Abstract
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal, hematopoietic stem cell disorder that manifests with hemolytic anemia and bone marrow failure. Eculizumab has been shown to improve anemia, decrease intravascular hemolysis, and reduce the risk of thrombosis.
Design and methods
This is a retrospective, single-center study of patients treated with eculizumab and categorized according to response criteria. Complete response (CR) was defined as transfusion independence with normal hemoglobin for age/sex, absence of symptoms, and lactate dehydrogenase <1.5 times the upper limit of normal. A good partial response (GPR) was defined as decreased transfusions from pretreatment and lactate dehydrogenase <1.5 upper limit of normal without thrombosis. These patients did not achieve normal hemoglobins for age and sex. A suboptimal response was defined as unchanged transfusion needs and persistent of symptoms.
Results
Thirty patients with PNH clones were treated with eculizumab and classified as complete responders (four patients), good partial responders (16), and suboptimal responders (10) over 863 patient-months of treatment. Complete responders had a decrease in red cell clone size, while good partial responders had an increase. Thirteen patients treated did not meet inclusion criteria for the clinical trials of eculizumab due to lack of transfusions or thrombocytopenia; eight had at least a GPR.
Conclusions
Eculizumab is efficacious in patients with PNH, but responses can vary and may depend on underlying marrow failure, underlying inflammatory conditions and red cell clone size following treatment. Normalization of hemoglobin with decrease in red cell clone size may predict CR.
Keywords: eculizumab, paroxysmal nocturnal hemoglobinuria, bone marrow failure
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, clonal, hematopoietic stem cell disorder that manifests with a complement-mediated hemolytic anemia, bone marrow failure, and a propensity for thrombosis (1–3). Somatic mutations in PIG-A, a gene whose product is required for the synthesis of glycosylphosphatidyinositol (GPI) anchors, are found in all patients with PNH (4–6). The GPI anchor is a lipid moiety that tethers dozens of proteins to cellular membrane; thus, PNH cells have a marked deficiency or absence of all GPI-anchored proteins. Hemolysis in PNH is a direct consequence of CD55 and CD59 deficiency. These GPI-anchored proteins protect normal red cells at different stages of the complement cascade. CD55 regulates the formation and stability of the C3 and C5 convertases (4), whereas CD59 blocks the formation of the membrane attack complex (MAC) (5). The chronic hemolytic anemia in PNH is largely mediated by the alternative pathway of complement that is in a state of continuous activation through a process known as tick-over (6, 7). Increased activation of complement due to surgery, stress, infections, or inflammatory disorders can lead to severe paroxysms of hemolysis. However, iron deficiency from intravascular hemolysis and varying degrees of underlying bone marrow failure may also contribute to the anemia in patients with PNH.
Eculizumab is an FDA-approved humanized monoclonal antibody for the treatment of PNH and in clinical trials has been shown to improve anemia, decrease intravascular hemolysis, reduce the risk for thrombosis, and markedly improve quality of life for most patients with PNH (8–10). The language used by the FDA for its approval is broad and states that it is indicated ‘for the treatment of patients with PNH to reduce hemolysis’. However, this drug does not improve bone marrow failure nor is it curative. After an initial 1-month loading period, the drug is administered intravenously every 14 ± 2 d. Eculizumab blocks terminal complement activation by recognizing C5 and preventing formation of the MAC (11, 12). Eculizumab compensates for the CD59 deficiency on PNH red cells, but it does not compensate for the CD55 deficiency. In patients receiving eculizumab, PNH red cells begin to accumulate C3 on their cell surface, which leads to opsonins that are recognized by the reticuloendothelial system resulting in extravascular hemolysis (13, 14). This phenomenon is not observed in untreated patients with PNH because complement activation on red cells in the absence of eculizumab leads to rapid elimination due to the MAC. Laboratory evidence of extravascular hemolysis in eculizumab-treated patients includes an elevated reticulocyte count, a normal to mildly elevated LDH, varying degrees of anemia, and in some cases, a direct Coombs test that is positive for C3 but not IgG. While many of these patients remain asymptomatic and do not require therapy, others have symptomatic anemia and require chronic blood transfusions.
Eculizumab costs more than $400,000 US dollars annually, making it one of the world’s most expensive drugs. Thus, it is important to identify the factors that are responsible for suboptimal response to eculizumab therapy to make informed treatment decisions. In this study, we performed a retrospective analysis on 30 eculizumab-treated patients to identify factors associated with suboptimal response to treatment.
