Abstract
Objectives
Parallel comparison with 0.15% ganciclovir (GCV) ophthalmic gel to evaluate the effectiveness and safety of 0.15% GCV in situ ophthalmic gel for the treatment of herpes simplex keratitis (HSK).
Methods
This was a multicenter, randomized, investigator-masked, parallel group study. HSK patients were randomly divided into two groups, with the corresponding treatment of 0.15% GCV ophthalmic gel or 0.15% GCV in situ ophthalmic gel. Symptoms and signs were observed before administration, and 3 (±1), 7 (±1), 14 (±2), and 21 (±3) days after the administration. The clinical effective rate was considered as the primary outcome. The safety profile was evaluated by AEs, visual acuity, and ocular tolerance.
Results
The clinical effective rate in the per-protocol (PP) dataset for the treatment group and the control group were 95.10% and 93.00%, respectively (P = 0.5282). The noninferiority test showed significant differences (P = 0.000305, P < 0.025), indicating that the tested drug was noninferior to the control. Patients in the PP dataset of both groups experienced decreases in the total scores of clinical indicators. Ocular AEs were few but similar between the two groups. There were no significant differences between patients’ visions between the two groups before and after administration in the safety analysis set. In terms of drug tolerance, the rates of patients without transient blurred vision during all the visits in the treatment group were higher than those for the control group (P < 0.05). During the third and fourth visits, the rates of patients with eye itching were 4.08% and 1.22% in the treatment group, and 13.59% and 8.14% in the control group, respectively (P < 0.05). During the second visit, the rates of patients with eye irritation were 14.42% in the treatment group and 25.71% in the control group (P < 0.05).
Conclusion
The 0.15% GCV in situ ophthalmic gel was effective and safe for the treatment of HSK, and was not inferior to 0.15% GCV ophthalmic gel. The 0.15% GCV in situ ophthalmic gel presented superior ocular tolerance.
Keywords: virus keratitis, herpes simplex virus 1, ganciclovir in situ ophthalmic gel, treatment
Introduction
Herpes simplex keratitis (HSK) is the most common type of virus keratitis, which remains a major cause of visual morbidity. The patients generally have the symptoms of ophthalmodynia, phengophobia, tearing, foreign body sensation, signs of ciliary congestion, corneal infiltration/ulcer, or corneal edema. For the treatment of HSK, antivirus drugs are generally administered during early symptoms to prevent virus proliferation. Ganciclovir (GCV) is a highly-efficient broad-spectrum antivirus drug.1 It is a derivative of acycloguanosine, which is transformed into triphosphate-GCV in vivo. Via competitive inhibition, triphosphate deoxyguanosine embeds into the virus deoxyribonucleic acid (DNA), and consequently inhibits the DNA polymerase of the herpes simplex virus (HSV), slows the replication of virus DNA chains, and inhibits the synthesis of virus DNA.2–4 GCV is effective for the treatment of HSK, but its distribution coefficient is so low that its topical use holds a certain degree of limitation.5 What is worse, eye drops generally need frequent administration because of their short retention time. To overcome these problems, some researchers have developed many alternatives to eye drops, such as ointment, microspheres, and gels.6,7 These dosage forms literally improve antivirus effectiveness, but their discomfort and blurred vision cause poor tolerance. GCV in situ gel can offer the advantages of combining the merits of both solutions and gels. GCV in situ ophthalmic gel is administered in a solution state, goes through phase transition due to different pH values, and then forms into a semisolid with nonchemical cross-linking.8 Therefore, the retention time is prolonged and the bioavailability is consequently enhanced while discomfort and visual blurring are alleviated.
This clinical study was conducted in multiple centers nationwide, in a randomized and single-blind method. The effectiveness and safety of 0.15% GCV in situ gel for HSK was in parallel comparison with that of 0.15% GCV ophthalmic gel.
Methods
Study design
This study was a multicenter, randomized, single-blind, parallel-group clinical trial consisting of a 3-week treatment phase. A total of 226 HSK patients were recruited from five clinical centers in the People’s Republic of China from April 2009 to June 2011. Written, informed consent was obtained from all participants before inclusion in the study. This study was approved by each center’s Institutional Review Board and was conducted in accordance with good clinical practices and the Declaration of Helsinki.
The HSK patients included in this study were aged 18–65 years, with the symptoms of punctiform, arborization, or map-shaped changes in cornea. HSK patients were excluded if they were taking antivirus drugs, glucocorticoids, anti-inflammatory medicines, or epithelial repair drugs. Patients with discoid keratitis, concurrent uveitis, serious corneal decompensation, or ocular allergic disease were excluded. Patients who had a previous history of serious heart, lung, liver, or kidney dysfunction were also excluded. Pregnancy and breastfeeding women were excluded. Patients who had participated in any other clinical trials within 3 months were excluded. Patients with mental illness were excluded at the discretion of the researchers.
