Abstract
Objectives
The purpose of this study was to document and analyze intraneural vascular flow within the median nerve using power and spectral Doppler sonography and to determine the relationship of this vascular flow with diagnosis of carpal tunnel syndrome based on electrodiagnostic testing.
Methods
Power and spectral Doppler sonograms in the median nerve were prospectively collected in 47 symptomatic and 44 asymptomatic subjects. Doppler studies were conducted with a 12-MHz linear transducer. Strict inclusion criteria were established for postexamination assessment of waveforms; routine quality assurance was completed; electrodiagnostic tests were conducted on the same day as sonographic measurements; and the skin temperature was controlled. Included waveforms were categorized by location and averaged by individual for comparative analysis to electrodiagnostic testing.
Results
A total of 416 waveforms were collected, and 245 were retained for statistical analysis based on strict inclusion criteria. The mean spectral peak velocity among all waveforms was 4.42 (SD, 2.15) cm/s. At the level of the pisiform, the most consistent data point, mean peak systole, was 3.75 cm/s in symptomatic patients versus 4.26 cm/s in asymptomatic controls. Statistical trending showed an initial increase in the mean spectral peak velocity in symptomatic but diagnostically negative cases, with decreasing velocity as diagnostic categories progressed from mild to severe.
Conclusions
An inverse relationship may exist between intraneural vascular flow in the median nerve and an increasing severity of carpal tunnel syndrome based on nerve conduction results. Randomized controlled trials are needed to determine whether spectral Doppler sonography can provide an additive benefit for diagnosing the severity of carpal tunnel syndrome.
Keywords: carpal tunnel syndrome, electrodiagnostics, median mononeuropathy, musculoskeletal sonography
Clinical evaluation of median nerve disorders has long relied on electrodiagnostic testing. With electrical stimulation provided to the nerve, electrodiagnostic testing measures various parameters related to the transmission of this electrical impulse within the nerve.1 When the nerve is compressed or is in various stages of demyelination, the transfer of electrical impulses along the nerve can become impeded. Because of this ability to measure physiologic changes, guidelines for the diagnosis of work-related carpal tunnel syndrome commonly lead to a confirmed diagnosis by electrodiagnostic testing2:
Patient history;
-
Physical examination of the patient:
Gathering personal characteristics;
Sensory examination;
Manual muscle testing of the upper arm;
Provocative testing;
-
Electrodiagnostic testing:
Nerve conduction studies; and
Electromyography (EMG).
A plethora of research provides sensitivity and specificity data supporting the utility of electrodiagnostic studies for the diagnosis of carpal tunnel syndrome.3–6 However, abnormal electrodiagnostic test results do not necessarily equate to carpal tunnel syndrome and are not necessarily predictive of future disorders.3 Furthermore, there is debate regarding the variability in protocols, diagnostic threshold values, and overall diagnostic accuracy of electrodiagnostic testing for diagnosing carpal tunnel syndrome.7 In addition to these limitations, when diagnosing carpal tunnel syndrome, electrodiagnostic testing evaluates secondary conditions that may only appear in more chronic stages of the disorder. Although reported symptoms and provocative testing may be able to identify acute cases, the subjectivity of these measures results in very poor reliability and diagnostic accuracy.8–10 The limitations of electrodiagnostic testing and lack of a reliable step in diagnostic guidelines to identify acute cases provide an opportunity for continued investigation of complementary or alternative diagnostic methods.
Despite some limitations, high-resolution sonography may be an appropriate alternative diagnostic tool because it can be used to visualize median nerve enlargement and increased hypoechogenicity throughout various stages of carpal tunnel syndrome.11 Attempts to calculate the diagnostic accuracy of measurements with sonography across all research literature have been challenging because of variability in methods and equipment parameters.12 At best, a rigorous systematic review of all available literature indicated that, when used alone, gray scale sonographic measurements had sensitivity of 29.4% to 100% and specificity of 47% to 100%.13 Similarly, hypervascularity, hyperechogenicity, and other qualitative measures have been used, but the results of these methods alone result in sensitivity and specificity as low as 50%14 and 65%15 respectively. Improved accuracy has been suggested by combinations of sonographic measures of the median nerve cross-sectional area, hypo-echogenicity, and hypervascularity. This combined-measures method resulted in 90% agreement with the clinical presentation of carpal tunnel syndrome,16 which could provide the necessary evidence for combining complementary methods to improve accuracy.
Although electrodiagnostic testing may currently remain the reference standard for carpal tunnel syndrome diagnosis, further evaluation and refinement of innovative sonographic methods are warranted to provide complementary diagnostic information. As a foundation for further development of a complementary method, this research evaluated the relationship of power and spectral Doppler measures of intraneural vascularity as a means for diagnosis of carpal tunnel syndrome compared to electrodiagnostic testing.
Materials and Methods
This prospective research was approved by The Ohio State University Biomedical Institutional Review Board, and all participants provided written consent. In addition to consent, patients acknowledged understanding of the Health Insurance Portability and Accountability Act and provided permission to the researchers for access to results of electrodiagnostic testing.