Methods
The protocol for review of these patients was approved by the institutional review board of the Johns Hopkins hospital. This was a retrospective study performed via chart review and contact of outside physicians in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Patient population
Johns Hopkins Hospital is a tertiary referral hospital where a total of 73 patients were diagnosed with a PNH clone at JHH between 2005 and 2012. Thirty patients with PNH were prescribed eculizumab and followed over that time period. Five patients were treated on either the TRIUMPH (8) or SHEPHERD (9) trials and reported previously in the context of the trial manuscripts. The diagnosis of PNH was made or confirmed by peripheral blood flow cytometry on granulocytes, monocytes, and red cells (15, 16). Data collected for analysis included patient demographics, medical history, classification of PNH phenotype per the International PNH Interest Group (IPIG) guidelines (2), PNH erythrocyte and granulocyte clone size, transfusion needs, thrombosis history, laboratory values of hemolysis (LDH, reticulocyte count), and vital status. Complications of the therapy were also documented. All information was obtained from the electronic patient records of the Johns Hopkins Hospital and records from referring physicians. Data were collected through April 2012 when this analysis was performed. Patients were classified according to the IPIG (2) as (i) classical PNH in patients who have clinical and laboratory evidence of hemolysis and no evidence of another bone marrow failure disorder, (ii) PNH in the setting of another specified bone marrow disorder (PNH/AA) for patients with clinical and laboratory evidence of hemolysis and documented evidence of another bone marrow failure disorder, and (iii) Subclinical PNH (PNHsc) for patients with no clinical and laboratory evidence of hemolysis despite a detectable population of GPI-AP deficient cells but do have documented evidence of another bone marrow failure disorder.
Treatment
The decision to initiate eculizumab therapy was made by one of the authors (RAB) in conjunction with each individual patient. Eculizumab therapy was recommended for patients with a large PNH granulocyte clone who had disabling fatigue, thrombosis, red cell transfusion dependence due to hemolysis, frequent pain paroxysms, renal insufficiency, or other end-organ complications from disease (11). All patients were vaccinated against Neisseria meningitidis prior to receiving the first dose of eculizumab. Throughout the study, patients received transfusions with packed RBCs if medically indicated. Patients received eculizumab using a 25- to 45-min intravenous (IV) infusion as previously described (11). Briefly, an induction dose of eculizumab 600 mg was administered for every 7 ± 2 d for four doses and then eculizumab 900 mg for 7 ± 2 d later, followed by a maintenance dose of eculizumab 900 mg for every 14 ± 2 d.
Response criteria
The patients who were treated with eculizumab were evaluated by the authors for response criteria involving improvement in anemia, PNH symptoms, and thrombosis. Complete response (CR) was defined as transfusion independence with normal hemoglobin for age and sex for 6 months or greater; the patients had to be free of PNH-related symptoms including thromboses and smooth muscle dystonias. Also, their LDH values had decreased to <1.5 time the upper limit of normal. A good partial response (GPR) was defined as a decrease in transfusions from pretreatment and LDH level <1.5 upper limit of normal without thrombosis. These patients did not achieve normal hemoglobins for age and sex. There was also PNH-related symptom improvement in these patients. A suboptimal response was defined as unchanged transfusion needs and persistent of PNH symptoms.
Statistical analysis
The tables and figures were made using stata10 IC (College Station, TX, USA). Descriptive statistics were performed using this software as well. The Student’s t-test was employed for comparisons. Overall survival was estimated using the Kaplan–Meier method.
Results
Patient demographics
From January 2005 through March 2012, there were 73 patients at JHH identified as having a population of GPI-AP-deficient granulocytes of 0.1% or greater. Thirty of these patients were treated longitudinally with eculizumab. Of the 30 eculizumab-treated patients, 21 (70%) met IPIG criteria for classical PNH, 9 (30%) had PNH/AA. Of the 43 patients who did not receive eculizumab, two patients (4.7%) had classical PNH, 14 patients (32.6%) had PNH/AA, and 27 (62.8%) had scPNH. The untreated patients with classical PNH were largely asymptomatic despite large PNH clones and thus did not require therapy. One patient had 50% PNH granulocytes and 56% type III PNH erythrocytes; the other patient had 91% PNH granulocytes and 29% type III erythrocytes. Median size of the PNH red cell clone in the untreated patients was 1% (range, 0–56%), whereas the median size of the PNH red cell clone in the patients treated with eculizumab was 37.5% (range, 1–88%) (P = 0.0000). Median value of the absolute reticulocyte count in the untreated patients was 48.7 K/cu mm (range, 5.1–446.2), whereas the median value of the absolute reticulocyte count in the patients treated with eculizumab was 151.1 K/cu mm (range, 8.2–451.8; P = 0.0002). Median value of the LDH in the untreated patients was 223 U/L (range, 85–2304), whereas the median value of the LDH in the patients treated with eculizumab is 1488.5 U/L (range, 329–4835; P = 0.0000).
Ultimately 30 of the original 73 (41.67%) of patients were treated continuously with eculizumab. See Table 1 for demographics. The median granulocyte and erythrocyte clone sizes were 86.5% and 37.5%, respectively. Of these 30 patients, 27 (90%) had LDH levels >1.5 upper limit of normal prior to therapy. Only eight patients (26.7%) had not been transfused prior to the start of therapy. Of the nine PNH/AA patients treated with eculizumab, three evolved to classical PNH before treatment and would have satisfied eligibility criteria for the SHEPHERD trial. Of the remaining six, three were treated because of life-threatening thrombosis in conjunction with a large PNH granulocyte clone, and three were treated because of symptomatic anemia with evidence of intravascular hemolysis (Table 2).
Table 1.