Study treatments and assessments
Eligible patients for this study were randomly assigned in a 1:1 ratio by a computer-generated randomization list to investigator-masked treatment with either 0.15% GCV in situ ophthalmic gel (Shenyang SINQI Pharmaceutical Co, Ltd, Shenyang, Liaoning, People’s Republic of China) or 0.15% GCV ophthalmic gel (Hubei Keyi Pharmaceutical Co, Ltd, Wuhan, Hubei, People’s Republic of China). Patients randomly assigned were instructed to self-administer one drop of 0.15% GCV ophthalmic gel or 0.15% GCV in situ ophthalmic gel in the conjunctival sac each time, four times a day for 3 weeks.
Randomization was performed by the order of entrance to the study and based on a previous list generated by computer. Sealed, opaque envelopes guaranteed allocation concealment. For the purposes of masking, labels on the commercial bottles of 0.15% GCV in situ ophthalmic gel and 0.15% GCV ophthalmic gel were replaced with investigational labels, and bottles were packaged in identical kit boxes in an attempt to mask patients. However, due to differences in the appearance of the bottles, patients were not fully masked. To ensure that the investigators were masked, a masked designee at each site dispensed the kit boxes to patients according to the randomization list, and retrieved the kits from patients at the end of the study; investigators were not present during the study treatment dispensation to and retrieval from patients. Unmasked designees also instructed patients on the proper instillation of the study medication.
The investigator attained the informed consent of patients during the first visit. Demographic data and other baseline characteristics of patients were collected; and the symptoms and signs were quantified. Patients were revisited 3 (±1), 7 (±1), 14 (±2), and 21 (±3) days after administration. During the revisits, relevant data were gathered, which included symptoms and signs of the eyes, visual acuity (VA), adverse events (AEs), and tolerance tests.
Outcome measures
Indicators for effectiveness consisted of ophthalmalgia, photophobia, tearing, foreign body sensation, and blurred vision (symptoms), as well as conjunctival congestion, corneal infiltration/ulcer, and corneal edema (signs). Ocular signs were evaluated using a slit-lamp biomicroscope and graded on a 0 to 4 scale (0 = absent, 1 = trace, 2 = mild, 3 = moderate, and 4 = severe). Ocular symptoms were graded on a 0 to 4 scale (0 = absent, 1 = trace, 2 = mild, 3 = moderate, and 4 = severe) and assessed by the investigator through direct patient inquiry.
All efficacy variables were evaluated for the HSK eyes at each visit. The effectiveness of both the 0.15% GCV in situ ophthalmic gel and the GCV ophthalmic gel was evaluated according to the changes of the total scores of clinical indicators (TSI) and therapeutic index. The criteria are shown in Table 1.
Table 1.
Criteria for the evaluation of effectiveness
| Grades | Evaluation of effectiveness |
|---|---|
| Significantly effective | All the indicators decreased to 0 |
| Effective | Significant improvement in all the indicators (TIa ≥ 60%) |
| Slightly effective | Improvement in the indicators (30% ≤ TI 60%) |
| Ineffective | No improvement or exacerbation in all the indicators (TI < 30%) |
Notes:
TI was calculated according to the following equation: TI (%) = (TSI before administration − TSI after administration)/TSI before administration × 100%; TSI = total symptoms’ scores + total signs’ scores.
Abbreviations: TI, therapeutic index; TSI, total scores of clinical indicators.
The clinical effective rate (CER) was calculated according to the following equation:
| (1) |
CER was used as the major indicator to test effectiveness. As for the indicators for safety, they included all the AEs, visual tests, and tolerance tests. The relevance between the drugs and the AEs would be considered to be independent, possibly independent, possibly dependent, highly possibly dependent, and dependent. For the visual test, the international standard decimal VA chart was utilized. The tolerance tests including transient blurred vision, eye irritation, and itching, which were graded from 0 to 4 (0 = comfortable; 1 = occasional discomfort without influence on daily life; 2 = regular discomfort with slight influence on daily life; 3 = frequent discomfort with serious influence on daily life; and 4 = lasting discomfort with serious influence on daily life).
