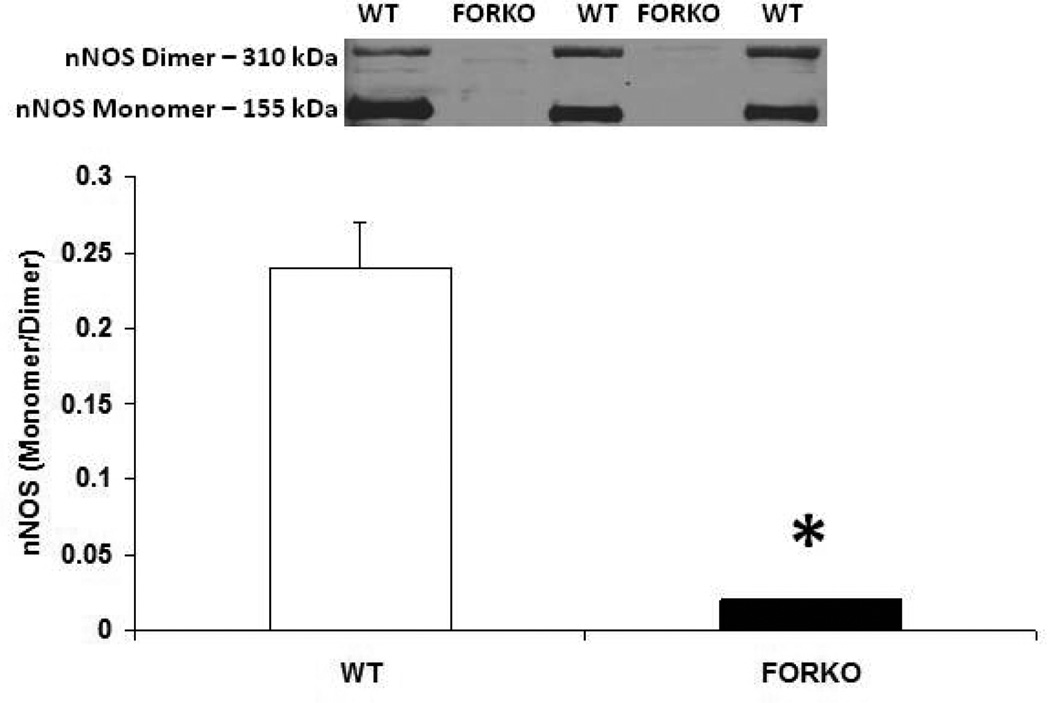
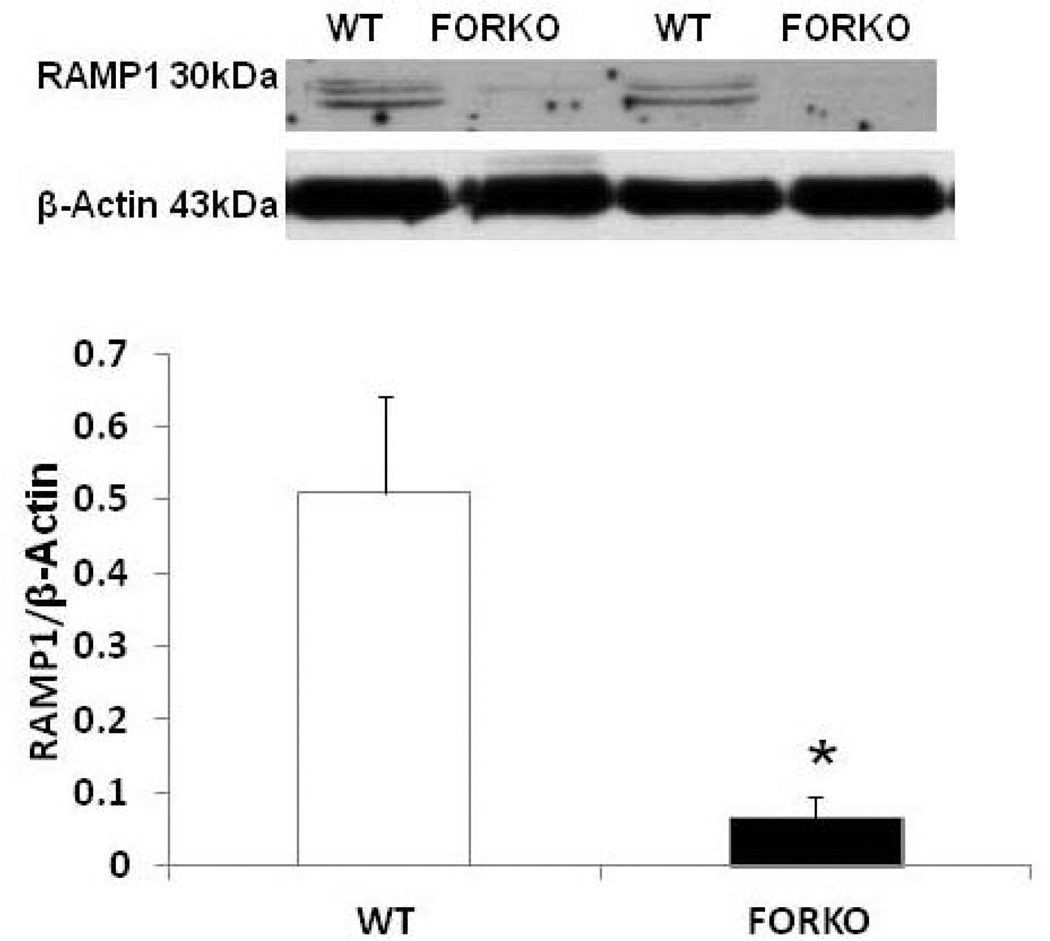
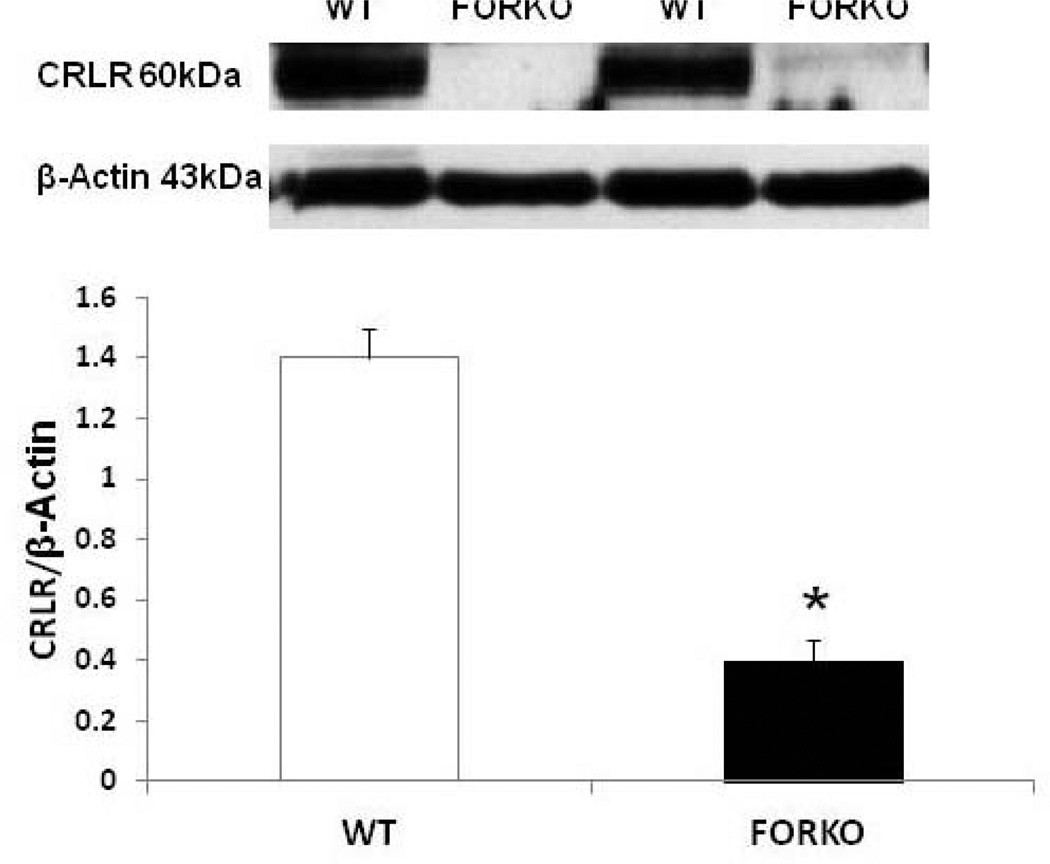
The metabolic syndrome represents a specific clustering of cardiovascular risk factors including obesity. It is highly prevalent in the United States, and currently affects approximately one in four adults. Hypertension in women increases fourfold the risk of subsequent cardiovascular disease, and leads to approximately 35% of all cardiovascular events (53). The presence of hypertension has a significantly greater effect on CHD in women than in men. African Americans-especially AA women- appear to be particularly predisposed to the development of the metabolic syndrome. AAs also have the highest mortality rate due to CHD of any ethnic group (54). Reports indicate that AAs have a 3-to-5-times higher cardiovascular mortality rate compared to whites (55). Although the exact mechanisms for developing this disease are not known, reduced levels of sex steroid hormones such as estrogens and/or vitamin D may be responsible for the increased CHD in this population (56). Others have shown a link between vitamin D and estrogens in the vasculature suggesting that these two factors both independently or inter-dependently regulate cardiovascular functions and normalize blood pressure via several endogenous potent vasodilators including prostaglandins (PGs), nitric oxide (NO) and calcitonin gene related peptide (CGRP) (57) (Figures 1–2). For example vitamin D or estradiol supplementation improves vascular NO and regulates blood pressure (58). Recent studies indicate that the enzymes responsible (59) for vitamin D synthesis are colocalized with calcitonin gene related peptide containing neurons (I-CGRP) in the dorsal root ganglia. We and others have demonstrated that the vascular protective effects of sex steroid hormones, namely estradiol-17beta and progesterone, involve CGRP (60). However, it is unclear whether sex hormones and/or vitamin D regulate the blood pressure directly or indirectly and if so, what molecular mechanisms are involved. Sun exposure, as an indirect index of vitamin D skin synthesis, has been reported to be inversely associated with blood pressure and the prevalence of hypertension. In addition, ultraviolet light exposure lowers blood pressure. This further supports the fact that estrogen receptors, vitamin D receptors (VDR) and the enzyme, 1-alpha-hydroxylase (1alpha-OHD), a