Demographics of patients treated with eculizumab
| Age at diagnosis –median (range) | 34 (16–77) |
| % Male | 48 |
| % Classical PNH | 67 |
| Enrolled on SHEPHERD | 2 |
| Enrolled on TRIUMPH | 3 |
| Median LDH prior to start of treatment (U/L) | 1488.5 (157–4835) |
| Median size of PNH clone (granulocyte; %) | 86.5 (40–100) |
| Median size of PNH clone (erythrocyte; %) | 37.5 (1–88) |
| Median Hb prior to start of treatment (g/dL) | 8.6 (6.3–14.5) |
| Median platelets prior to start of treatment (K/cu mm) | 136.5 (16–402) |
| Median absolute reticulocyte count prior to start of treatment (K/cu mm) | 151.1 (8.2–451.8) |
| Median time from diagnosis to treatment (months, range) | 18 (0–288) |
PNH, paroxysmal nocturnal hemoglobinuria.
Table 2.
Patients who would not meet criteria for inclusion in the SHEPHERD trial
| Age/Sex | Disease phenotype |
Criteria not met | Indication for treatment with eculizumab |
RBC clone size at initiation (%) |
Granulocyte clone size at initiation (%) |
Absolute neutrophil Count (/cu mm) |
Platelet count at initiation (K/cu mm) |
LDH at initiation (U/L) |
Absolute reticulocyte count at initiation (K/cu mm) |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 31 M | Classical | Thrombocytopenia due to liver failure | Thrombosis, liver failure, renal failure, Anemia from hemolysis, transfusions | 83 | 99 | 4620 | 29 | 1591 | 129.2 | GPR Successful liver transplant |
| 24 F | PNH/AA | Thrombocytopenia due to bone marrow failure, red cell clone <10% | Thrombosis | 1 | 65 | 160 | 18 | 157 | 8.2 | GPR Successful bridge to BMT for SAA |
| 77 F | PNH/AA | Thrombocytopenia due to bone marrow failure, red cell clone <10% | Anemia from hemolysis, transfusions | 8 | 86 | 550 | 24 | 1085 | 68.1 | SR Deceased from bone marrow failure |
| 28 M | PNH/AA | Thrombocytopenia due to bone marrow failure | Anemia from hemolysis, transfusions; PNH sx | 13 | 71 | 880 | 18 | 2023 | NA | SR Decreased intravascular hemolysis but persistent transfusional needs from underlying BMF |
| 63 M | PNH/AA | Thrombocytopenia due to bone marrow failure | Anemia from hemolysis, transfusions; PNH sx | 7 | 58 | 1070 | 15 | 417 | 32.6 | SR No change in transfusional needs due to BMF, discontinued drug after 4 months |
| 28 F | PNH/AA | Thrombocytopenia due to bone marrow failure | Anemia from hemolysis, desire for pregnancy; PNH sx | 34 | 72 | 2120 | 28 | 529 | 69.2 | GPR Ongoing mild bone marrow failure |
| 51 F | PNH/AA | Thrombocytopenia due to bone marrow failure | Splenic infarct, transfusions | 11 | 56 | 1150 | 27 | 568 | 151.1 | SR Decreased intravascular hemolysis, no further thromboses but persistent transfusional needs from underlying BMF. Required initiation of cyclosporine |
| 32 M | Classical | No transfusions required | Anemia from hemolysis, PNH sx | 28 | 88 | 2730 | 257 | 2555 | 299.5 | GPR Remains on treatment |
| 18 F | Classical | No transfusions required | Anemia from hemolysis, PNH sx | 37 | 93 | 1130 | 122 | 1767 | 169.7 | GPR Remains on treatment |
| 47 F | Classical | No transfusions required | Thrombosis, Anemia from hemolysis, PNH sx | 43 | 90 | 3070 | 207 | 1440 | 160.9 | GPR Remains on treatment |
| 22 F | Classical | No transfusions required | Anemia from hemolysis, PNH sx | 77 | 92 | 2630 | 193 | 924 | 266.1 | GPR Remains on treatment |
| 28 M | Classical | No transfusions required | Anemia from hemolysis, PNH sx | 51 | 98 | 5170 | 65 | 888 | 109.7 | CR Remains on treatment |
| 63 M | Classical | No transfusions required, | Anemia from hemolysis, thromboses | 16 | 78 | 4760 | 264 | 981 | 142.4 | SR Remains on treatment |
BMF, bone marrow failure; CR, complete response; GPR, good partial response; SR, suboptimal response; PNH, paroxysmal nocturnal hemoglobinuria. PNH sx: fatigue, pain, smooth muscle dystonias.