Statistics
SAS software V8.2 (SAS Institute Inc, Cary, NC, USA) was used for the statistical analysis. The numerical data normally distributed were expressed as the mean ± standard deviation. Efficacy data collected on the HSK eye were analyzed. The more severe eye was selected as the study eye if both eyes had HSK. Analysis of covariance (ANCOVA), Kruskal–Wallis H test, or the χ2 test were conducted on the demographic and clinical characteristics of the intent-to-treat (ITT) dataset patients. The Cochran–Mantel–Haenszel χ2 test was utilized to calibrate multicenter effects, and to compare the indicators of the effectiveness and CER for both groups. A total of 10% of the CER of the control group was considered to be an acceptable deviation (Δ). P ≤ 0.025 for the noninferiority test would indicate that the tested drug was not inferior to the control. The TSI and the decrease of TSI from baseline (DTB) were compared with ANCOVA calibrating the center effect in the ITT dataset and the per-protocol (PP) dataset, respectively. Safety profiles were assessed by evaluating all reported AEs, change in VA, and the rate of tolerance tests in the safety analysis set (SS). AEs were categorized into relevant and irrelevant. AE rates were compared by Fisher’s direct test, and the tolerance rates were performed using the Cochran–Mantel–Haenszel χ2 test. VA was analyzed using an ANCOVA model. A P-value of <0.05 was considered statistically significant.
Results
Patients’ descriptions
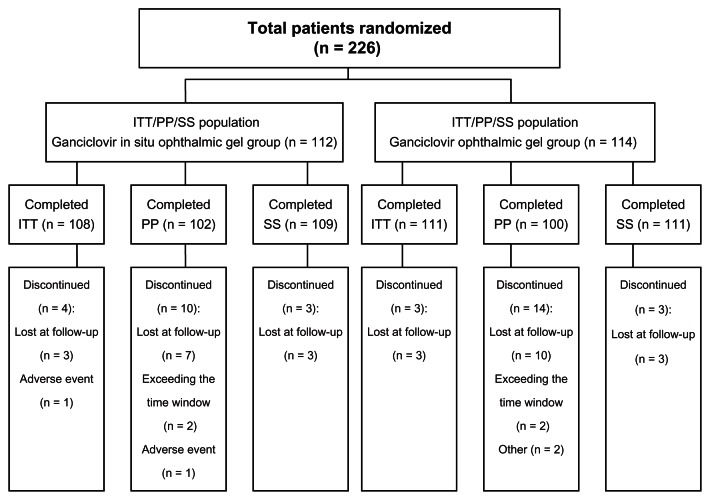
A total of 226 patients from the five centers were randomly divided into either the treatment group (n = 112) or control group (n = 114) (Figure 1). In all, 219 patients were in the ITT data set, with 108 in the treatment group, and 111 in the control group. Seven patients were excluded for different reasons: AEs (one in the treatment group), or loss of follow-up (three in the treatment group and three in the control group). A total of 202 patients were in the PP data set, with 102 in the treatment group and 100 in the control group. Moreover, 24 patients were excluded for different reasons: AEs (one in the treatment group), loss of follow-up (seven in the treatment group and ten in the control group), noncompliance (one in the control group), exceeding the time window during the last visit (two in the treatment group and two in the control group), or the intake of other drugs (one in the control group). In all, 220 patients were in the SS, with 109 in the treatment group and 111 in the control group. Six patients were excluded for loss of follow-up (three in the treatment group and three in the control group).
Figure 1.
Patients’ descriptions.
Abbreviations: n, number; ITT, intent-to-treat; PP, per-protocol; SS, safety analysis set.
Patient baseline characteristics
The baseline characteristics of patients in the treatment group and control group were similar. Comparing the ITT datasets of the treatment group and control group after calibrating the center effect, there were no significant differences between the baselines of demography, gender, physical examination, allergy history, TSI, and VA (P > 0.05) (Table 2). It was noted that all the patients were Chinese. The average age of patients in the treatment group was 43.83 years, and that for patients in the control group was 44.54 years. The TSI of the treatment and control groups before administration were 17.75 ± 5.71 and 17.98 ± 5.45 (P = 0.678). The average baseline vision of the treatment group and the control group were 0.50 ± 0.26 and 0.54 ± 0.27 (P = 0.297).
Table 2.