Participants were recruited from both a clinical patient population and a convenient nonpatient population. Symptomatic patients with signs and symptoms of carpal tunnel syndrome were recruited when presenting to a neurology clinic for electrodiagnostic testing. Symptomatic patients were included when symptoms of numbness or tingling in the median nerve distribution were present for at least 3 weeks. Asymptomatic nonclinical control participants had no reports or clinical signs or symptoms of median nerve disorders. Exclusion criteria for both symptomatic patients and asymptomatic controls were a history of broken bones or surgery in the wrist or hand, permanently placed shunts or objects in the wrist or hand, a history of systemic neurologic or uncontrolled thyroid disorders, and pregnancy. Because of the methods and participant availability, matching of asymptomatic controls was unable to be completed. Data were collected from both the dominant and nondominant wrists that met inclusion criteria.
A Synergy tower (CareFusion Corporation, Middleton, WI) was used to obtain electrodiagnostic testing data for all symptomatic patients according to previously described testing procedures.17 Orthodromic sensory responses were collected for averaged distal sensory nerve action potentials and the sensory nerve action potential amplitude, velocity, and latency. Distal motor latency, distal and proximal compound muscle action potential amplitudes, and conduction velocity were recorded. Needle EMG was completed in the abductor pollicis brevis. To reduce error and rule out confounding diagnoses, the skin temperature at the fingers was greater than 34°C for all patients before initiating electrodiagnostic testing; additional nerve conduction study data were collected on the ulnar nerve; and needle EMG was used to evaluate additional muscles.
Based on American Association of Electrodiagnostic Medicine guidelines,4 nerve conduction study results were considered diagnostic when the sensory conduction velocity was less than 50 m/s across the carpal tunnel; the sensory nerve action potential amplitude was less than 10 µV; distal motor latency was greater than 4.2 milliseconds; and the compound muscle action potential amplitude was less than 4.0 mV. The results of electrodiagnostic testing were used to categorize data obtained from wrists of symptomatic patients into 4 groups:
Negative: absence of any electrical diagnostic criterion;
Mild: reduction in the sensory conduction velocity with normal motor responses and EMG results;
Moderate: sensory nerve abnormalities combined with prolonged distal motor latency but normal EMG results; and
Severe: absence of sensory responses coupled with motor nerve changes or abnormal EMG results.
Sonographic examinations were performed on all participants with a LOGIQi hand-carried unit and a 12-MHz linear transducer (GE Healthcare, Milwaukee, WI). Equipment parameters were evaluated with a weekly quality control program using a general-purpose urethane tissue-mimicking phantom (CIRS, Inc, Norfolk, VA). Quality control measurements were then taken by the same sonographer, who ensured accuracy in maximum penetration, axial resolution, and lateral resolution.
A gray scale scanning protocol for the median nerve18 was amended to include interrogation of vascular flow within the median nerve. Images and waveforms were collected by 2 researchers (K.D.E. and S.C.R.) with training in scanning of the median nerve and established laboratory reliability.18 Spectral Doppler waveforms were collected in a longitudinal view of the carpal tunnel region. Anatomic landmarks were used to define regional boundaries within the carpal tunnel. The proximal carpal tunnel (ie, inlet) was identified as the region immediately proximal to the distal edge of the radius. The mid carpal tunnel region included the area anterior to the lunate and capitate bones. The distal carpal tunnel (ie, outlet) was identified as the area of the median nerve immediately distal to the capitate bone. Each region was interrogated thoroughly in all participants, and as many waveforms as possible were collected within each region.