Overall survival and response
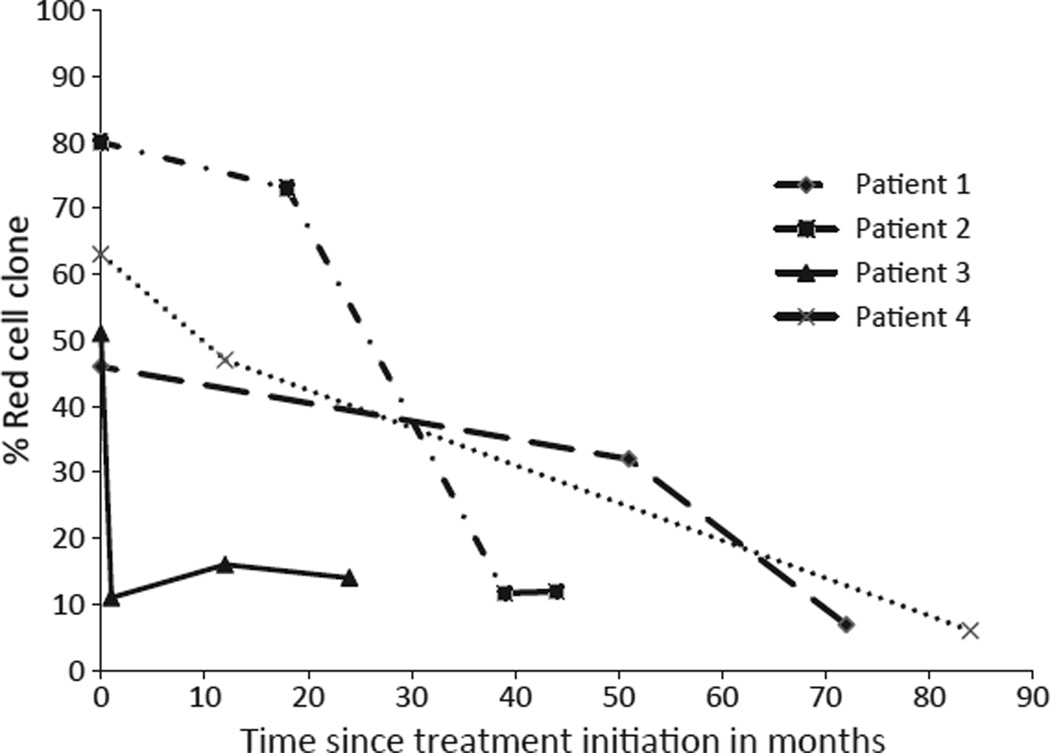
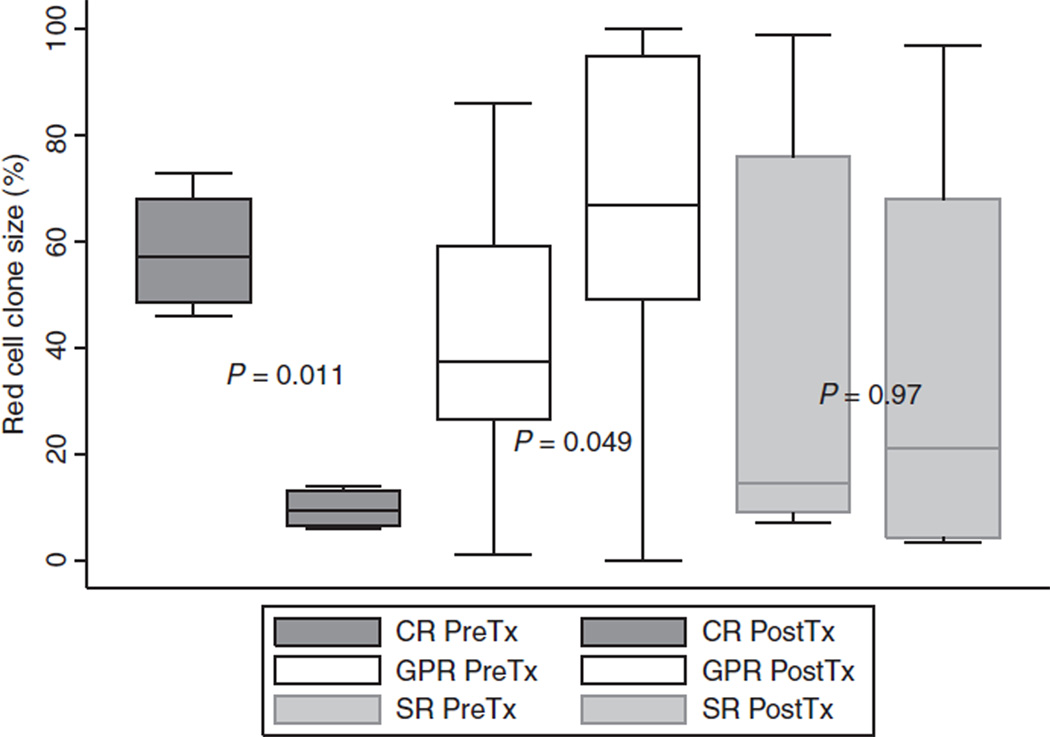
A total of 863 patient-months of treatment with eculizumab are reviewed here from a single institution. With a median follow-up of 24 (range, 6–80) months, the overall survival was 96.66% with a single death in this cohort. A complete hemoglobin response was achieved in four (13.3%) of the patients. (Table 3A) All four of these patients had a significant decrease in the size of their red cell clone after treatment with eculizumab. The kinetics of the decline in PNH erythrocytes are shown in Fig. 1. A good partial hemoglobin response was achieved in 16 (53.3%), and ten patients (33.3%) had a suboptimal response. See Table 3B, C for the characteristics of these patients. A boxplot is shown in Fig. 2 comparing the median red cell clone size changes from pre- to post-treatment between the complete responders, good partial responders, and suboptimal responders. The PNH erythrocyte clone decreased in all four of the complete responders; it increased in all patients with GPRs.
Table 3.