Demographic and clinical characteristics of study patients (ITT data set)
| Variables | Ganciclovir in situ ophthalmic gel (n = 108) | Ganciclovir ophthalmic gel (n = 111) | P-value |
|---|---|---|---|
| Sex, n (%) | 0.2106 | ||
| Male | 70 (64.81) | 63 (56.76) | |
| Female | 38 (35.19) | 48 (43.24) | |
| Age, mean ± SD, years | 43.83 ± 13.37 | 44.54 ± 12.15 | 0.6997 |
| Chinese race, n (%) | 108 (100.0) | 111 (100.0) | |
| Physical examination, n (%) | 0.5853 | ||
| Normal | 107 (99.07) | 109 (98.20) | |
| Abnormal | 1 (0.93) | 2 (1.80) | |
| Allergy history, n (%) | 0.2705 | ||
| Negative | 98 (90.74) | 105 (94.59) | |
| Positive | 10 (9.26) | 6 (5.41) | |
| TSI of the baseline, mean ± SD | 17.75 ± 5.71 | 17.98 ± 5.45 | 0.6782 |
| Baseline VA | |||
| Mean ± SD | 0.50 ± 0.26 | 0.54 ± 0.27 | 0.2970 |
Abbreviations: ITT, intent-to-treat; n, number; SD, standard deviation; TSI, total scores of clinical indicators; VA, visual acuity.
Effectiveness analysis
Clinical effectiveness
The major indicator for this study was the CER. After 21 days of treatment, the PP dataset indicated that the CERs of the treatment group and the control group were 95.10% and 93.00%, respectively (P = 0.5282), while the 95% confidence interval of the CERs were (90.91, 99.29) and (88.00, 98.00), respectively. While setting 10% of the CER of the control group as the critical point (Δ = 9.30%), the noninferiority test indicated that the tested drug was not inferior to the control drug (P = 0.000305; P < 0.025) (Table 3).
Table 3.
Clinical effectiveness of treatment group and control group (PP/ITT dataset)
| Variables | PP dataset | ITT dataset | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Ganciclovir in situ ophthalmic gel group | Ganciclovir ophthalmic gel group | P-value | Ganciclovir in situ ophthalmic gel group | Ganciclovir ophthalmic gel group | P-value | |||
| Clinical effectiveness | χ2 = 0.6331 | 0.4262 | χ2 = 0.49 | 0.4836 | ||||
| Total (%) | 102 (100.00) | 100 (100.00) | 108 (100.00) | 111 (100.00) | ||||
| Significantly effective (%) | 47 (46.08) | 43 (43.00) | 47 (43.52) | 45 (40.54) | ||||
| Effective (%) | 50 (49.02) | 50 (50.00) | 51 (47.22) | 53 (47.75) | ||||
| Slightly effective (%) | 4 (3.92) | 6 (6.00) | 5 (4.63) | 6 (5.41) | ||||
| Ineffective (%) | 1 (0.98) | 1 (1.00) | 5 (4.63) | 7 (6.31) | ||||
| Total clinical effectiveness | χ2 = 0.3978 | 0.5282 | χ2 = 0.3503 | 0.5540 | ||||
| CER (%) | 97 (95.10) | 93 (93.00) | 98 (90.74) | 98 (88.29) | ||||
| CIER (%) | 5 (4.90) | 7 (7.00) | 10 (9.26) | 13 (11.71) | ||||
| Confidence interval of 95% CER (%) | (90.91, 99.29) | (88.00, 98.00) | Z = 3.427 | 0.000305 | (85.27, 96.21) | (82.31, 94.27) | Z = 2.836 | 0.0022872 |
Abbreviations: PP, per-protocol; ITT, intent-to-treat; CER, clinical effective rate; CIER, clinical ineffective rate.
Change of total scores of clinical indicators from baseline
The average TSI of the treatment group and the control group in the PP dataset were 17.86 and 17.91 before administration (P = 0.8983), respectively. After administration, the TSI for both groups decreased gradually. Three, 7, 14, and 21 days after the administration, the corresponding scores were 14.25, 9.75, 5.41, and 2.06 for the treatment group, and 13.82, 9.41, 5.30, and 2.32 for the control group (P > 0.05), respectively (Table 4). After treatment, the extent of the DTB became greater with time. Three, 7, 14 and 21 days after the administration, the DTB was 3.61, 8.11, 12.45, and 15.80 for the treatment group, and 4.09, 8.50, 12.61, and 15.59 for the control group. However, there were no significant differences between the two groups (P > 0.05) (Table 4).
Table 4.