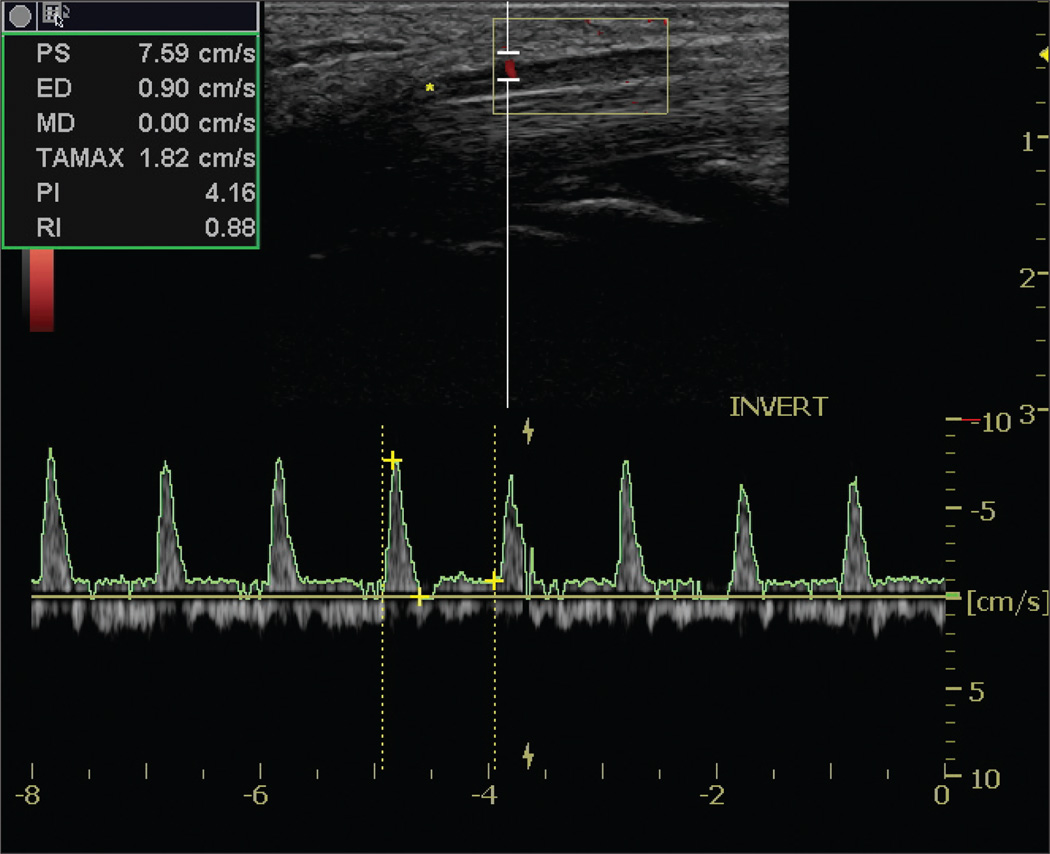
Spectral waveforms were generated from the power Doppler streaks that appeared within the median nerve (Figure 1). The mean power Doppler pulse repetition frequency was 0.46 kHz, and the mean overall gain was 20.39 dB. The mean spectral Doppler pulse repetition frequency was 1.87 kHz; the acoustic output was 100%; and the mean overall gain was 20.82 dB. The mean spectral Doppler depth was 0.4 cm along the perpendicular axis of the cursor, relative to the nerve, with a mean gate width of 2.0 mm (equipment range, 1–16 mm). Angle correcting the interrogation was impossible because the orientation of the corresponding arteriole was represented by an active power Doppler pixel.
Figure 1.
Sample waveform taken within the median nerve in the distal carpal tunnel region.
Sonographic data were collected in both wrists of the symptomatic patients and asymptomatic controls. In the patients, sonographic examinations were completed immediately after electrodiagnostic testing to reduce error due to temperature changes or other effects of time passing between examinations. Sonographers were unable to be blinded to the participant group but were blinded to the results of the electrodiagnostic testing. To minimize error and individual bias, all waveforms were evaluated by 2 sonographers (K.D.E. and K.R.V.), who were blinded to the group designation, nerve conduction results, provocative test results, and gray scale sonographic study results. Every duplex waveform was reviewed according to a priori quality inclusion criteria19:
Must have at least 3 cardiac cycles in the sample;
Must have the spectral Doppler gate positioned within the median nerve;
Must have more than some pulsatility (ie, the waveform has consistent positive deflections throughout the spectral segment);
Must have an optimized gray scale image; and
Must have more signal than noise in the spectral waveform tracing.
Waveforms that met all 5 criteria based on both evaluators were included in further analysis. The peak systolic and end-diastolic velocities for every included waveform were recorded by an automatic trace. When the sonographers noted that the equipment’s automatic trace did not accurately capture systolic peaks, the trace was manually adjusted according to evaluator consensus. When multiple values were recorded at the same anatomic location within the nerve of one wrist, the arithmetic average was calculated for the peak systolic and end-diastolic velocities for use in the analysis.
Retained waveforms were categorized by the location within the carpal tunnel region in which spectral Doppler data were collected. Descriptive statistics were calculated to describe the spectral Doppler data set and average spectral Doppler value in each region and were compared between symptomatic and asymptomatic groups with a t test. Symptomatic patient data were further divided by diagnostic categorization, and analysis of variance (ANOVA) was used to determine differences among the groups. Polynomial trend lines were fitted to the average spectral Doppler value by region across diagnostic categories. All data were analyzed using SPSS version 19 software (IBM, Somers, NY).
Results
Power and spectral Doppler sonograms in the median nerve were prospectively collected in 47 symptomatic patients and 44 asymptomatic control participants. The symptomatic patients were slightly older, had a larger body mass index, and had a slightly squarer wrist, which were statistically significant (Table 1). Data were collected from 83 wrists in both groups (166 total), and 416 spectral waveforms were obtained from various carpal tunnel regions across both groups. A total of 245 waveforms were retained for further analysis after application of the quality inclusion criteria by the evaluators.19 Of these included waveforms, 48 wrists of symptomatic patients and 42 wrists of asymptomatic controls were represented. No acceptable spectral waveforms were noted in the remaining 77 wrists (46%). Most waveforms (97%) across the entire participant population showed spectral broadening with systolic forward flow into diastole.
