Abstract
Introduction
Combination antiretroviral therapy has enabled HIV infected persons to reach older ages in high numbers. Hepatic and renal changes that normally occur with advancing age occur earlier and with higher incidence in HIV-infected individuals. A limited number of prospective controlled studies have demonstrated small reductions (17% to 41%) in lopinavir, atazanavir, and lamivudine clearance in older versus younger adults. A much larger number of retrospective studies in adults (age range ~20 to 60 years), including all antiretroviral drugs, have evaluated age as a covariate for pharmacokinetics. Most studies did not detect substantial associations between drug exposures and age.
Areas Covered
This review summarizes antiretroviral drug pharmacokinetics in older persons. The authors review articles from PubMed (search terms: elderly, antiretroviral, pharmacokinetics) in addition to the bibliographies of those selected.
Expert Opinion
The evidence to date does not support major pharmacokinetic changes in adults between ~20 and 60 years of age. However, additional prospective, well-controlled studies are needed in more persons > 60 years, including those with frailty and comorbidities, with assessment of unbound drug clearance, and incorporation of adherence, pharmacogenetics, and concomitant medications. Until then, guidelines for drug-drug interactions and dosing in renal and hepatic impairment should be followed in older HIV infected individuals.
Keywords: antiretroviral therapy, clinical pharmacology, elderly, HIV, pharmacokinetics
1. Introduction
As a result of the effectiveness of antiretroviral therapy, HIV infected persons are reaching older ages in high numbers.[*1, 2] In the next several years, the average age for HIV infected persons will surpass 50 years, ushering in a new era of HIV management, heavily influenced by considerations for older persons.[3]
Medication use in older persons is complicated by end-organ dysfunction, slowed drug elimination, and polypharmacy of comorbidities with an elevated risk of drug-drug interactions, all contributing to unpredictable drug responses in older persons.[4] The onset of end-organ dysfunction and comorbidities in older HIV infected persons is earlier than in those without HIV infection, by approximately 10 years.[*5, *6] Thus, these general end-organ deficits and concerns are of particular importance among those aging with HIV infection. Prompted by these general concerns, the FDA has designated adults 65 or older as a unique patient population, signifying the need for informed drug use decisions in this population.[7] Understanding pharmacokinetic differences in older persons underlies the basis to make informed treatment decisions. When this information is not available, the following standard statement is required for the FDA-approved product label, “Clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.” This standard statement (or similar wording) occupies all antiretroviral drug labels, because pharmacokinetic and pharmacodynamics information in older adults is lacking for nearly all antiretroviral drugs. Therefore, clinicians who treat older persons with antiretroviral therapy have little guidance in dose adjustment, drug interactions, or safe monitoring.
This review will discuss pharmacokinetic considerations for antiretroviral drugs in older patients and examine the published prospective and retrospective studies evaluating the association between older age and antiretroviral drug pharmacokinetics. The PubMed database was searched with the following terms: “elderly, HIV, antiretroviral, pharmacokinetics”. Titles and abstracts were evaluated for pharmacokinetic studies in adults that included age in the analysis. Articles were selected if age was examined as a covariate in pharmacokinetic studies. Additionally, articles were selected from the bibliographies of these studies, and drug package inserts were evaluated for additional pharmacokinetic information in older patients.