(A) Complete Responder profiles, (B) Good Partial Responder profiles, (C) Suboptimal Responder profiles
| Age/ Sex |
Disease phenotype |
Length of Dx (in months) |
Indication for treatment with eculizumab |
Pre-tx Hb (g/dL) |
Post-tx Hb (g/dL) |
Pre-tx LDH (U/L) |
Post-tx LDH (U/L) |
Pre-tx Retic (K/cu mm) |
Post-tx Retic (K/cu mm) |
Pre-tx RBC clone size (%) |
Post-tx RBC clone size (%) |
Pre-tx Gran clone size (%) |
Post-tx Gran clone size (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||||||
| 33 M | Classical | 13 | Anemia from hemolysis | 8.6 | 17.4 | 1670 | 274 | NA | 133.5 | 46 | 6.9 | 87 | 24 |
| 31 M | AA/PNH | 82 | Thrombosis, anemia from hemolysis | 7.4 | 15.6 | 771 | 311 | 82.6 | 150.7 | 73 | 11.9 | 99 | 99 |
| 28 M | Classical | 4 | Thrombosis, anemia from hemolysis | 10.1 | 16.2 | 888 | 261 | 110 | 109.7 | 51 | 14 | 98 | 99 |
| 36 M | Classical | 192 | PNH sx, Thrombosis, anemia from hemolysis | 8.4 | 15.9 | 3490 | 135 | 324.3 | 101.1 | 63 | 6 | 99 | 23 |
| (B) | |||||||||||||
| 27 M | Classical | 2 | Anemia from hemolysis, PNH sx | 8.9 | 12.7 | 6218 | 329 | 180 | 164.6 | 6.66 | 49 | 73 | 87 |
| 35 F | Classical | 84 | Anemia from hemolysis, PNH sx | 6.6 | 10.3 | H | 196 | 20 | NA | 54 | NA | 99 | NA |
| 56 F | AA/PNH | 288 | Anemia from hemolysis, PNH sx | 8.4 | 8.9 | 2042 | 223 | 101.5 | 79.5 | 25 | 44 | 85 | 96 |
| 21 M | Classical | 60 | Anemia from hemolysis, PNH sx | 9.6 | 12 | 1969 | 279 | 208.9 | 162.3 | 64 | NA | 96 | NA |
| 31 M | Classical | 168 | Thrombosis, anemia from hemolysis | 11 | 12.81 | 1591 | 209 | 129.2 | 129.8 | 83 | 100 | 99 | 99 |
| 64 F | Classical | 108 | Anemia from hemolysis, PNH sx | 9.2 | 11.7 | 1583 | 247 | 161.4 | 86.5 | 86 | 99.7 | 87 | 75.9 |
| 24 F2 | AA/PNH | 36 | Thrombosis | 9.9 | 14.7 | 157 | 168 | 8.2 | 0.9 | 1 | 0 | 65 | 0 |
| 32 M | Classical | 13 | Anemia from hemolysis, PNH sx | 12.1 | 12.6 | 2555 | 240 | 299.5 | 325.1 | 28 | NA | 88 | NA |
| 22 F | Classical | 122 | Anemia from hemolysis, PNH sx | 5.8 | 10.9 | 924 | 195 | 266.1 | 179.8 | 77 | 94 | 92 | 99 |
| 18 F | Classical | 26 | Anemia from hemolysis, PNH sx | 6.3 | 10 | 1760 | 234 | 219.8 | 179.8 | 13 | 95 | 55 | 99 |
| 28 F | AA/PNH | 12 | Anemia from hemolysis, PNH sx | 11.1 | 9.3 | 529 | 180 | 69.2 | 68 | 34 | NA | 72 | NA |
| 48 M | Classical | 36 | Anemia from hemolysis, PNH sx | 6.8 | 10.3 | 2343 | 317 | 190.1 | 160.4 | 38 | 68 | 41 | 62 |
| 47 F | Classical | 2 | Thrombosis, anemia from hemolysis, PNH sx | 10 | 10.9 | 1440 | 268 | 160.9 | 5.1 | 43 | 55 | 90 | 91 |
| 18 F | Classical | 4 | Anemia from hemolysis | 5.1 | 10.0 | 1767 | NA | 169.7 | NA | 37 | 66 | 93 | 98 |
| 48 M | Classical | 204 | Anemia from hemolysis | 7.6 | 10.5 | 4835 | 277 | 451.8 | 321.8 | 42 | 99 | 97 | 96 |
| 57 M | Classical | 150 | Anemia from hemolysis | 11.1 | 10.1 | 1537 | NA | 174 | NA | 34 | NA | 90 | NA |
| (C) | |||||||||||||
| 77 F | AA/PNH | 9 | Anemia from hemolysis | 7.8 | 11.41 | 1085 | 303 | 68.1 | 113.9 | 8 | 7.91 | 86 | 59 |
| 40 F | AA/PNH | 12 | Thrombosis | 8.2 | 8.2 | 622 | 252 | 112.2 | 114.8 | 9 | 97 | 66 | 97 |
| 16 F | Classical | 24 | Thrombosis, anemia from hemolysis | 9.2 | 9.8 | 282 | 362 | 251.1 | NA | 76 | NA | 97 | NA |
| 28 M | Classical | 1 | Anemia from hemolysis, PNH sx | 8.2 | 7.9 | 2023 | 1637 | NA | NA | 13 | NA | 71 | NA |
| 63 M | AA/PNH | 36 | Anemia from hemolysis, PNH sx | 8.5 | 8.5 | 417 | 278 | 32.6 | 28 | 7 | 4 | 58 | 72 |
| 35 M | AA/PNH | 2 | Thrombosis, anemia from hemolysis | 10 | 7.6 | NA | 919 | NA | 84 | 80 | 4.51 | 75 | 92 |
| 46 F | Classical | 3 | Thrombosis, anemia from hemolysis | 8.1 | 9.1 | 1551 | 200 | 139.7 | 222.9 | 32 | 50 | 83 | 94 |
| 39 M2 | Classical | 72 | Thrombosis | 9.9 | 8.1 | H | 185 | 351.4 | 340 | 99 | 86 | 99 | 100 |
| 63 M | Classical | 14 | Thrombosis, anemia from hemolysis | 10.4 | 10.91 | 981 | 189 | 142.8 | 187.3 | 16 | 341 | 78 | 96 |
| 51 F | AA/PNH | 4 | Thrombosis, anemia from hemolysis | 9.1 | 11.71 | 568 | 222 | 151.1 | 106.3 | 11 | 3.2 | 56 | 18 |
PNH, paroxysmal nocturnal hemoglobinuria; H, hemolyzed; NA, not available (for patient with treatment initiated at primary institution but followed elsewhere, only information received at time of analysis).