Table 1.
Demographic Characteristics of Participants by Group
| Characteristic | Symptomatic Patients (n=47) |
Asymptomatic Controls (n=44) |
Test and Statistic |
df | P |
|---|---|---|---|---|---|
| Age, y (SD) | 45.6 (10.6) | 40.0 (12.1) | t = 2.355 | 89 | .021 |
| Female/male, n | 37/10 | 30/14 | χ2 = 1.301 | 1 | .254 |
| Right/left hand dominance, n | 44/3 | 37/7 | χ2 = 2.108 | 1 | .146 |
| Body mass index, kg/m2 (SD) | 32.0 (7.4) | 27.4 (7.0) | t = 2.980 | 89 | .004 |
| Wrist ratio (SD) | 0.732 (0.041) | 0.710 (0.041) | t = 3.535 | 164 | .001 |
These 245 waveforms were categorized by carpal tunnel region. When multiple waveforms within a region from one individual wrist were available, the peak systolic data for that region were averaged. This data reduction resulted in 123 peak systolic measurements to be used in further comparative analysis. Of the 123 peak systolic measurements, 27 were recorded proximally, 42 in the mid carpal tunnel, and 54 in the distal portion of the tunnel. The average peak systole was 3.90 (SD, 1.38), 4.25 (SD, 1.77), and 3.98 (SD, 1.74) cm/s in each region, respectively. Because the number of averaged peak systolic measurements that were able to be included varied across the groups, no statistical comparison of the averages among the regions was conducted.
Although the lack of high-quality waveforms limited one-to-one comparison among the regions, the averaged peak systolic measurements in each region were evenly distributed between patient and control groups. The symptomatic patient group contributed 14 of the 27 measurements in the proximal region, 24 of the 42 measurements in the mid region, and 28 of the 54 measurements in the distal region. The mean peak systole was significantly higher in the control group than in the patient group in the proximal region (t= –2.278; 25 df; P=.032; Table 2), with no significant difference noted between the groups in the other regions.
Table 2.
Comparison of Mean Spectral Peak Measurements by Location in the Carpal Tunnel Between Patient and Control Groups
| Parameter | Symptomatic Patients |
Asymptomatic Controls |
t | df | P |
|---|---|---|---|---|---|
| Proximal peak systole, cm/s (SD) | 3.34 (0.94) | 4.50 (1.64) | −2.278 | 25 | .032 |
| Mid peak systole, cm/s (SD) | 4.54 (1.63) | 3.87 (1.35) | 1.430 | 40 | .161 |
| Distal peak systole, cm/s (SD) | 3.78 (1.84) | 4.20 (1.73) | −0.859 | 52 | .394 |
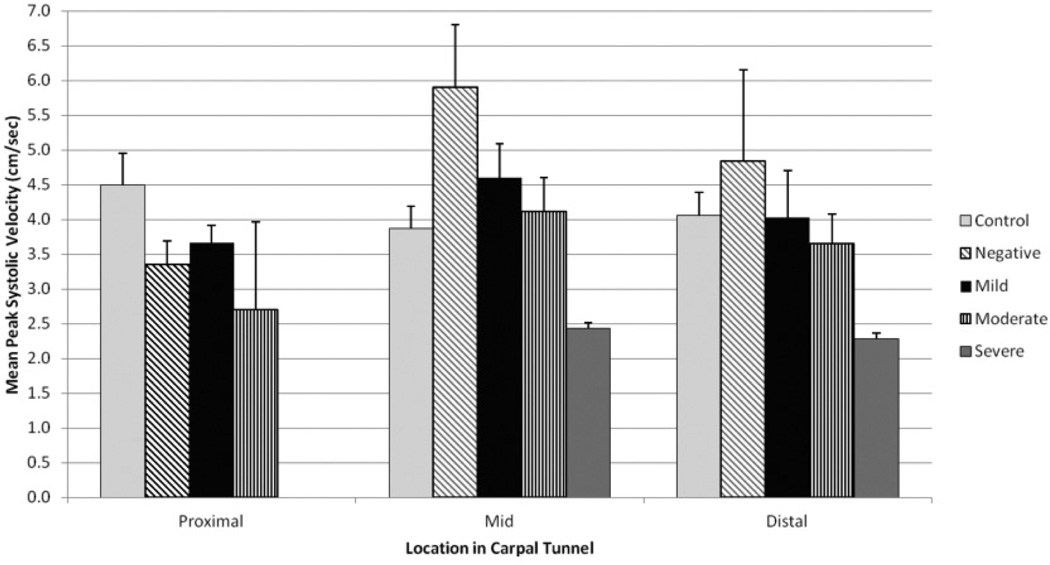
The measurements obtained from the symptomatic patients were subdivided into diagnostic categories based on electrodiagnostic test results. Mean peak systolic measurements were highest in wrists that were symptomatic but considered diagnostically negative by electrodiagnostic testing. In this category, the mean peak systole was 5.90 (SD, 1.79) cm/s in the mid carpal tunnel and 4.84 (SD, 2.27) cm/s in the distal portion of the carpal tunnel. The lowest mean peak systolic measurements were noted in wrists that were categorized as having severe carpal tunnel syndrome, with values of less than 2.50 cm/s. Figure 2 displays the mean peak systolic measurements among the diagnostic categories within the symptomatic patient group along with the mean peak systolic measurements obtained in each region from the symptomatic controls. Results of ANOVA did not indicate any significant differences among the diagnostic groups, which may have been limited because of the small numbers within the subdivided groups.