Change of total symptom and sign scores after administration (ITT/PP dataset)
| Variables | ITT | PP | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Treatment groupa | Control groupb | F-value | P-value | Treatment groupa | Control groupb | F-value | P-value | |
| Baseline | ||||||||
| TSI, mean ± SD | 17.75 ± 5.71 | 17.98 ± 5.45 | 0.1726 | 0.6782 | 17.86 ± 5.80 | 17.91 ± 5.52 | 0.02 | 0.8983 |
| Visit 1 (3rd day), mean ± SD | ||||||||
| TSI | 14.19 ± 5.80 | 14.03 ± 5.11 | 0.0155 | 0.9009 | 14.25 ± 5.92 | 13.82 ± 5.15 | 0.21 | 0.6447 |
| DTB | 3.56 ± 3.51 | 3.95 ± 4.03 | 0.5795 | 0.4473 | 3.61 ± 3.59 | 4.09 ± 3.86 | 0.67 | 0.4147 |
| Visit 2 (7th day), mean ± SD | ||||||||
| TSI | 9.91 ± 5.08 | 9.90 ± 4.77 | 0.0093 | 0.9231 | 9.75 ± 5.16 | 9.41 ± 4.40 | 0.12 | 0.7279 |
| DTB | 7.84 ± 4.72 | 8.08 ± 5.27 | 0.1318 | 0.7169 | 8.11 ± 4.69 | 8.50 ± 4.87 | 0.22 | 0.6420 |
| Visit 3 (14th day), mean ± SD | ||||||||
| TSI | 5.71 ± 4.31 | 6.06 ± 4.91 | 0.4706 | 0.4935 | 5.41 ± 4.16 | 5.30 ± 3.98 | 0.04 | 0.8329 |
| DTB | 12.04 ± 5.58 | 11.92 ± 5.97 | 0.0114 | 0.9153 | 12.45 ± 5.33 | 12.61 ± 5.37 | 0.00 | 0.9945 |
| Visit 4 (21st day), mean ± SD | ||||||||
| TSI | 2.50 ± 3.32 | 3.16 ± 4.80 | 2.24 | 0.1361 | 2.06 ± 2.71 | 2.32 ± 3.09 | 0.70 | 0.4033 |
| DTB | 15.25 ± 6.05 | 14.82 ± 6.57 | 0.19 | 0.6609 | 15.80 ± 5.59 | 15.59 ± 5.78 | 0.08 | 0.7771 |
Notes:
Treatment group = ganciclovir in situ ophthalmic gel group;
control group = ganciclovir ophthalmic gel group.
Abbreviations: ITT, intent-to-treat; PP, per-protocol; TSI, total scores of clinical indicators; SD, standard deviation; DTB, decrease of total scores of clinical indicators from baseline.
Safety profile
Adverse events
In the SS, the rate of AEs in the treatment group was 1.83% (2/109), and the percentage in the control group was 0% (0/111) (P = 0.244). No serious AEs were observed. For the two AEs in the treatment group, this was proven to be irrelevant to the tested drug. In detail, one of the AEs was due to iridocyclitis (independent of the tested drug), and the other was because of dizziness (possibly independent of the tested drug).
Vision changes
There were no significant differences between patients’ visions in the treatment group and control group before administration (P = 0.297) and after administration (P > 0.05) in the SS (Table 5). The results indicated that the tested drug had no negative influence on patients’ vision.
Table 5.
Change of VA after administration (SS)
| VA | Treatment groupa | Control groupb | t-value | P-value |
|---|---|---|---|---|
| Baseline, mean ± SD | 0.50 ± 0.26 | 0.54 ± 0.27 | 1.046 | 0.297 |
| Visit 1 (3rd day), mean ± SD | 0.52 ± 0.27 | 0.56 ± 0.24 | 0.999 | 0.319 |
| Visit 2 (7th day), mean ± SD | 0.57 ± 0.28 | 0.61 ± 0.23 | 1.088 | 0.278 |
| Visit 3 (14th day), mean ± SD | 0.63 ± 0.27 | 0.68 ± 0.23 | 1.540 | 0.125 |
| Visit 4 (21st day), mean ± SD | 0.69 ± 0.29 | 0.74 ± 0.20 | 1.245 | 0.215 |
Notes:
Treatment group = ganciclovir in situ ophthalmic gel group;
control group = ganciclovir ophthalmic gel group.
Abbreviations: VA, visual acuity; SS, safety analysis set; SD, standard deviation.
Tolerance
Analysis of the SS dataset indicated that most patients reported comfort after administration, while a few patients reported slight discomfort that lasted for a short time. During the visits, the rates of patients not reporting transient blurred vision were 87.04% (visit 1), 91.35% (visit 2), 91.84% (visit 3), and 95.12% (visit 4) in the treatment group, and 72.97% (visit 1), 75.24% (visit 2), 81.55% (visit 3), and 86.05% (visit 4) in the control group (P < 0.05) (Table 6). During the second visit, the rates of patients reporting slight eye irritation were 14.42% in the treatment group and 25.71% in the control group (P = 0.0422) (Table 7). The rates of patients reporting slight eye itching were 4.08% (third visit) and 1.22% (fourth visit) for the treatment group, and 13.59% (third visit) and 8.14% (fourth visit) for the control group (P < 0.05) (Table 8). Apparently, the 0.15% GCV in situ ophthalmic gel could offer more comfort to patients than the 0.15% GCV ophthalmic gel.