key enzyme for vitamin D synthesis, are present in the vasculature. Further the decrease in vascular 1alpha-OHD and VDR (Figures 3, 4) in female FORKO (follicle stimulating hormone receptor knockout) mice, a model of hypertension and menopause, was associated with a reduction in neuronal NO synthase (nNOS) dimerization as well as a reduction in CGRP receptor components such as CRLR and RAMP1 (Figures 5–7). This data further support the hypothesis that a cross-talk between sex hormones and vitamin-D may play a pivotal role in regulating vascular functions, particularly in women.
Figure 1.
Proposed schematic diagram for involvement of vitamin D in the regulation of vascular functions. Vitamin D regulates estrogen (E2) synthesis in gonads by maintaining calcium homeostasis, and E2 binds to its receptors on endothelial cells and smooth muscle cells to regulate vascular functions. In addition, vitamin D activates vitamin D receptors (VDR) on endothelial cells and vascular smooth muscle cells to regulate endogenous vasodilators, including prostaglandins (PGs), nitric oxide (NO) and calcitonin gene related peptide (CGRP). Vitamin D deficiency results in NO-mediated endothelial dysfunction, and supplementation of vitamin D attenuates this symptom. In addition, vitamin D plays a pivotal role in the down-regulation of renin-angiotensin-aldosterone system (RAAS) by the kidney. Lower vitamin D levels may lead to an impaired RAAS and abnormalities in vascular relaxation that eventually lead to the development of hypertension.
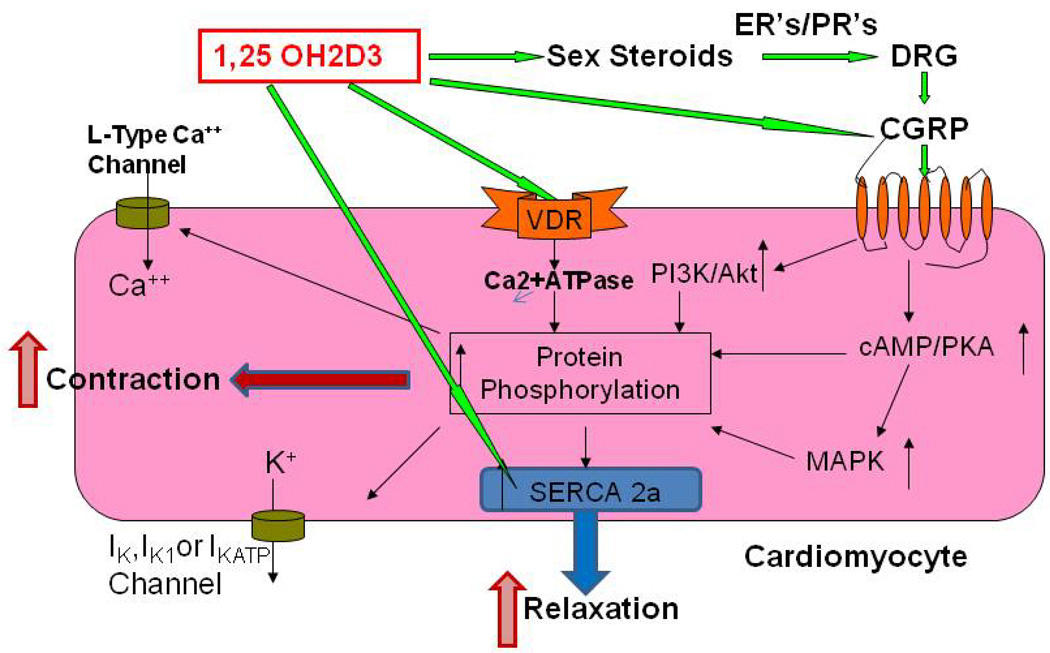
Figure 2.
Proposed schematic diagram for involvement of vitamin D in the myocardial contractility. Cardiomyocytes express vitamin D receptors (VDR), estrogen receptors (ER’s), progesterone receptors (PR’s) and CGRP receptors. Vitamin D regulates myocyte function through the biosynthesis of steroid hormone and CGRP. In addition, vitamin D has both genomic and nongenomic effects on the cardiomyocyte functions. VDR has been found to localize to t-tubules in the heart, and is in association with Serca-2, the sarcoplasmic reticulum Ca2+-ATPase, suggesting a role of VDR in regulating cardiac contractility by a direct interaction with Serca-2. Thus, vitamin D exerts an immediate effect on signal transduction mediators and ion channels in the cardiomyocyte, and plays an important role in not only heart structure, but also regulation of its function.
Figure 3.