Figure 2.
Mean Doppler peak systolic measurements taken by location and subdivided by electrodiagnostic testing categories.
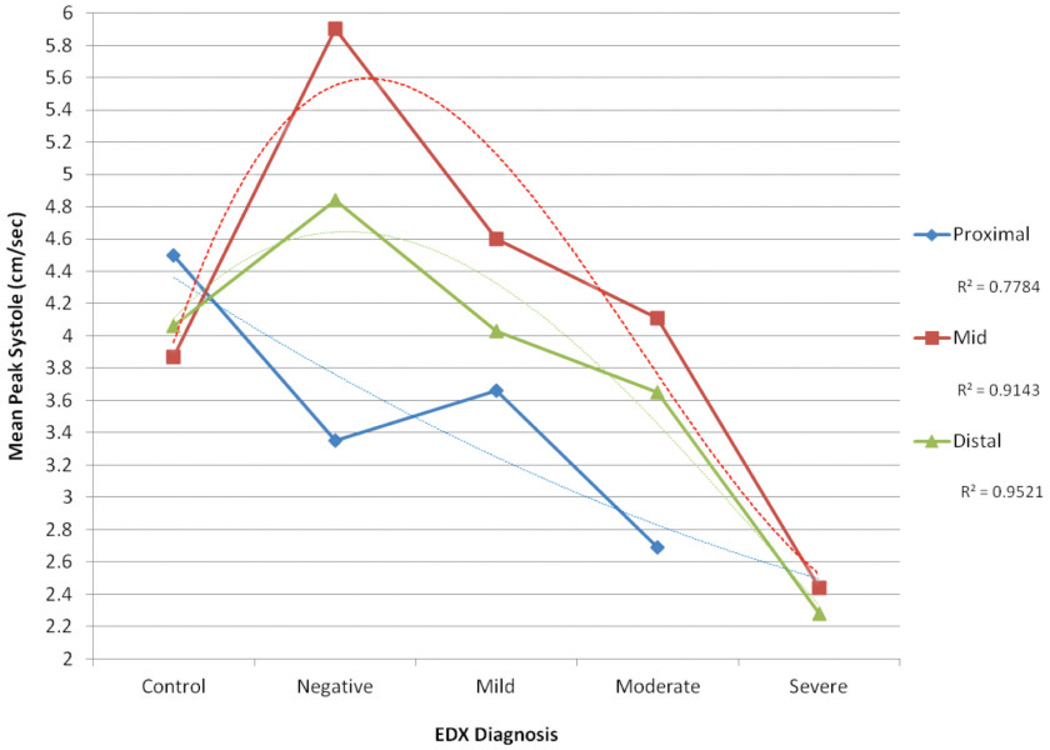
Although significant differences were not noted, a trend was noted in the differences in mean peak systolic measurements collected within the mid carpal tunnel region because the ANOVA results approached significance. To investigate possible trends that could be further explored with a larger data set, trend estimations were completed by fitting polynomial trend lines to the data. In the mid (R2 =0.91) and distal (R2 =0.95) carpal tunnel regions, the mean peak systole was noted to be increased in the symptomatic patients with negative electrodiagnostic test results versus the asymptomatic controls. Across all 3 regions, as the severity of the carpal tunnel syndrome diagnosis increased, the mean peak systole decreased (Figure 3).
Figure 3.
Mean spectral peak systolic measurements for each location relative to the carpal tunnel by diagnostic category based on nerve conduction testing with polynomial trending (dashed line). EDX indicates electrodiagnostic testing.