Table 6.
Rates of patients reporting transient blurred vision
| Scores | Treatment group | Control group | Chi-square | P |
|---|---|---|---|---|
| First visit | ||||
| N (missing) | 108 (1) | 111 (0) | 6.21 | 0.0127a |
| 0 | 94 (87.04%) | 81 (72.97%) | ||
| 1 | 13 (12.04%) | 28 (25.23%) | ||
| 2 | 1 (0.93%) | 2 (1.80%) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Second visit | ||||
| N (missing) | 104 (5) | 105 (6) | 8.18 | 0.0042b |
| 0 | 95 (91.35%) | 79 (75.24%) | ||
| 1 | 8 (7.69%) | 25 (23.81%) | ||
| 2 | 1 (0.96%) | 1 (0.95%) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Third visit | ||||
| N (missing) | 98 (11) | 103 (8) | 4.54 | 0.0330c |
| 0 | 90 (91.84%) | 84 (81.55%) | ||
| 1 | 8 (8.16%) | 19 (18.45%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Fourth visit | ||||
| N (Missing) | 82 (27) | 86 (25) | 3.99 | 0.0458d |
| 0 | 78 (95.12%) | 74 (86.05%) | ||
| 1 | 4 (4.88%) | 12 (13.95%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
Notes:
First visit P < 0.05;
second visit P < 0.05;
third visit P < 0.05;
fourth visit P < 0.05.
Abbreviation: N, number.
Table 7.
Rates of patients reporting eye irritation
| Scores | Treatment group | Control group | Chi-square | P |
|---|---|---|---|---|
| First visit | ||||
| N (missing) | 108 (1) | 111 (0) | 1.873 | 0.1711a |
| 0 | 83 (76.85%) | 79 (71.17%) | ||
| 1 | 23 (21.30%) | 27 (24.32%) | ||
| 2 | 2 (1.85%) | 5 (4.50%) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Second visit | ||||
| N (missing) | 104 (5) | 105 (6) | 5.4447 | 0.0196b |
| 0 | 89 (85.58%) | 78 (74.29%) | ||
| 1 | 15 (14.42%) | 27 (25.71%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Third visit | ||||
| N (missing) | 98 (11) | 103 (8) | 0.6324 | 0.4265c |
| 0 | 84 (85.71%) | 85 (82.52%) | ||
| 1 | 14 (14.29%) | 18 (17.48%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
| Fourth visit | ||||
| N (missing) | 82 (27) | 86 (25) | 0.0686 | 0.7934d |
| 0 | 71 (86.59%) | 74 (86.05%) | ||
| 1 | 11 (13.41%) | 12 (13.95%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
Notes:
First visit P > 0.05;
second visit P < 0.05;
third visit P > 0.05;
fourth visit P > 0.05.
Abbreviation: N, number.
Table 8.
Rates of patients reporting eye itching
| Scores | Treatment group | Control group | Chi-square | P |
|---|---|---|---|---|
| First visit | ||||
| N (missing) | 108 (1) | 111 (0) | 1.8029 | 0.1794a |
| 0 | 95 (87.96%) | 91 (81.98%) | ||
| 1 | 12 (11.11%) | 18 (16.22%) | ||
| 2 | 1 (0.93%) | 2 (1.80%) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| Second visit | ||||
| N (missing) | 104 (5) | 105 (6) | 0.4777 | 0.4895b |
| 0 | 93 (89.42%) | 92 (87.62%) | ||
| 1 | 11 (10.58%) | 12 (11.43%) | ||
| 2 | 0 (0.00) | 1 (0.95) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| Third visit | ||||
| N (missing) | 98 (11) | 103 (8) | 6.4948 | 0.0108c |
| 0 | 94 (95.92%) | 89 (86.41%) | ||
| 1 | 4 (4.08%) | 14 (13.59%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| Fourth visit | ||||
| N (missing) | 82 (27) | 86 (25) | 5.0726 | 0.0243d |
| 0 | 81 (98.78%) | 79 (91.86%) | ||
| 1 | 1 (1.22%) | 7 (8.14%) | ||
| 2 | 0 (0.00) | 0 (0.00) | ||
| 3 | 0 (0.00) | 0 (0.00) | ||
| 4 | 0 (0.00) | 0 (0.00) | ||
Notes:
First visit P > 0.05;
second visit P > 0.05;
third visit P < 0.05;
fourth visit P < 0.05.
Abbreviation: N, number.