Effect of chronic depletion of estrogens on the vascular vitamin D metabolizing enzyme, 1α-hydroxylase (1α-OHD) protein expression. Representative immunoblot and densitometric analysis data for 1α-OHD were depicted in figure 3. Groups of wild type control (WT) and follicle stimulating hormone receptor knockout female mice (FORKO; a model of hypertension and menopause128,129) were sacrificed and thoracic aortas were used for the westernblot analysis. The values are means ± SE for 3 animals in each group. P<0.05 compared to WT group.
Figure 4.
Effect of chronic depletion of estrogens on the vascular vitamin D receptor (VDR) protein expression. Representative immunoblot and densitometric analysis data for VDR were depicted in figure 4. Groups of wild type control (WT) and follicle stimulating hormone receptor knockout female mice (FORKO; a model of hypertension and menopause128, 129) were sacrificed and thoracic aortas were used for the westernblot analysis. The values are means ± SE for 3 animals in each group. P<0.05 compared to WT group.
Figure 5.
Effect of chronic depletion of estrogens on vascular neuronal nitric oxide synthase enzyme (nNOS) protein expression. Representative immunoblot and densitometric analysis data for vascular nNOS alpha were depicted in figure 5. Groups of wild type control (WT) and follicle stimulating hormone receptor knockout female mice (FORKO; a model of hypertension and menopause128, 129) were sacrificed and thoracic aortas were used for the westernblot analysis. The values are means ± SE for 3 animals in each group. P<0.05 compared to WT group.
Figure 7.
Effect of chronic depletion of estrogens on vascular CGRP receptor component, receptor activity modifying protein 1 (RAMP1) protein expression. Representative immunoblot and densitometric analysis data for vascular RAMP1 were depicted in figure 7. Groups of wild type control (WT) and follicle stimulating hormone receptor knockout female mice (FORKO; a model of hypertension and menopause128, 129) were sacrificed and thoracic aortas were used for the westernblot analysis. The values are means ± SE for 3 animals in each group. P<0.05 compared to WT group.
An increased activation of the RAAS plus endothelial dysfunction has been suggested as contributors to the development of hypertension (61). Vitamin D supplementation may protect subjects from hypertension by suppressing RAAS, restoring endothelial dysfunction, and reversing effects on calcium metabolism, including prevention of secondary hyperparathyroidism (62).
Specifically, supplementation with vitamin D has been shown to improve endothelial function in patients with type 2 diabetes (63). Unpublished studies from our laboratory indicate that non-calcemic 3-epi-isomer-vitamin D3 is more potent than 1,25 OH2D3 (known to possess high calcemic properties) in restoring reduced endothelial nitric oxide synthase (eNOS) dimerization in hyperglycemic human umbilical vascular endothelial cells (HUVEC) in-vitro. These data suggest that non-calcemic vitamin D analogs may be safe and useful in improving altered NO and thus control endothelial function at least in diabetic patients. Both VDR and 1 alpha-hydroxylase knock-out mice develop hypertension and myocardial hypertrophy (64). In addition, supplementation with vitamin D attenuates both the elevated blood pressure and impaired vascular relaxation in response to acetylcholine in spontaneous hypertensive rats (65). Finally, both endothelium-dependent and endothelium-independent vascular relaxation has been shown to be impaired in AA compared to non-AA subjects (66). Taken together, the above literature thus far suggests that the lower vitamin D levels observed in the AA population may potentially be related to impaired RAAS as well as abnormalities in vascular relaxation which may provide a link to the development of hypertension.
There are substantial gender differences in the incidences of hypertension, heart failure and coronary artery disease. The main risk factors for heart failure and coronary artery disease are diabetes and hypertension in both men and postmenopausal women (67). Ventricular and vascular stiffening (68), atrial fibrillation with hypertension and dilated cardiomyopathy (69) and drug induced prolongation of the electrocardiogram QT interval (70) were reported to occur more often in women than in men and the reverse was true for ischemic heart disease (71). Estrogen and testosterone were reported to have rapid nongenomic effects on ion channels and sarcolemmal currents (72), and their receptors were found to be colocalized in vascular smooth muscle and endothelial cells (73). Interactions between estrogen related receptors and other nuclear receptors such as the peroxisome proliferator activated receptors (PPARS) (74), as well as estrogen, testosterone and other sex steroids with gene loci on the X-chromosome may contribute to the male and postmenopausal predilections for cardiovascular disease and to the protection thought to be afforded by estrogen in premenopausal women.
5. SEX HORMONES AND RENAL DISEASE
Epidemiological studies show that men progress faster to end stage renal disease when they have conditions such as hypertensive glomerulosclerosis, polycystic kidney disease, or autoimmune glomerulonephritis. Even an age-related renal decline is faster in men than women, although women begin catching up after menopause (75). The mechanisms of action by which sex steroids prevent renal injury are multifactorial.
5.1. Sex hormones and the glomerular capillary pressure
Estradiol is thought to be renoprotective in part by decreasing the mitogenic effects of multiple growth factors that participate in glomerulosclerosis (76). Although the mechanism of action is unclear, it is felt that sex steroid-induced differences in glomerular capillary pressure may play a role. When men and women were both given an infusion of angiotensin (Ang) II, both groups had a similar response; there was an increase in blood pressure and a decrease in the effective renal plasma flow. However the resultant increase in estimated glomerular filtration rate (eGFR) was only maintained in men. In women the eGFR declined in parallel with the effective renal plasma flow and there was no increase in filtration fraction (FF). It can be surmised that the Ang II mediated increase in glomerular capillary pressure was greater in males than in females, and that the resultant increase in FF could lead to renal injury (75).
It is well established that inhibition of the RAAS delays the progression of renal disease. Testosterone may promote renal injury by stimulating the production of Ang II (75). In contrast estrogen decreases the angiotensin type 1 receptor (AT1) in many tissues including kidney. In addition estrogen increases angiotensinogen levels, and decreases plasma renin levels. The findings of the Women’s Health Initiative suggest these changes are lost after menopause (75).
5.2. Estradiol and testosterone effects on oxidative stress
There is also evidence that oxidative stress is linked to renal diseases such as drug-induced nephrotoxicity, IgA nephropathy (77), ischemia-reperfusion injury (78) and diabetic nephropathy (79). Estradiol is an antioxidant, while androgens appear to increase oxidative stress as evidenced by the fact that in human studies, men have higher levels of indicators of oxidative stress than age matched premenopausal women (80).
Recent reports demonstrated a synergistical role of 1, 25-dihydroxyvitamin D (3) and 17beta-estradial in proliferation and differentiation of osteoblasts, and this coordinated regulation might depend on the upregulation of vitamin D receptor in osteoblasts by 17beta-estradial. (81) Studies also suggested that increased androgenicity, characterized by high testosterone and low SHBG levels, is associated with an adverse CVD risk factor profile in postmenopausal women. (82)
5.3. Estradiol and the endothelial system
There is a paucity of data about the interaction of the sex hormones and the endothelial system. Since estradiol can down regulate AT1 receptor expression, it theoretically should increase endothelial markers such as NO and protect against renal injury, however such studies have not been performed (75). Estrogens play a pivotal role in a large number of physiological processes, including the cardiovascular system. Both acetylcholine-induced and flow-dependent vasodilation are preserved or potentiated by estrogen treatment in both animal models and humans. (83) E2 increases the endothelial production of nitric oxide and prostacyclin and prevents early atheroma through endothelial-mediated mechanisms. Furthermore, estrogens promote endothelial healing, as well as angiogenesis. Estrogen actions are essentially mediated by 2 molecular targets: estrogen receptor-alpha (ER-alpha) and ER-beta. An analysis of mouse models targeting ER-alpha or ER-beta demonstrated a prominent role of ER-alpha in vascular biology.