Discussion
This study was originally conceived as a means for evaluating additional techniques that might augment the sonographic evaluation of patients with suspected carpal tunnel syndrome. The literature has been building with respect to the use of color, power, and spectral Doppler sonography to evaluate the wrist, hand, and fingers of symptomatic patients, but these cohort studies seemed to lack rigorous quantitative assessment of the vascularity of nerve tissue. Our pilot work has been done to develop scanning protocols and reproducible measures that might contribute to the use of Doppler sonography to evaluate the onset of nerve compression and resulting hyperemia in the nerve. Rather than investing in a single measure for evaluating the patient with suspected carpal tunnel syndrome, we used both power and spectral Doppler sonography to compare the results to the clinical reference standard for diagnosis of carpal tunnel syndrome: nerve conduction. In short, our focus has been on ways to add Doppler measurements for diagnosis and harness these for maximum diagnostic value. Several published studies have recommended that continued research should focus on increasing the detection rate for carpal tunnel syndrome through the use of multiple diagnostic tests.20,21 Interestingly, a similar study completed by Akcar et al22 used power Doppler imaging in an attempt to raise the diagnostic efficiency of sonography; however, they had difficulty in recording intraneural flow in their healthy volunteers. Certainly, sonography is a very op-erator-dependent modality, and it takes training to perfect the consistent detection of intraneural vascularity. We did have limited success with our spectral Doppler protocol; however, it was not possible to record Doppler tracings at every anatomic location in all our patients. This limited success is encouraging, but it does point to the value of training and further development of equipment to consistently detect this type of low blood flow within the nerve. This study provides added evidence for detecting intraneural vascular flow with power and spectral Doppler sonography in asymptomatic as well as symptomatic patients; however, further work is needed to increase the consistency and make the data comparable to the current clinical reference standard.
This study was a preliminary determination of the value of using power and spectral Doppler sonography to determine the intraneural vascular flow in the median nerve and relate it to the progression of this compressive disorder. It has been suggested that provocative testing could be used to determine which patients should progress to electrodiagnostic testing as a way to obtain a conclusive diagnosis. We have found that provocative testing combined with sonography did not provide a strong indicator of inflammatory changes around the median nerve.18 This finding does not mean that the level of evidence to support the combined use of provocative testing, sonography, and electrodiagnostic testing is exhausted. This study would suggest that it may be possible to use power and spectral Doppler sonography to diagnostically screen those patients to remove the negative and severe and more fully concentrate on the level of severity of carpal tunnel syndrome in mild and moderate cases. A study by Pastare et al23 also found that combining gray scale sonography with electrodiagnostic testing raised the diagnostic sensitivity for detecting carpal tunnel syndrome. The data from our study indicate that an inverse relationship may exist between intraneural vascular flow and progressive loss of sensory and motor innervations. Symptomatic individuals with negative diagnostic findings may initially have a higher peak systole than other diagnostic groups and asymptomatic controls because of the inflammatory process involved in the acute stages of nerve entrapment. In this stage, blood flow may be increased to assist in repairing damaged tissues, but demyelination of the nerve has not yet begun to occur, so electrodiagnostic testing cannot detect any deficits. As the disorder becomes more chronic, demyelination begins to occur, which leads to definite changes in electrodiagnostic testing. As chronicity increases, tissue loses its perfusion, and the peak systole will decline. There may be diagnostic value in the detection of intraneural vascular flow in the mid and distal portions of the carpal tunnel as a good indicator of negative and severe carpal tunnel syndrome.
The combination of spectral Doppler sonography and provocative testing may assist the clinician in sorting out symptomatic patients who either currently have negative findings or are already at a severe stage. This process likely would need further investigation, much like a study conducted by Naranjo et al,24 which combined gray scale cross-sectional area measurements with provocative testing. Their data indicated that this combination was not effective and did not increase the sensitivity of sonography. Interestingly, El Miedany et al25 suggested that an algorithm for diagnosing carpal tunnel syndrome should be used that begins with a history and clinical examination. They suggested that this step be followed by gray scale evaluation of the wrist to screen patients with abnormalities from those without. Furthermore, they suggested that the next tier of evaluation should be the use of electrodiagnostic testing for grading the severity of carpal tunnel syndrome. Our study may suggest that Doppler examinations could add diagnostic information about the severity of the nerve and perhaps the location of the injury.
Two recently published studies assessed a cohort of asymptomatic and symptomatic patients with electrodiagnostic testing, gray scale sonography, and power Doppler sonography to likewise determine the severity of carpal tunnel syndrome comparatively.26,27 Both studies selected a method of counting pixels that were associated with the region of interest and related those quantitative scores to the electrodiagnostic test results. In the stronger methodological study, Ghasemi-Esfe et al27 reported that 100% of their patients with moderate and severe carpal tunnel syndrome had intraneural vascularity. These results are very encouraging, and with more participants in the severe category, the use of spectral Doppler sonography to confirm the flow associated with power Doppler pixels would provide further confirmatory evidence of the utility of spectral Doppler sonography.
This study had some limitations. The results must be carefully interpreted because the strict inclusion criteria for waveforms limited the ability to have one-to-one comparisons in all groups and regions, and the subdivision of the sample by electrodiagnostic categories diminished the statistical power of the analysis. In the case of the severe carpal tunnel syndrome diagnostic category, only 2 mean spectral peak measurements qualified. Although the trending is encouraging, these pilot data could be indicative of an important diagnostic variable, and a replication of this study would be encouraged. Increased numbers of participants will correspondingly add to the number of acceptable waveforms for analysis and categorization. Additionally, the data from this study were collected with a hand-carried sonographic unit, and the inability to obtain high-quality waveforms across all participants and in all regions may have been limited by the technology. It may be important to use more sophisticated equipment or develop methods to enhance the ability to consistently obtain high-quality spectral Doppler waveforms. Replication of these results would also depend on the sophistication of the sonographers recording the data and the decisions reached through consensus regarding recording of the data.