Discussion
HSK is commonly caused by HSV 1. The virus can lurk on Gasser’s ganglion, and can repeatedly become active under fever, injury, excessive ultraviolet, hormone abuse, immune hypofunction during menstrual period, surgery, and emotional fluctuations.9 It is reported that HSV 1 can also repeatedly lurk on the cornea and become active under immune hypofunction,10 causing neurotrophic ulcers in the cornea, corneal opacity, secondary glaucoma, inconvertible damage to vision, and even blindness.11,12 Thus, antiviral therapy is of great importance to the prevention and treatment of HSK. The 0.15% GCV ophthalmic gel has been used for the treatment of HSK since 1996, and has been widely spread in nearly 30 countries. It was approved by the Food and Drug Administration in the US in 2009.13,14 The effectiveness of 0.15% GCV ophthalmic gel for the prevention and treatment of HSK has already been proven.15–17 Based on the balanced distribution of the baseline data of the two groups of subjects, the two groups were comparable. This study demonstrated that 0.15% GCV in situ ophthalmic gel was not inferior to 0.15% GCV ophthalmic gel. The PP dataset indicated that the 0.15% GCV in situ ophthalmic gel and the 0.15% GCV ophthalmic gel both showed significant effectiveness, with evident decreases in TSI in both groups, although there was no significant difference between the two groups. After the calibration of center effect, the total clinical effectiveness was 95.10% for the treatment group and 93.00% for the control group. The noninferiority test indicated that the tested drug was not inferior to the control drug. All these results indicated that the 0.15% GCV in situ ophthalmic gel was effective for the treatment of HSK.
In addition, indicators of safety showed that the tested drug had no negative influence on the patients’ vision. The rates of AEs were 1.83% for the treatment group and 0% for the control group, and there were no serious AEs reported, indicating the safety of both the tested drug and the control drug. Patients who administered the gel sometimes experienced discomfort, blurred vision, or difficulty of dividing doses because of its high viscosity. It was reported that 29.1% of the patients taking the GCV ophthalmic gel would suffer from moderate or significant blurred vision;18 However, the 0.15% GCV in situ gel is administered in a solution state, it then goes through a phase transition due to its different pH values, and then it forms into a semisolid with nonchemical cross-linking. Compared with traditional eye drops, the gel can prolong retention time and then increase drug concentration at the administration site.19–22 It can also overcome the disadvantages of the gel to a great extent (discomfort, difficulty to divide doses, and blurred vision). Rheology testing proved that the pH sensitivity of the in situ gel is of a pseudoplastic characteristic, which is appropriate for ophthalmic topical application.23 The gel is of lower viscosity than oculentum,24 but it still exhibits poor spreadability and inaccurate dose division. In this study, the rates of patients who did not report blurred vision in the treatment group significantly exceeded that of the control group during all of the visits. The maximum rate of patients reporting blurred vision was 27.03% in the control group, while that in the treatment group was only 12.96%. During the second visit, the rates of patients reporting slight eye irritation were lower in the treatment group. The treatment group also reported lower rates of eye itching during the third and fourth visits. All these results indicated that the 0.15% GCV in situ ophthalmic gel is a promising alternative to the GCV ophthalmic gel for the treatment of HSK.
Conclusion
The 0.15% GCV in situ ophthalmic gel is significantly effective and safe for the treatment of HSK, and it is not inferior to the 0.15% GCV ophthalmic gel. The 0.15% GCV in situ ophthalmic gel also presented superior ocular tolerance, and it can offer more comfort than the 0.15% GCV ophthalmic gel.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. The authors had no financial interests in this study.