6. SEX HORMONES AND DIABETES
Results from a large clinical trial of 27,805 patients with type I diabetes mellitus indicated that the male sex was an independent risk factor associated with the development of CKD. In this study the female protective effect was lost in the presence of diabetes. There was also no gender difference in the development of CKD in patients with the metabolic syndrome (75).
7. REPLACEMENT STRATEGIES AND OUTCOMES
7.1. Vitamin D replacement and renal disease
Abnormal bone mineral metabolism with bone pain and osteodystrophy is present in all patients with stage 5 CKD. Severe osteodystrophy is less common in the earlier stages of CKD, but an elevation of parathyroid hormone, measured by intact parathyroid hormone (iPTH) levels has been reported (84–92). The K/DOQI guidelines recommend routine measurement of iPTH, phosphorous and calcium to manage and prevent metabolic bone disease (50). For patients with an elevated iPTH, the 25-OHD level should be assessed and replaced if a vitamin D deficiency (serum 25-OHD levels <30 ng/ml) is identified. Muntner et al (90) analyzed NHANES data for iPTH levels, and noted that iPTH levels were higher and vitamin D levels lower in ≤ stage 3 CKD. The 25-OHD level reduction caused a compensatory increase in iPTH in persons with normal renal function. Therefore reversing a vitamin D deficiency reduced the frequency of secondary hyperparathyroidism and metabolic bone disease (90). Consequently, vitamin D supplementation may be necessary to prevent these diseases.
A double blind, randomized, placebo controlled trial to evaluate the safety of paricalcitol in CKD stages 3 and 4 was conducted. In a subset of 118 patients that had baseline proteinuria, the odds of a reduction of proteinuria was 3.2 times greater (95% CI 1.5–6.9) for oral paricalcitoltreated patients than for placebo patients (93). Of the 57 paricalcitol patients, 29 (51%) had a reduction in proteinuria compared to only 15 of the 61 (25%) placebo patients (P=0.004). This suggests that vitamin D may possibly be important in maintaining the structural integrity of the kidney. More studies need to be done using less costly vitamin D preparations to evaluate if they are equally effective in reducing proteinuria.
It is recommended that patients with stage 5 CKD be treated with activated vitamin D. Older preparations, calcitriol and alfacalcidol, have been associated with increased serum calcium and phosphorus levels. Calcium and phosphorus elevations may cause calcification in the arteries and tissues. Newer preparations, including paricalcitol and doxecalciferol are supposed to reduce stimulation of parathyroid hormone in CKD patients but not appreciably increase calcium and phosphorus levels. The Cochrane Collaborative study did a metanalysis of 60 studies and found that patients receiving the older activated vitamin D compounds were not at increased risk of developing hypercalcemia or hyperphosphaturia (94). In those few studies that did see these changes, the results did not reach statistical significance. To the contrary, newer compounds were associated with more hypercalcemia (94).
7.2. Estrogens and progestins
It is well recognized that postmenopausal women of all ethnicities exhibit higher incidences of hypertension, heart failure and coronary artery disease than premenopausal women and that these outcomes can be explained by estrogen deficiency, although there is renewed interest in the related role of androgens (95). It is unclear whether deficiencies in estrogen synthesis, estrogen receptor expression or estrogen-related functional changes, some perhaps related to functions of the RAAS, contribute to the effects of postmenopausal estrogen deficiency (96). Postmenopausal women could be at higher risk for coronary artery disease because of lower plasminogen activator inhibitor-1 (PAI1) levels. PAI1 levels are reported to be correlated with lower plasma levels of estrogen and lower PAI-1 levels are reported to be associated with hypertension and heart failure, thereby suggesting that PAI1 may serve as a useful biomarker for the risk of cardiovascular disease (73). Cardiomyocytes express G-protein coupled estrogen receptors which are reported to decrease cardiac sarcolemmal K+ currents (97), prevent cardiac fibrosis (98) and provide cardioprotection from ischemia reperfusion injury (99). Knockout mice lacking the G-coupled estrogen receptor-2 exhibit systemic hypertension as well as right and left ventricular hypertrophy (100, 101). Estradiol-induced stimulation of G-protein coupled estrogen receptors resulted in cGMP mediated phosphorylation and inactivation of calcium-activated K+ channels in human coronary artery myocytes independent of the endothelium (102), known mechanisms related to NO-mediated vascular smooth muscle relaxation. Regions of human atherosclerotic plaques had higher rates of DNA methylation of the promoter region of the estrogen-beta receptor gene than non-atherosclerotic regions (103), suggesting a role for epigenetic mechanisms. In normotensive experimental animals, estrogens were shown to relax coronary artery smooth muscle (104) by superoxide-mediated (105), NO-mediated and protein kinase C related mechanisms (106). Paradoxically, the estrogen receptor antagonist tamoxifen also appeared to relax coronary artery smooth muscle by an endothelium, NO-dependent mechanism (107). On the other hand, attenuation of vascular smooth muscle relaxation is shown to occur in spontaneously hypertensive female rats (108). In humans, hormone replacement therapy with estrogen is reported to be associated with adverse cardiovascular events (109). Ethinyl estradiol and some progestins were reported to activate the RAAS (110).
7.3. Androgens: testosterone and dehydroepiandrosterone (DHEA)
Testosterone antagonizes vascular smooth muscle relaxation produced by adenosine, thereby increasing vascular resistance (111). Although the mechanisms remain unclear, the low coronary blood flow associated with high LDL, high VLDL and low HDL levels correlated with the risk for development of systemic hypertension and heart failure. High pulse pressure is shown to be inversely correlated with penile blood flow and to be a predictor of low plasma testosterone, erectile dysfunction and coronary artery disease (112). Low blood flow to medullary vasomotor centers has been proposed as a mechanism for essential hypertension (113) and perhaps chronic renal disease. Low plasma testosterone and high estradiol are reported to be associated with development of the metabolic syndrome triad of obesity, diabetes and hypertension (114). The presence of a vertex pattern of androgenic alopecia appears to be a correlate of the metabolic syndrome and atherosclerosis in men and successful treatment with finasteride suggested involvement of an increased activity of 5-alpha reductase as an important mechanism (115). In young women, high plasma testosterone levels are associated with high incidences of coronary artery disease (116). In women with polycystic ovary syndrome, plasma testosterone appears to be positively correlated with systemic blood pressure (117). Recent studies demonstrated large beneficial effects of DHEA administration on Mental Rotation, Subject-Ordered Pointing, Fragmented Picture Identification, Perceptual Identification, and Same-Different Judgment.(118) Moreover, DHEA administration enhanced serum levels of DHEA, DHEAS, testosterone, and estrone, and regression analyses demonstrated that levels of DHEA and its metabolites were positively related to cognitive performance on the visual-spatial tasks in the DHEA condition.
The incidence of chronic renal diseases is associated with postmenopausal loss of, and is improved by treatment with estrogen (119). The precursors for estrogens are the androgenic hormones testosterone and DHEA and low DHEA levels were reported to be associated with endothelial dysfunction, renal injury and increased cardiovascular mortality, thereby providing a rationale for DHEA supplementation in postmenopausal women (120). Administration of DHEA to ovariectomized rats protected against hypertension-induced kidney injury via upregulation of the sigma-1R receptor and stimulation of Akt-eNOS signaling (121).
7.4. Oxytocin
Modulations in expression of oxytocin and its receptors may also contribute to various predilections for hypertension, heart failure and coronary artery disease. Decrements in oxytocin receptor activity are reported to contribute to cardiac pathology by decreases in left ventricular ejection fraction and increases in collagen content, indicative of fibrosis (122). Oxytocin receptors have been demonstrated in cardiomocytes (123) and in the capillary endothelium expressing CD31 colocalized with NO (124). In ovariectomized spontaneously hypertensive rats, genistein-stimulated increases in synthesis of oxytocin receptors were reported to be associated with decrements in mean arterial blood pressure, heart weight, collagen content and brain natriuretic peptide (122).