Values for post-treatment performed after minimum of 6 months from initiation of treatment.
Transfused value.
S/p bone marrow transplantation.
Figure 1.
Decrease in complete responder red cell clone over time. Graphical representation of the four patients who were complete responders with their red cell clone size plotted over time in months. Each patient started with a red cell clone >45% and all decreased to <15% after therapy.
Figure 2.
Comparison of red cell clone size pre- and post-treatment with eculizumap between complete responders, good partial responders, and suboptimal responders. A boxplot representation of the median and interquartile ranges of the clones size of the patients in each response category. CR, complete response; GPR, good partial response; SR, suboptimal response; tx, treatment.
The Food and Drug Administration (FDA) approved eculizumab for the treatment of PNH; however, clinical trials of eculizumab have had relatively strict inclusion criteria (9, 17). Of the two phase III clinical trials of eculizumab for PNH, SHEPHERD had the more liberal inclusion criteria. Patients were required to have had at least one or more transfusions in the past 2 yr for anemia or anemia-related symptoms. A PNH type III RBC proportion ≥10% assessed by flow cytometry, platelets ≥30 000/µL, and LDH levels ≥1.5 times the upper limit of the normal range were also required. Thus, we assessed this cohort of eculizumab-treated patients for SHEPHERD eligibility. Seventeen patients met SHEPHERD eligibility criteria, and 13 (43.3%) did not, due to platelet counts <30 000/µL (seven patients) or red cell transfusion independence (six patients). These patients were prescribed eculizumab to treat life-threatening thrombosis, intravascular hemolysis, or both. The thrombocytopenia was related to bone marrow failure in six patients. One patient had thrombocytopenia due to liver failure and hypersplenism. Two of the seven patients with platelet counts <30 000/µL appeared to benefit from eculizumab therapy. The patient with classical PNH and liver failure due to Budd –Chiari syndrome was able to receive a successful orthotopic liver transplant and remained on eculizumab 5 yr later; he has discontinued anticoagulation with no further thrombotic episodes. The 24-yr-old woman with refractory SAA and mesenteric thrombosis associated with a large PNH clone (65% PNH granulocytes) was treated with eculizumab for 8 wk prior to receiving a matched unrelated allogeneic BMT for refractory SAA. Eculizumab was administered to prevent further thromboses and to treat severe PNH pain crises. Anticoagulation was relatively contraindicated prior to BMT due to the patient being refractory to platelet transfusions. Eculizumab was discontinued after the patient received her allograft. She currently has normal peripheral blood counts with no PNH cells.
Discontinuation and dose modifications
Five patients (16.7%) in this cohort discontinued eculizumab therapy. Three patients who discontinued therapy received an allogeneic bone marrow transplant. Of these three, two received a BMT for severe bone marrow failure (one also had mesenteric thrombosis); the other had classical PNH and severe intravascular hemolysis despite a dose of 1500 mg of eculizumab every 12 d. All three patients were classified as suboptimal responders, but eculizumab may have allowed successful ‘bridging’ to BMT. The two other suboptimal responders also had moderate to severe bone marrow failure; neither patient was eligible for BMT due to age, comorbidities, or both. Two patients with classical PNH required dose adjustments after having been on the eculizumab for 24 and 3 months, respectively. This was in the context of recurrent or breakthrough hemolysis that was not addressed easily with standard eculizumab dosing. Both patients were transiently controlled (1–3 months) with 1200 mg every 12 d, but eventually experienced a return of symptoms and evidence of intravascular hemolysis on days 9 or 10. Similarly, increasing the dose to 1500 mg every 12–14 d also resulted in transient control of symptoms and intravascular hemolysis, but both patients experienced a return of PNH symptoms and signs of intravascular hemolysis even at this dose by days 10 or 11. Interestingly, these two patients had co-existent autoimmune diseases: one had Crohn’s disease and the other had rheumatoid arthritis. There are two additional patients in the cohort with autoimmune diseases: one with Graves’ disease and another with Crohn’s disease, both of who are categorized as suboptimal responses. They have not had dose increases because eculizumab controlled their intravascular hemolysis; however, the patients remain transfusion dependent without abatement in their symptoms or transfusion requirements. The patient with Graves’ disease remains transfusion dependent and has significant extravascular hemolysis, while patient with the Crohn’s disease has severe bone marrow failure.