References
- 1.Bruno B, Gooley T, Hackman RC, Davis C, Corey L, Boeckh M. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9(5):341–352. doi: 10.1016/s1083-8791(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 2.Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ. The selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Reproduced from Proc Natl Acad. Sci U S A. 1977;74:5716–5720. Rev Med Virol. 1999;9(3:):147–152. doi: 10.1002/(sici)1099-1654(199907/09)9:3<147::aid-rmv255>3.0.co;2-p. discussion 152–153. [DOI] [PubMed] [Google Scholar]
- 3.Shiota H, Naito T, Mimura Y. Anti-herpes simplex virus (HSV) effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG) in rabbit cornea. Curr Eye Res. 1987;6(1):241–245. doi: 10.3109/02713688709020098. [DOI] [PubMed] [Google Scholar]
- 4.Castela N, Vermerie N, Chast F, et al. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: intraocular penetration and efficacy. J Ocul Pharmacol. 1994;10(2):439–451. doi: 10.1089/jop.1994.10.439. [DOI] [PubMed] [Google Scholar]
- 5.Tirucherai GS, Dias C, Mitra AK. Corneal permeation of ganciclovir: mechanism of ganciclovir permeation enhancement by acyl ester prodrug design. J Ocul Pharmacol Ther. 2002;18(6):535–548. doi: 10.1089/108076802321021081. [DOI] [PubMed] [Google Scholar]
- 6.Castela N, Vermerie N, Chast F, et al. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: intraocular penetration and efficacy. J Ocul Pharmacol. 1994;10(2):439–451. doi: 10.1089/jop.1994.10.439. [DOI] [PubMed] [Google Scholar]
- 7.Fresta M, Fontana G, Bucolo C, et al. Ocular tolerability and in vivo bioavailability of poly(ethylene glycol) (PEG)-coated polyethyl-2-cyanoacrylate nanosphere-encapsulated acyclovir. J Pharm Sci. 2001;90(3):288–297. doi: 10.1002/1520-6017(200103)90:3<288::aid-jps4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CG, Zhu YP, Frier M, Rao LS, Gilchrist P, Perkins AC. Ocular contact time of a carbomer gel (GelTears) in humans. Br J Ophthalmol. 1998;82(10):1131–1134. doi: 10.1136/bjo.82.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elftman MD, Hunzeker JT, Mellinger JC, Bonneau RH, Norbury CC, Truckenmiller ME. Stress-induced glucocorticoids at the earliest stage of herpes simplex virus-1 infection suppress subsequent antiviral immunity, implicating impaired dendritic cell function. J Immunol. 2010;184(4):1867–1875. doi: 10.4049/jimmunol.0902469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabbara KF. Treatment of herpetic keratitis. Ophthalmology. 2005;112(9):1640. doi: 10.1016/j.ophtha.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Green LK, Pavan-Langston D. Herpes simplex ocular inflammatory disease. Int Ophthalmol Clin. 2006;46(2):27–37. doi: 10.1097/00004397-200604620-00005. [DOI] [PubMed] [Google Scholar]
- 12.Tullo A. Pathogenesis and management of herpes simplex virus keratitis. Eye (Lond) 2003;17(8):919–922. doi: 10.1038/sj.eye.6700564. [DOI] [PubMed] [Google Scholar]
- 13.Colin J. Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes. Clin Ophthalmol. 2007;1(4):441–453. [PMC free article] [PubMed] [Google Scholar]
- 14.Zirgan®(ganciclovir) [package insert] Rochester, NY: Bausch and Lomb, Inc; 2009. [Google Scholar]
- 15.Kaufman HE, Haw WH. Ganciclovir ophthalmic gel 0.15%: safety and efficacy of a new treatment for herpes simplex keratitis. Curr Eye Res. 2012;37(7):654–660. doi: 10.3109/02713683.2012.692846. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman HE. Ganciclovir: a promising topical antiviral gel for herpetic keratitis. Expert Rev Ophthalmol. 2009;4(4):367–375. [Google Scholar]
- 17.Croxtall JD. Ganciclovir ophthalmic gel 0.15%: in acute herpetic keratitis (dendritic ulcers) Drugs. 2011;71(5):603–610. doi: 10.2165/11207240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Hoh HB, Hurley C, Claoue C, et al. Randomised trial of ganciclovir and acyclovir in the treatment of herpes simplex dendritic keratitis: a multicentre study. Br J Ophthalmol. 1996;80(2):140–143. doi: 10.1136/bjo.80.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu J, Feng X, Yuan H, et al. Study of ocular pharmacokinetics of in situ gel system for S(−)-satropane evaluated by microdialysis. J Pharm Biomed Anal. 2008;48(3):840–843. doi: 10.1016/j.jpba.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Mundada AS, Avari JG. In situ gelling polymers in ocular drug delivery systems: a review. Crit Rev Ther Drug Carrier Syst. 2009;26(1):85–118. doi: 10.1615/critrevtherdrugcarriersyst.v26.i1.30. [DOI] [PubMed] [Google Scholar]
- 21.Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186–193. doi: 10.1016/j.ejpb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Development of dorzolamide hydrochloride in situ gel nanoemulsion for ocular delivery. Drug Dev Ind Pharm. 2010;36(11):1330–1339. doi: 10.3109/03639041003801885. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S, Himmelstein KJ. Modification of in situ gelling behavior of carbopol solutions by hydroxypropyl methylcellulose. J Pharm Sci. 1995;84(3):344–348. doi: 10.1002/jps.2600840315. [DOI] [PubMed] [Google Scholar]
- 24.Edsman K, Carlfors J, Harju K. Rheological evaluation and ocular contact time of some carbomer gels for ophthalmic use. Int J Pharm. 1996;137(2):233–241. [Google Scholar]