A randomized, double-blind, placebo-controlled trial was conducted at three Italian university medical centers to assess the effects of the administration of the phytoestrogen genistein (54 mg/d) on some predictors of cardiovascular risk in osteopenic, postmenopausal women (125). The results suggested that 54 mg genistein plus calcium, vitamin D3, and a healthy diet was associated with favorable effects on both glycemic control and some cardiovascular risk markers in a cohort of osteopenic, postmenopausal women.
Studies exploring the effect of calcium intake or calcium supplementation on cardiovascular risk suggest that systolic blood pressure increases under low calcium intake and decreases with calcium supplementation (126). A lower calcium intake has been associated with an increased risk of stroke. Negative correlations between vitamin D levels and the risk of hypertension, myocardial infarction, and stroke have been reported in several observational studies. However, there is a lack of randomized clinical trials primarily addressing the effect of these parameters on CVD. Therefore, the real impact of calcium and vitamin D on cardiovascular outcomes remains to be documented by appropriate experimental data.
8. PERSPECTIVE
Metabolic syndrome represents a specific clustering of cardiovascular risk factors including obesity. Several lines of evidence suggest that overweight and diabetes are most common cause of hypertension and gastroparesis due to at least in part due to impairment in vascular as well as stomach nitric oxide system. Metabolic syndrome is highly prevalent in the United States, and currently affects approximately one in four adults. Hypertension in women increases the risk of subsequent cardiovascular disease by fourfold, and leads to approximately 35% of all cardiovascular events (53). The presence of hypertension has a significantly greater effect on CHD in women than in men. African Americans-especially AA women appear to be particularly predisposed to the development of the metabolic syndrome. AAs also have the highest mortality rate due to CHD of any ethnic group (54). Reports indicate that AAs have a 3-to-5-times higher cardiovascular mortality rate compared to whites (55). Although the exact mechanisms for developing this disease are not known, reduced levels of sex steroid hormones such as estrogens and/or of vitamin D may be responsible for the increased CHD in this population (56). Others have shown a link between vitamin D and estrogens in the vasculature suggesting that these two factors both independently or inter-dependently regulate cardiovascular functions through their receptors and normalize blood pressure via several endogenous potent vasodilators including PGs, nitric oxide NO and CGRP (57).
It is evident that vitamin D improves eNOS expression in vascular cells and that vitamin D metabolizing enzymes appear to be colocalized with I-CGRP neurons in the dorsal root ganglia. Sex steroid hormones, in particular estradiol-17beta and/or soy isoflavones (estrogenic compounds), regulate eNOS- or nNOS-mediated neuroendocrine and vascular smooth muscle function through the NMDA (N-methyl-D-aspartate) receptor channels, calcium/calmodulin and HSP-90 signaling. On the other hand, sex steroid hormones regulate cardiovascular functions via endothelium-independent mechanisms such as CGRP. Our findings including others support the existing hypothesis that sex steroid hormones and vitamin D may regulate renocardiovascular as well as gut motility functions through a number of mechanisms including NO and CGRP. Large clinical trials are needed to unravel the beneficial effects of natural hormones/activation of hormone receptors, and/or vitamin D/VDR analogs on the kidneys, blood pressure and rest of the body, and to determine their place in the treatment of menopause.
Figure 6.
Effect of chronic depletion of estrogens on vascular CGRP receptor component, calcitonin like receptor (CRLR) protein expression. Representative immunoblot and densitometric analysis data for vascular CRLR were depicted in figure 6. Groups of wild type control (WT) and follicle stimulating hormone receptor knockout female mice (FORKO; a model of hypertension and menopause128, 129) were sacrificed and thoracic aortas were used for the westernblot analysis. The values are means ± SE for 3 animals in each group. P<0.05 compared to WT group.
ACKNOWLEDGEMENTS
This work has been supported by a Meharry Translational Research Pilot Grant (MeTRC- NIH/NCRR; 1 U54RR026140-01) to Dr. Gangula, NIH/NCRR grant I U54RR026138 to Dr. Nicholas and in part by grant GM08016-38 NIGMS/NIH to Dr. Haddad. All of the authors contributed to the preparation of this manuscript. We thank Kalpana Ravella, Vijayakumar Chinnathambi (Gangula’s lab; FORKO experiments) and Sharon Chakradhari (Dr. Al-Hendy lab; FORKO breeding and supply mice) for technical assistance. We thank Dr. Diana Marver for a review of the manuscript.
Abbreviations
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- CGRP
calcitonin gene related peptide
- CRLR
calcitonin receptor like receptor
- RAMP1
receptor activity modifying protein 1
- RAAS
renin-angiotensin-aldosterone system
- SPF
sun protection factor
- PG
prostaglandin
- HSP-90
heat shock protein-90
- NMDA
N-methy-D-aspartate
- CHD
coronary heart disease
- AA
African-American
- TNF-α
tumor necrosis factor-alpha
- IL-10
interleukin-10
- CKD
chronic kidney disease
- VDR
vitamin D receptor
- ER
estrogen receptor
- PR
progesterone receptor
- E2
estradiol-17B
- P4
progesterone
- HUVEC
human umbilical vascular endothelial cells
- GFR
glomerular filtration rate
- FF
filtration fraction
- Ang
angiotensin
- AT1
angiotensin type 1
- iPTH
intact parathyroid hormone
- K/DOQ1
Kidney Disease Outcome Quality Initiation
- PAI-1
plasminogen actiator inhibitor-1
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- VLDL
very low density lipoprotein
- DHEA
dehydroepiandrosterone
- AC
adenylate cyclase
- PKA
protein kinase-A
- FORKO
follicle-stimulating hormone receptor knockout
- WT
wild type
REFERENCES
- 1.Wilson PW, Kannel WB, Sibershatz H, D’Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. J Am Med Assoc. 1996;275:1571–1576. [PubMed] [Google Scholar]
- 3.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Androne AS, Hryniewicz K, Hudaihed A. Comparison of metabolic vasodilation in response to exercise and ischemia and endothelium-dependent flow-mediated dilation in African-American versus non-African-American patients with chronic heart failure. Am J Cardiol. 2006;97:685–689. doi: 10.1016/j.amjcard.2005.09.115. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, Stevenson J. British Menopause Society Council. Primary prevention of coronary heart disease in women. Menopause Int. 2008;14:40–45. doi: 10.1258/mi.2007.007037. [DOI] [PubMed] [Google Scholar]
- 6.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 8.Working Group of the Australian and New Zealand Bone and Mineral Society. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005;182:281–285. doi: 10.5694/j.1326-5377.2005.tb06701.x. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zittermann A, Gummert JF, Borgermann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. 2009;12:634–639. doi: 10.1097/MCO.0b013e3283310767. [DOI] [PubMed] [Google Scholar]