As noted in the “Materials and Methods,” because of the inability to identify the directionality of the very small vessels, angle correction was not possible; therefore, velocity measurements were limited. Similarly to obtaining velocity measurement in a transcranial Doppler examination, angle correction is only possible when a discernible vessel is seen. This factor is a pitfall for moving this research forward, and as for transcranial Doppler sonography, studies continue on the percent error associated with the spectral Doppler angle error. In this study, the superficial vessels that were detected in the median nerve needed to be acquired at angles between 30° and 60° to maintain a 5% error in the computation of velocity.28,29 The challenge remains to resolve the angle of intraneural vessels and increase the computed spectral Doppler velocities.
In addition, it would also be advisable to collect data from more than one clinical site to ensure that patient variety is maximized and be truly representative of patients with carpal tunnel syndrome. Continued research is needed to obtain higher-level evidence for a noninvasive approach to determining the severity of carpal tunnel syndrome. It has been strongly suggested that higher-order research designs are needed to appropriately evaluate the diagnostic tests that might be used for patients with vague and nonspecific symptoms inherent in this compressive disorder.30 The results of randomized clinical trials that incorporate provocative testing, gray scale sonography, and Doppler imaging, as well as electrodiagnostic testing, are needed to further refine the present clinical algorithm for diagnosing the severity of carpal tunnel syndrome.
In conclusion, an inverse relationship may exist between the measurement of intraneural vascular flow and nerve condition velocities. Continued research devoted to refining diagnostic guidelines for carpal tunnel syndrome could be focused on how to improve the diagnostic yield as well as balance the use of health care resources. Sonographic evaluation may be an economical, painless diagnostic step that can further refine a clinician’s capability for determining carpal tunnel syndrome.
Acknowledgments
We thank Xiaobai Li, PhD, for biostatisical support and funding provided by The Ohio State University's Center for Clinical and Translational Science, funded by the National Institutes of Health.
Abbreviations
- ANOVA
analysis of variance
- EMG
electromyography
References
- 1.Rempel D, Evanoff B, Amadio PC, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. 1998;88:1447–1451. doi: 10.2105/ajph.88.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons Clinical Guideline on Diagnosis of Carpal Tunnel Syndrome. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007. [Google Scholar]
- 3.US Department of Health and Human Services, Agency for Healthcare Research and Quality. [Accessed November 6, 2011];National Guideline Clearinghouse website. http://www.guideline.gov/.
- 4.American Association of Electrodiagnostic Medicine. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. [Accessed November 6, 2011];American Association of Electrodiagnostic Medicine website. http://www.aanem.org/getmedia/7ddc9ef9-ee91-4b48-9c1a-53454313001e/CTS.pdf.aspx.
- 5.Jordan R, Carter T, Cummins C. A systematic review of the utility of electrodiagnostic testing in carpal tunnel syndrome. Br J Gen Pract. 2002;52:670–673. [PMC free article] [PubMed] [Google Scholar]
- 6.Strickland JW, Gozani SN. Accuracy of in-office nerve conduction studies for median neuropathy: a meta-analysis. J Hand Surg Am. 2011;36:52–60. doi: 10.1016/j.jhsa.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Lew HL, Date ES, Pan SS, Wu P, Ware PF, Kingery WS. Sensitivity, specificity, and variability of nerve conduction velocity measurements in carpal tunnel syndrome. Arch Phys Med Rehabil. 2005;86:12–16. doi: 10.1016/j.apmr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Salerno DF, Franzblau A, Werner RA, et al. Reliability of physical examination of the upper extremity among keyboard operators. Am J Ind Med. 2000;37:423–430. doi: 10.1002/(sici)1097-0274(200004)37:4<423::aid-ajim12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Kuhlman KA, Hennessey WJ. Sensitivity and specificity of carpal tunnel syndrome signs. Am J Phys Med Rehabil. 1997;76:451–457. doi: 10.1097/00002060-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Mondelli M, Passero S, Giannini F. Provocative tests in different stages of carpal tunnel syndrome. Clin Neurol Neurosurg. 2001;103:178–183. doi: 10.1016/s0303-8467(01)00140-8. [DOI] [PubMed] [Google Scholar]