Nine patients had single episodes of breakthrough hemolysis during their course. These are in the setting of active infection and distinct from the above discussion of recurrent hemolysis. One patient had documented bacteremia with Neisseria meningitides. Two additional patients had possible Neisserial infections but took ciprofloxacin doses at home prior to cultures drawn on admission. Six patients had viral syndromes and presented with fevers and evidence of transiently increased intravascular hemolysis with elevated LDH and decreased hemoglobin levels from baseline. In all but one breakthrough, intravascular hemolysis lasted for less than a single 14-d dosing interval and hemoglobin and LDH returned to baseline levels. The remaining patient had documented Cytomegalovirus (CMV) mononucleosis with a prolonged course of increased breakthrough intravascular hemolysis that lasted for 8 wk. Breakthrough intravascular hemolysis caused her hemoglobin to fall to 4.5 g/dL (baseline 11.0 g/dL) and her LDH level to rise to 716 IU/L (baseline <120 IU/L). During her 10-d hospitalization, she required 3 units of packed red cells. Her intravascular hemolysis resolved, and her hemoglobin and LDH levels returned to baseline following ganciclovir treatment that eradicated her CMV viremia.
Thrombotic events
Prior to eculizumab therapy, 10 of 30 patients (33%) had experienced total of 14 separate thrombotic events. All of these patients were on anticoagulation before eculizumab therapy. Seven of these ten patients (70%) discontinued anticoagulation after a year or more without thromboses. One patient experienced a deep vein thrombosis 1 yr after initiation of eculizumab and was anticoagulated for an additional 6 months and then discontinued anticoagulation with no further thromboses.
Discussion
Eculizumab is the only FDA-approved drug for the treatment of PNH. In patients with classical PNH, eculizumab has been shown to decrease the need for transfusions, prevent intravascular hemolysis, improve quality of life, and prevent thrombosis (18). The data presented here differ from previous reports because they represent more of a ‘real world’ experience and include patients who would not have been eligible for eculizumab on clinical trials. Response was evaluated based on improvements in anemia, PNH symptoms, and thrombosis. This is the largest cohort of patients treated in the United States at a single institution. We found that hemoglobin response to eculizumab is highly variable and may be influenced by bone marrow failure, the size of the PNH red cell clone, and underlying inflammatory conditions. Moreover, we demonstrate that even patients who would not have met eligibility criteria for previous eculizumab trials, especially patients with thrombosis, may benefit from terminal complement inhibition.
There are 13 patients in this series who would not have been eligible to receive the drug based upon the original SHEPHERD trial inclusion criteria. All six classical patients with PNH who were not requiring transfusions prior to eculizumab therapy responded to treatment with abatement of thrombosis, PNH symptoms, or both. Fewer patients with severe thrombocytopenia benefited from eculizumab; however, it did appear to benefit at least two of these seven patients: one with classical PNH and liver failure and another with AA/PNH who developed mesenteric vein thrombosis. In both cases, eculizumab therapy was used as a ‘bridge’ to allow these patients to undergo either liver or bone marrow transplantation. Thus, we believe that regardless of the platelet count, eculizumab therapy is indicated in PNH patients with life-threatening thrombosis. It is also important to point out that not every patient with a large PNH clone or patients with classic PNH need to start on eculizumab therapy as evidenced by the two patients with classical PNH and large PNH clones who are asymptomatic for over 5 yr and have not required eculizumab therapy.
The majority of eculizumab-treated patients experienced improved quality of life and became red cell transfusion independent; however, most responders continued to have laboratory evidence of extravascular hemolysis (elevated reticulocyte count and mild-moderate anemia). Interestingly, four patients achieved normal hemoglobin levels without evidence of extravascular hemolysis. The reduction in the percentage of PNH red cells means that only a small percentage of the red cell mass is susceptible to extravascular hemolysis in these patients because the increase in C3 deposition in PNH patients treated with eculizumab only occurs on the PNH red cells (14).
Understanding the mechanism whereby patients with PNH on eculizumab achieve a CR is important because development of novel complement inhibitors that prevent both intravascular and extravascular hemolysis is underway (19). All four patients who achieved CR experienced a decline in the percentage PNH red cells following eculizumab therapy. In contrast, all patients who achieved a GPR (ongoing extravascular hemolysis) experienced an increase in the size of the PNH red cell clone. It is unclear whether eculizumab directly contributed to the reduction in the PNH clone because spontaneous remissions have been reported in up to 10% of patients with PNH (8, 9). Indeed, two of these four patients also experienced a reduction in the percentage of PNH granulocytes, suggesting that the percentage of normal stem cells relative to the PNH stem cell clone had increased. The other two patients who experienced a marked reduction in the percentage of PNH red cells continued to have 99% PNH granulocytes, suggesting that eculizumab therapy somehow produced an environment that was more favorable to the normal erythroid progenitors. The PNH erythrocyte clone size in the partial and suboptimal responders was smaller than that of the complete responders as well as smaller than the corresponding granulocyte clone. This is a feature of transfused red cells in the sample tested and may suggest that more heavily transfused patients had worse bone marrow failure and hemolysis and eculizumab is not able to overcome it.