- 12.Zittermann A, Koerfer R. Protective and toxic effects of vitamin D on vascular calcification: clinical implications. Mol Aspects Med. 2008;29:423–432. doi: 10.1016/j.mam.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 14.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 15.Chapuy MC. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 17.Aaron JE. Frequency of osteomalacia and osteoporosis in fractures of the proximal femur. Lancet. 1974;1:229–133. doi: 10.1016/s0140-6736(74)92545-8. [DOI] [PubMed] [Google Scholar]
- 18.Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Pérez-Sacristán B. Suitability of ultra-high performance liquid chromatography for the determination of fat-soluble nutritional status (vitamins A, E, D, and individual carotenoids) Anal Bioanal Chem. 2010;397:1389–1393. doi: 10.1007/s00216-010-3655-2. [DOI] [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Huqhes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 20.Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Erikson EF. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–2658. doi: 10.1046/j.1365-2796.2000.00595.x. [DOI] [PubMed] [Google Scholar]
- 21.Boonen S, Bischoff-Ferrari HA, Cooper C, Lips P, Ljunqqren O, Meunier PJ, Reqinster JY. Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int. 2006;78:257–270. doi: 10.1007/s00223-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 22.Lips P, Hosking D, Liqquner K, Norquist JM, Wehren L, Maalouf G, Raqi-Eis S, Chandler J. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 23.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19:370–378. doi: 10.1359/JBMR.0301240. [DOI] [PubMed] [Google Scholar]
- 24.Tangpricha V, Koutkia P, Rieke SM, TC Chen, Perez AA, Holick MF. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr. 2003;77:1478–1483. doi: 10.1093/ajcn/77.6.1478. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Luc MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 27.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 28.Martini LA, Wood RJ. Vitamin D and blood pressure connection: update on epidemiologic, clinical, and mechanistic evidence. Nutr Rev. 2008;66:291–297. doi: 10.1111/j.1753-4887.2008.00035.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MA, Davey A, Park S, Hausman DB, Poon LW. Age, race and season predict vitamin D status in African American and white octogenarians and centenarians. J Nutr Health Aging. 2008;12:690–695. doi: 10.1007/BF03028616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mheid IAl, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58:186–192. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 32.Argacha JF, Egrise D, Pochet S, Fontaine D, Lefort A, Libert F, Goldman S, van de Borne P, Berkenboom G, Moreno-Reyes R. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol. 2011;58:65–71. doi: 10.1097/FJC.0b013e31821c832f. [DOI] [PubMed] [Google Scholar]
- 33.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, Post W, O’Connell JR, Hixson JE, Kardia SL, Sun YV, Jhun MA, Wang X, Mehta NN, Li M, Koller DL, Hakonarson H, Keating BJ, Rader DJ, Shuldiner AR, Peyser PA, Reilly MP, Mitchell BD. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2010;30:2648–2654. doi: 10.1161/ATVBAHA.110.211805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu E, Meigs JB, Pittas AG, Economos CD, McKeown NM, Booth SL, Jacques PF. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham offspring study. Am J Clin Nutr. 2010;91:1627–1633. doi: 10.3945/ajcn.2009.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Schooten FJ, Hirvonen A, Maas LM, De Mol BA, Kleinjans JC, Bell DA, Durrer JD. Putative susceptibility markers of coronary artery disease: association between VDR genotype, smoking, and aromatic DNA adduct levels in human right atrial tissue. FASEB J. 1998;12:1409–1417. doi: 10.1096/fasebj.12.13.1409. [DOI] [PubMed] [Google Scholar]
- 36.Testa A, Mallamaci F, Benedetto FA, Pisano A, Tripepi G, Malatino L, Thadhani R, Zoccali C. Vitamin D receptor (VDR) gene polymorphism is associated with left ventricular (LV) mass and predicts left ventricular hypertrophy (LVH) progression in end-stage renal disease (ESRD) patients. J Bone Miner Res. 2010;25:313–319. doi: 10.1359/jbmr.090717. [DOI] [PubMed] [Google Scholar]
- 37.Ban Y, Taniyama M, Ban Y. Vitamin D receptor gene polymorphism is associated with Graves' disease in the Japanese population. J Clin Endocr Metab. 2000;85:4639–4643. doi: 10.1210/jcem.85.12.7038. [DOI] [PubMed] [Google Scholar]
- 38.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 42.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 43.Hughes PJ, Lee JS, Reiner NE, Brown G. The vitamin D receptor-mediated activation of phosphatidylinositol 3-kinase (PI3Kalpha) plays a role in the 1alpha,25-dihydroxyvitamin D3-stimulated increase in steroid sulphatase activity in myeloid leukaemic cell lines. J Cell Biochem. 2008;103:1551–1572. doi: 10.1002/jcb.21545. [DOI] [PubMed] [Google Scholar]
- 44.Regulska M, Leskiewicz M, Budziszewska B, Kutner A, Basta-Kaim A, Kubera M, Jaworska-Feil L, Lason W. Involvement of PI3-K in neuroprotective effects of the 1,25-dihydroxyvitamin D3 analogue - PRI-2191. Pharmacol Rep. 2006;58:900–907. [PubMed] [Google Scholar]
- 45.Wang J, Zhao Y, Kauss MA, Spindel S, Lian H. Akt regulates vitamin D3-induced leukemia cell functional differentiation via Raf/MEK/ERK MAPK signaling. Eur J Cell Biol. 2009;88:103–115. doi: 10.1016/j.ejcb.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y, Xu L, Liang X, Liang Y. 1,25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol. 2009;30:34–43. doi: 10.1159/000200769. [DOI] [PubMed] [Google Scholar]
- 47.Tan J, Lu J, Huang W, Dong Z, Kong C, Li L, Gao L, Guo J, Huang B. Genome-wide analysis of histone H3 lysine9 modifications in human mesenchymal stem cell osteogenic differentiation. PLoS One. 2009;4:e6792. doi: 10.1371/journal.pone.0006792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, Slatopolsky E. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol. 2010;121:188–192. doi: 10.1016/j.jsbmb.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG. Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension. 2008;52:1106–1112. doi: 10.1161/HYPERTENSIONAHA.108.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4) Suppl 3:S1–S201. [PubMed] [Google Scholar]
- 51.Brown AJ. Therapeutic uses of vitamin D analogues. Am J Kidney Dis. 2001;38(5) Suppl 5:S3–S19. doi: 10.1053/ajkd.2001.28111. [DOI] [PubMed] [Google Scholar]
- 52.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 53.Griffin FC, Gadegbeku CA, Sowers MR. Vitamin D and subsequent systolic hypertension among women. Am J Hyperten. 2011;24:316–321. doi: 10.1038/ajh.2010.226. [DOI] [PubMed] [Google Scholar]
- 54.Williams RA. Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc. 2009;101:536–540. doi: 10.1016/s0027-9684(15)30938-x. [DOI] [PubMed] [Google Scholar]