- 11.Bussières AE, Peterson C, Taylor JA. Diagnostic imaging guideline for musculoskeletal complaints in adults—an evidence-based approach, part 2: upper extremity disorders. J Manipulative Physiol Ther. 2008;31:2–32. doi: 10.1016/j.jmpt.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Descatha A, Huard L, Duval S. Letter to the editor: the sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: a meta-analysis. Clin Orthop Relat Res. 2011;469:901–902. doi: 10.1007/s11999-010-1761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roll SC, Case-Smith J, Evans KD. Diagnostic accuracy of ultrasonography vs electromyography in carpal tunnel syndrome: a systematic review of literature. Ultrasound Med Biol. 2011;37:1539–1553. doi: 10.1016/j.ultrasmedbio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang LY, Leong CP, Huang YC, Hung JW, Cheung SM, Pong YP. Best diagnostic criterion in high-resolution ultrasonography for carpal tunnel syndrome. Chang Gung Med J. 2008;31:469–476. [PubMed] [Google Scholar]
- 15.Mallouhi A, Pultzl A, Trieb T, Piza H, Bodner G. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240–1245. doi: 10.2214/AJR.04.1715. [DOI] [PubMed] [Google Scholar]
- 16.Rahmani M, Ghasemi Esfe AR, Vaziri-Bozorg SM, Mazloumi M, Khalilzadeh O, Kahnouji H. The ultrasonographic correlates of carpal tunnel syndrome in patients with normal electrodiagnostic tests. Radiol Med. 2011;116:489–496. doi: 10.1007/s11547-011-0632-6. [DOI] [PubMed] [Google Scholar]
- 17.Roll SC, Evans KD, Li X, Freimer M, Sommerich CM. Screening for carpal tunnel syndrome using sonography. J Ultrasound Med. 2011;30:1657–1667. doi: 10.7863/jum.2011.30.12.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roll SC, Evans KD. Feasibility of using a hand-carried sonographic unit for investigating median nerve pathology. J Diagn Med Sonography. 2009;25:241–249. [Google Scholar]
- 19.Evans KD, Volz KR, Hutmire C, Roll SC. Morphologic characterization of intraneural flow associated with median nerve pathology. J Diagn Med Sonography. 2012;28:11–19. doi: 10.1177/8756479311426777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claes F, Meulstee J, Claessen-Oude Luttikhus TL, Huygen PL, Verhagen WI. Usefulness of additional measures of the median nerve with ultrasonography. Neurol Sci. 2010;31:721–725. doi: 10.1007/s10072-010-0258-9. [DOI] [PubMed] [Google Scholar]
- 21.Lewis C, Mauffrey C, Newman S, Lambert A, Hull P. Current concepts in carpal tunnel syndrome: a review of the literature. Eur J Orthop Surg Traumatol. 2010;2:445–452. [Google Scholar]
- 22.Akcar N, Ozkan S, Mehmetoglu O, Calisir C, Adapinar B. Value of power Doppler and gray-scale US in the diagnosis of carpal tunnel syndrome: contribution of cross-sectional area just before the tunnel inlet as compared with the cross-sectional area in the tunnel. Korean J Radiol. 2010;11:632–639. doi: 10.3348/kjr.2010.11.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastare D, Therimadasamy AK, Lee E, Wilder-Smith EP. Sonography versus nerve conduction studies in patients referred with a clinical diagnosis of carpal tunnel syndrome. J Clin Ultrasound. 2009;37:389–393. doi: 10.1002/jcu.20601. [DOI] [PubMed] [Google Scholar]
- 24.Naranjo A, Ojeda S, Mendoza D, Francisco F, Quevedo JC, Erausquin C. What is the diagnostic value of ultrasonography compared to physical evaluation in patients with idiopathic carpal tunnel syndrome? Clin Exp Rheumatol. 2007;25:853–859. [PubMed] [Google Scholar]
- 25.El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology (Oxford) 2004;43:887–895. doi: 10.1093/rheumatology/keh190. [DOI] [PubMed] [Google Scholar]
- 26.Karadag O, Kalyoneu U, Akdogan A, et al. Sonographic assessment of carpal tunnel syndrome in rheumatoid arthritis: prevalence and correlation with disease activity [published online ahead of print September 17,2011] Rhematol Int. doi: 10.1007/s00296-011-1957-0. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi-Esfe AR, Khalilzadeh O, Vaziri-Bozorg SM, et al. Color and power Doppler US for diagnosing carpal tunnel syndrome and determining its severity: a quantitative image processing method. Radiology. 2011;261:499–506. doi: 10.1148/radiol.11110150. [DOI] [PubMed] [Google Scholar]
- 28.Zagzebski JA. Essentials of Ultrasound Physics. St Louis, MO: CV Mosby Co; 1996. Doppler instrumentation; pp. 87–108. [Google Scholar]
- 29.Hedrick WR, Hykes DL, Starchman DE. Ultrasound Physics and Instrumentation. 4th ed. St Louis, MO: CV Mosby Co; 2005. Doppler physics and instrumentation; pp. 205–220. [Google Scholar]
- 30.Bachmann LM, Juni P, Reichenbach S, Ziswiler HR, Kessels AG, Vogelin E. Consequences of different diagnostic gold standards in test accuracy research: carpal tunnel syndrome as an example. Int J Epidemiol. 2005;34:953–955. doi: 10.1093/ije/dyi105. [DOI] [PubMed] [Google Scholar]