We also observed clinical scenarios that appear to predict for either breakthrough hemolysis or a poor response to eculizumab. As previously reported, eculizumab does not improve underlying bone marrow failure (9). There were four patients in this study with a coexistent autoimmune disease (two Crohn’s, one Graves’ disease, and one rheumatoid arthritis), and all four of these patients had a suboptimal response. One of the patients with Crohn’s disease had underlying bone marrow failure, but the other three had classical PNH that was not controlled with eculizumab. Two of these patients (Crohn’s disease and rheumatoid arthritis) continued to have breakthrough intravascular hemolysis on day 10 after eculizumab in spite of dosage increases to 1500 mg every 12 d. The patient with Grave’s disease has extensive extravascular hemolysis and is requiring red cell transfusions every 4–6 wk. While the mechanism for this potential association is unclear, it is conceivable that chronic inflammatory states lead to increased complement activation that requires high dosages of eculizumab because standard doses resulted in incomplete C5 blockade. Indeed, transient breakthrough intravascular hemolysis was observed in nine patients following viral or bacterial infections. One patient acquired CMV-associated mononucleosis requiring treatment with ganciclovir. The illness lasted over 8 wk and was associated with extensive intravascular hemolysis despite being well controlled on a stable dose of eculizumab (900 mg every 14 d) for over 4 yr; breakthrough hemolysis resolved with eradication of the CMV viremia. Breakthrough intravascular hemolysis was also observed in the patient who had massive ascites and required a liver transplant. Breakthrough in this circumstance was probably related to an increased volume of distribution. No further breakthrough was seen following liver transplantation.
The major limitations of this study are the relatively small patient sample, the retrospective analysis, and the possibility of selection bias in choosing which patients required therapy. However, treatment decisions were made by just one of the authors and based on published recommendations (11). Moreover, PNH is a rare disease, and future prospective studies with eculizumab in PNH may not be performed given that the drug has been approved already by the FDA.
In conclusion, terminal complement inhibition benefits most patients with classical PNH, but hemoglobin responses are highly variable and may depend on the degree of underlying bone marrow failure, underlying inflammatory conditions and size of the PNH red cell clone following drug treatment. Normalization of the hemoglobin levels appears to predict for a decrease in the size of red cell clone. Furthermore, thrombosis in the setting of a large PNH clone, regardless of co-existent thrombocytopenia or severe bone marrow failure, should be considered an indication for terminal complement inhibition. Validation of these hypothesis generating findings should be validated by the International PNH registry (www.pnhregistry.com).
Acknowledgments
Acknowledgements and disclosures
The authors would like to thank the patients for allowing us to participate in their care. This work was supported by NIH grant P01 CA70970 to RAB and NIH grant 5K12HL08 7169-05 to AED.
Footnotes
Authors contributions
AED cared for the patients, collected, analyzed and interpreted the data, and drafted the manuscript. RAB cared for the patients, analyzed and interpreted the data, and drafted and reviewed the manuscript. DD cared for the patients and assisted with data collection. RAB serves on the international advisory board of Alexion Pharmaceuticals.
References
- 1.Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine (Baltimore) 1997;76:63–93. doi: 10.1097/00005792-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148:587–595. doi: 10.7326/0003-4819-148-8-200804150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 6.Lachmann PJ, Hughes-Jones NC. Initiation of complement activation. Springer Semin Immunopathol. 1984;7:143–162. doi: 10.1007/BF01893018. [DOI] [PubMed] [Google Scholar]
- 7.Pangburn MK, Muller-Eberhard HJ. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980;152:1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 10.Hillmen P, Elebute M, Kelly R, et al. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85:553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113:6522–6527. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 13.Hill A, Rother RP, Arnold L, Kelly R, Cullen MJ, Richards SJ, Hillmen P. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95:567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113:4094–4100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky RA, Mukhina GL, Li S, Nelson KL, Chiurazzi PL, Buckley JT, Borowitz MJ. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borowitz MJ, Craig FE, DiGiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT, Richards SJ. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78:211–230. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 17.Hillmen P, Muus P, Duhrsen U, et al. The terminal complement inhibitor eculizumab reduces thrombosis in patients with paroxysmal nocturnal hemoglobinuria (Abstract) Blood. 2006;106:40a–41a. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 18.Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 19.Fridkis-Hareli M, Storek M, Mazsaroff I, Risitano AM, Lundberg AS, Horvath CJ, Holers VM. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of the human complement alternative pathway-mediated diseases. Blood. 2011;118:4705–4713. doi: 10.1182/blood-2011-06-359646. [DOI] [PMC free article] [PubMed] [Google Scholar]