- 55.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK, Robb KJ. Twelve-year follow-up of American women's awareness of cardiovascular disease risk and barriers to heart health. Circ Cardiovasc Qual Outcomes. 2010;3:120–127. doi: 10.1161/CIRCOUTCOMES.109.915538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson MA, Davey A, Park S, Hausman DB, Poon LW. Georgia Centenarian Study. Age, race and season predict vitamin D status in African American and white octogenarians and centenarians. J Nutr Health Aging. 2008;12:690–695. doi: 10.1007/BF03028616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somjen D, Katzburg S, Baz M, Stern N, Posner GH. Modulation of the response to estradiol-17 beta of rat vascular tissues by a non-calcemic vitamin D analog. J Steroid Biochem Mol Biol. 2004;89–90:339–341. doi: 10.1016/j.jsbmb.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, Parkington HC. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011 Aug 1; doi: 10.1113/jphysiol.2011.214726. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat. 2011;41:1–12. doi: 10.1016/j.jchemneu.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gangula PR, Chauhan M, Reed L, Yallampalli C. Age-related changes in dorsal root ganglia, circulating and vascular calcitonin gene-related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett. 2009;454:118–123. doi: 10.1016/j.neulet.2009.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.George J, Struthers AD. Evaluation of the aldosterone-blocking agent eplerenone in hypertension and heart failure. Expert Opin Pharmacother. 2007;8:3053–3059. doi: 10.1517/14656566.8.17.3053. [DOI] [PubMed] [Google Scholar]
- 62.Sekkarie M. The impact of over-the-counter vitamin D supplements on vitamin D and parathyroid hormone levels in chronic kidney disease. Clin Nephrol. 2006;65:91–96. doi: 10.5414/cnp65091. [DOI] [PubMed] [Google Scholar]
- 63.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 65.Kähönen M, Näppi S, Jolma P, Hutri-Kähönen N, Tolvanen JP, Saha H, Koivisto P, Krogerus L, Kalliovalkama J, Pörsti I. Vascular influences of calcium supplementation and vitamin D-induced hypercalcemia in NaCl-hypertensive rats. J Cardiovasc Pharmacol. 2003;42:319–328. doi: 10.1097/00005344-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Houghton JL, Philbin EF, Strogatz DS, Torosoff MT, Fein SA, Kuhner PA, Smith VE, Carr AA. The presence of African American race predicts improvement in coronary endothelial function after supplementary L-arginine. J Am Coll Cardiol. 2002;39:1314–1322. doi: 10.1016/s0735-1097(02)01781-3. [DOI] [PubMed] [Google Scholar]
- 67.Regitz-Zagrosek V, Lehmkuhl E, Mahmoodzadeh S. Gender aspects of the role of the metabolic syndrome as a risk factor for cardiovascular disease. Gend Med. 2007;(4) Suppl B:S162–S177. doi: 10.1016/s1550-8579(07)80056-8. [DOI] [PubMed] [Google Scholar]
- 68.Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Sliwa K, Carrington MJ, Klug E, Opie L, Lee G, Ball J, Stewart S. Predisposing factors and incidence of newly diagnosed atrial fibrillation in an urban African community: insights from the Heart of Soweto Study. Heart. 2010;96:1878–1882. doi: 10.1136/hrt.2010.206938. [DOI] [PubMed] [Google Scholar]
- 70.Yang PC, Clancy CE. Effects of sex hormones on cardiac repolarization. J Cardiovasc Pharmacol. 2010;56:123–129. doi: 10.1097/FJC.0b013e3181d6f7dd. [DOI] [PubMed] [Google Scholar]
- 71.Wake R, Yoshiyama M. Gender differences in ischemic heart disease. Recent Pat Cardiovasc Drug Discov. 2009;4:234–240. doi: 10.2174/157489009789152249. [DOI] [PubMed] [Google Scholar]
- 72.Verrecchia F, Hervé JC. Nongenomic steroid action: Inhibiting effects on cell-to-cell communication between rat ventricular myocytes. Exp Clin Cardiol. 2001;6:124–131. [PMC free article] [PubMed] [Google Scholar]
- 73.Qiao X, McConnell KR, Khalil RA. Sex steroids and vascular responses in hypertension and aging. Gend Med. 2008;5(Suppl A):S46–S64. doi: 10.1016/j.genm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008;8:329–337. doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanes LL, Sartori-Valinotti JC, Reckelhoff JF. Sex steroids and renal disease: lessons from animal studies. Hypertension. 2008;51:976–981. doi: 10.1161/HYPERTENSIONAHA.107.105767. [DOI] [PubMed] [Google Scholar]
- 76.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 77.Ong-ajyooth LO, Parichatikanond P. The effect of alpha tocopherol on the oxidative stress and antioxidants in idiopathic IgA nephropathy. J Med Assoc Thai. 2006;89(Supplement 5):S164–S170. [PubMed] [Google Scholar]
- 78.Singh DCV, Chopra K. Carvedilol attenuates ischemia-reperfusion induced oxidative renal injury in rats. Fund Clin Pharmacol. 2004;18:627–634. doi: 10.1111/j.1472-8206.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- 79.Cetin HOS, Oktem F, Ciris M, Uz E, Asian C, Ozguner F. Novel evidence suggesting an anti-oxidant property for erythropoietin on vancomycin-induced nephrotoxicity in a rat model. Clin Exp Pharmacol Physiol. 2007;34:1181–1185. doi: 10.1111/j.1440-1681.2007.04695.x. [DOI] [PubMed] [Google Scholar]
- 80.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 81.Song L, Zhang X, Zhou Y. A synergetic role of 1,25-dihydroxyvitamin D(3) in 17β-estradial induced-proliferation and differentiation of osteoblastic MC3T3-E1 cells. Eur J Pharmacol. 2011;659:273–280. doi: 10.1016/j.ejphar.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Brand JS, van der Schouw YT. Testosterone, SHBG and cardiovascular health in postmenopausal women. Int J Impot Res. 2010;22:91–104. doi: 10.1038/ijir.2009.64. [DOI] [PubMed] [Google Scholar]
- 83.Arnal JF, Fontaine C, Billon-Galés A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 84.Brossard JH, Cloutier M, Roy L, Lepage R, Gascon-Barre M, D’Amour P. Accumulation of a non-(1–84) molecular form of parathyroid hormone (PTH) detected by intact PTH assay in renal failure: importance in the interpretation of PTH values. J Clin Endocrinol Metab. 1996;81:3923–3929. doi: 10.1210/jcem.81.11.8923839. [DOI] [PubMed] [Google Scholar]
- 85.Coburn JW, Elangovan L. Prevention of metabolic bone disease in the pre-end-stage renal disease setting. J Am Soc Nephrol. 1998;9(12 Suppl):S71–S77. [PubMed] [Google Scholar]
- 86.Coen G, Mazzaferro S, Ballanti P, Sardella D, Chicca S, Manni M, Bonucci E, Taqqi F. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: a cross-sectional study. Nephrol Dial Transplant. 1996;11:813–819. doi: 10.1093/oxfordjournals.ndt.a027404. [DOI] [PubMed] [Google Scholar]
- 87.Fajtova VT, Sayegh MD, Hickey N, Aliabadi P, Lazarus JM, LeBoff MS. Intact parathyroid hormone levels in renal insufficiency. Calcif Tissue Int. 1995;57:329–335. doi: 10.1007/BF00302067. [DOI] [PubMed] [Google Scholar]
- 88.Llach F, Massry SG, Singer FR, Kurokawa K, Kaye JH, Coburn JW. Skeletal resistance to endogenous parathyroid hormone in pateints with early renal failure. A possible cause for secondary hyperparathyroidism. J Clin Endocrinol Metab. 1975;41:339–345. doi: 10.1210/jcem-41-2-339. [DOI] [PubMed] [Google Scholar]
- 89.Martinez I, Saracho R, Montenegro J, Llach F. The importance of dietary calcium and phosphorous in the secondary hyperparathyroidism of patients with early renal failure. Am J Kidney Dis. 1997;29:496–502. doi: 10.1016/s0272-6386(97)90330-9. [DOI] [PubMed] [Google Scholar]
- 90.Muntner P, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB. Association of serum intact parathyroid hormone with lower estimated glomular filtration rate. Clin J Am Soc Nephrol. 2009;4:184–194. doi: 10.2215/CJN.03050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rix M, Andreassen H, Eskildsen P, Lanqdahl B, Olqaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56:1084–1093. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 92.Torres A, Lorenzo V, Hernandez D, Rodriquez JC, Concepcion MT, Rodriquez AP, Hernandez A, Bonis Ede, Daris E, Gonzalez-Posada JM, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int. 1995;47:1434–1442. doi: 10.1038/ki.1995.201. [DOI] [PubMed] [Google Scholar]
- 93.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68:2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 94.Palmer SC, Craig JC, Elder G, Macaskill P, Strippoli GFM. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2009;7:CD008175. doi: 10.1002/14651858.CD005633.pub2. [DOI] [PubMed] [Google Scholar]
- 95.Kaushik M, Sontineni SP, Hunter C. Cardiovascular disease and androgens: a review. Int J Cardiol. 2010;142:8–14. doi: 10.1016/j.ijcard.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 96.Krishnamurthi K, Verbalis JG, Zheng W, Wu Z, Clerch LB, Sandberg K. Estrogen regulates angiotensin AT1 receptor expression via cytosolic proteins that bind to the 5' leader sequence of the receptor mRNA. Endocrinology. 1999;140:5435–5438. doi: 10.1210/endo.140.11.7242. [DOI] [PubMed] [Google Scholar]
- 97.Gebeily GEl, Fiset C. Upregulation of ventricular potassium channels by chronic tamoxifen treatment. Cardiovasc Res. 2011;90:68–76. doi: 10.1093/cvr/cvq384. [DOI] [PubMed] [Google Scholar]
- 98.Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol. 2010;24:2152–2165. doi: 10.1210/me.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deschamps AM, Murphy E, Sun J. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med. 2010;20:73–78. doi: 10.1016/j.tcm.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc Natl Acad Sci U S A. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arias-Loza PA, Jazbutyte V, Pelzer T. Genetic and pharmacologic strategies to determine the function of estrogen receptor alpha and estrogen receptor beta in cardiovascular system. Gend Med. 2008;5(Suppl A):S34–S45. doi: 10.1016/j.genm.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Han G, Yu X, Lu L, Li S, Ma H, Zhu S, Cui X, White RE. Estrogen receptor alpha mediates acute potassium channel stimulation in human coronary artery smooth muscle cells. J Pharmacol Exp Ther. 2006;316:1025–1030. doi: 10.1124/jpet.105.093542. [DOI] [PubMed] [Google Scholar]
- 103.Kim J, Kim JY, Song KS, Lee YH, Seo JS, Jelinek J, Goldschmidt-Clermont PJ, Issa JP. Epigenetic changes in estrogen receptor beta gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim Biophys Acta. 2007;1772:72–80. doi: 10.1016/j.bbadis.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- 105.White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1468–H1475. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 107.Figtree GA, Webb CM, Collins P. Tamoxifen acutely relaxes coronary arteries by an endothelium-, nitric oxide-, and estrogen receptor-dependent mechanism. J Pharmacol Exp Ther. 2000;295:519–523. [PubMed] [Google Scholar]
- 108.Santos RL, Marin EB, Gonçalves WL, Bissoli NS, Abreu GR, Moysés MR. Sex differences in the coronary vasodilation induced by 17 β-oestradiol in the isolated perfused heart from spontaneously hypertensive rats. Acta Physiol (Oxf) 2010;200:203–210. doi: 10.1111/j.1748-1716.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 109.Shetty KD, Vogt WB, Bhattacharya J. Hormone replacement therapy and cardiovascular health in the United States. Med Care. 2009;47:600–606. doi: 10.1097/MLR.0b013e31818bfe9b. [DOI] [PubMed] [Google Scholar]
- 110.Elger W, Beier S, Pollow K, Garfield R, Shi SQ, Hillisch A. Conception and pharmacodynamic profile of drospirenone. Steroids. 2003;68:891–905. doi: 10.1016/j.steroids.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Ceballos G, Figueroa L, Rubio I, Gallo G, Garcia A, Martinez A, Yañez R, Perez J, Morato T, Chamorro G. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999;33:691–697. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 112.Corona G, Mannucci E, Lotti F, Fisher AD, Bandini E, Balercia G, Forti G, Maggi M. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med. 2009;6:285–293. doi: 10.1111/j.1743-6109.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 113.Garg VK, Pepper GM. Essential hypertension: could the basic defect be in blood supply of vasomotor centre? Med Hypotheses. 1995;45:287–291. doi: 10.1016/0306-9877(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 114.Traish AM, Kypreos KE. Testosterone and cardiovascular disease: an old idea with modern clinical implications. Atherosclerosis. 2011;214:244–248. doi: 10.1016/j.atherosclerosis.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 115.Duskova M, Hill M, Starka L. Changes of metabolic profile in men treated for androgenetic alopecia with 1 mg finasteride. Endocr Regul. 2010;44:3–8. doi: 10.4149/endo_2010_01_3. [DOI] [PubMed] [Google Scholar]
- 116.Sablik Z, Samborska-Sablik A, Bolińska-Sołtysiak H, Goch JH, Kula K. Hyperandrogenism as a risk factor of coronary artery disease in young women. Pol Arch Med Wewn. 2006;115:118–124. [PubMed] [Google Scholar]
- 117.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 118.Stangl B, Hirshman E, Verbalis J. Administration of dehydroepiandrosterone (DHEA) enhances visual-spatial performance in postmenopausal women. Behav Neurosci. 2011;125:742–752. doi: 10.1037/a0025151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fung MM, Poddar S, Bettencourt R, Jassal SK, Barrett-Connor E. A cross-sectional and 10-year prospective study of postmenopausal estrogen therapy and blood pressure, renal function, and albuminuria: the Rancho Bernardo Study. Menopause. 2011;18:629–637. doi: 10.1097/gme.0b013e3181fca9c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Labrie F. DHEA, important source of sex steroids in men and even more in women. Prog Brain Res. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- 121.Bhuiyan S, Fukunaga K. Stimulation of sigma-1 receptor by dehydroepiandrosterone ameliorates hypertension-induced kidney hypertrophy in ovariectomized rats. Exp Biol Med (Maywood) 2010;235:356–364. doi: 10.1258/ebm.2009.009177. [DOI] [PubMed] [Google Scholar]
- 122.Jankowski M, Wang D, Danalache B, Gangal M, Gutkowska J. Cardiac oxytocin receptor blockade stimulates adverse cardiac remodeling in ovariectomized spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H265–H274. doi: 10.1152/ajpheart.00487.2009. [DOI] [PubMed] [Google Scholar]
- 123.Danalache BA, Gutkowska J, Slusarz MJ, Berezowska I, Jankowski M. Oxytocin-Gly-Lys-Arg: a novel cardiomyogenic peptide. PLoS One. 2010;5:e13643. doi: 10.1371/journal.pone.0013643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kobayashi H, Yasuda S, Bao N, Iwasa M, KawamuraI I, Yamada Y, Yamaki T, Sumi S, Ushikoshi H, Nishigaki K, Takemura G, Fujiwara T, Fujiwara H, Minatoguchi S. Postinfarct treatment with oxytocin improves cardiac function and remodeling via activating cell-survival signals and angiogenesis. J Cardiovasc Pharmacol. 2009;54:510–519. doi: 10.1097/FJC.0b013e3181bfac02. [DOI] [PubMed] [Google Scholar]
- 125.Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, D'Anna R, Cannata ML, Gaudio A, Frisina A, Frisina N, Corrado F, Cancellieri F, Lubrano C, Bonaiuto M, Adamo EB, Squadrito F. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 126.Guessous I, Bochud M, Bonny O, Burnier M. Vitamin D and Cardiovascular Disease. Kidney Blood Press Res. 2011;34:404–417. doi: 10.1159/000328332. [DOI] [PubMed] [Google Scholar]
- 127.Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;41:1317–1324. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- 128.Belo NO, Sairam MR, Dos Reis AM. Impairment of the natriuretic peptide system in follitropin receptor knockout mice and reversed by estradiol: implications for obesity-associated hypertension in menopause. Endocrinology. 2008;149:1399–1406. doi: 10.1210/en.2007-0572. [DOI] [PubMed] [Google Scholar]
- 129.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mouse. Endocrinol. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]