2. Aging with HIV infection and antiretroviral drug pharmacokinetics
2.1 Epidemiology of HIV and aging
Antiretroviral therapy has enabled HIV infected people to live life spans that approach the general population.[8] Additionally, 10% to 20% of new HIV infections are in those over 50 years old.[2, 3] The Centers for Disease Control and Prevention have designated ≥ 50 years as “older” for HIV infected persons.[*9] The ≥ 50 years distinction differs from HIV-uninfected populations, where “older” is usually defined as ≥ 65 years, and the distinction does not appear to be based upon well-established physiological definitions.[*9, 10] At the end of 2009, approximately 40,300 HIV infected persons in the United States were 65 or older, and 155,700 were 55–64 demonstrating that further aging definitions will be needed in the near future.[3] Aging is also relevant for sub-Saharan Africa where 3 million persons with HIV-infection are 50 years or older.[11, 12] These numbers are expected to increase steadily in the ensuing years, as the age demographic continues to shift.[11, 13]
More important than chronological age is the presence of co-morbid conditions, and declines in physiological function with older age, including renal and hepatic function.[14] Multiple studies suggest that significant age-related co-morbidities appear as much as 10 years earlier compared with HIV-negative persons.[*5, 15] Earlier appearance of comorbidities in HIV infected individuals supports an earlier “older” age designation (i.e. 50 versus 65 years). The most common co-morbidities include hypertension, diabetes, cardiovascular disease, cancer, osteoporosis, frailty, cognitive decline, and hepatic and renal dysfunction.[*1, 16] The reasons for the earlier onset of these conditions are likely multifactorial, related in part to chronic immune activation from HIV and other chronic viral infections, life-style habits (smoking, alcohol, recreational drugs), and adverse effects from antiretroviral drugs.[2, 17] Chronic immune activation is also associated with frailty, which is a condition of vulnerability in older persons, often associated with a decline in muscle strength and depletion of end organ functional reserve.[18] This translates into heightened sensitivity to physiological stressors, which could include drug therapy, drug-drug interactions, or pharmacogenetic sensitivity to medications. Studies using a frailty-related phenotype have reported early occurrence and increased incidence of frailty in HIV infected individuals.[*6, 15] Frailty has been associated with pharmacokinetic changes and higher intra- and inter-patient pharmacokinetic variability.[14]
2.2 General pharmacology considerations for antiretroviral drugs
Twenty-five antiretroviral drugs from classes with six different mechanisms of action are currently available for clinical use.[*19] The classes and individual drugs exhibit wide variation in disposition and routes of elimination. Nucleoside analog reverse transcriptase inhibitors (NRTIs) are generally eliminated unchanged in urine, and require phosphorylation in cells, by cellular kinases and phosphotransferases, to elicit pharmacologic effect. Other drug classes undergo gut and hepatic metabolism. Cytochrome P450 3A plays the largest part in protease inhibitor metabolism, whereas 3A, 2B6, 2C9 and 2C19 are involved in the metabolism of non-nucleoside reverse transcriptase inhibitors (NNRTIs).[*19] The integrase inhibitors raltegravir and dolutegravir, which was recently submitted for regulatory approval, are predominantly metabolized by uridine 5′-diphospho-glucuronosyltransferase (UGT)-1A1. Antiretroviral drugs metabolized enterically and hepatically typically exhibit high protein binding to albumin and/or alpha-1 acid glycoprotein. Some antiretroviral drugs require an acidic environment for optimal dissolution and absorption (e.g. rilpivirine, atazanavir), and several antiretroviral drugs require special instructions for dosing relative to meals to optimize absorption. Numerous transporters contribute to the disposition and handling of all antiretroviral drugs in all classes, but this knowledge-base is still evolving. The proteins, enzymes, and transporters impacting antiretroviral drug pharmacokinetics, as well as absorption issues and dose adjustment requirements for hepatic or renal dysfunction are listed in Table 1 [Data from product information and refs [*19–22]]. Physiologic changes associated with aging or concomitant medications that impact any of these systems could alter antiretroviral drug pharmacokinetics in older patients.
Table 1.
General pharmacologic considerations for antiretroviral drugs [Data from product information and [19–22]]
| Antiretroviral drug class (agents) | Absorption considerationsa | % unchanged in urine | Metabolism Routes (as a class) | Renal/hepatic dose adjustments?b | Transporter proteins substrates (as a class) | Plasma protein bindingc |
|---|---|---|---|---|---|---|
| Pharmacokinetic enhancer (cobicistat) | Noned | 8.2% | CYP3A, CYP2D6 |
Renal: Yesd Hepatic: No |
pending | 97.5% |
| CCR5 inhibitor (maraviroc) | None | 8% | CYP3A4 |
Renal: Yes Hepatic: No |
ABCB1, OATP1B1 | 76% |
| Fusion inhibitore (enfuvirtide) | N/A | N/A | N/A |
Renal: No Hepatic: No |
N/A | 92% (predominantly albumin) |
| Integrase inhibitors (raltegravir) (elvitegravir) |
Raltegravir: None Elivtegravir: ↑ with food |
6.7 to 9% | UGT1A1, CYP3A4 |
Renal: Yesd Hepatic: No |
ABCB1, OAT1 | 83% to >98% |
| NNRTI (efavirenz) (delavirdine) (etravirine) (nevirapine) (rilpivirine) |
Efavirenz: ↑ with foodf Delavirdine: None Etravirine: ↑ with food Nevirapine: None Rilpivirine: ↑ with food, ↓ with antacid |
< 5% | CYP2B6, CYP3A, CYP2C9, CYP2C19, UGT2B7 |
Efavirenz: Renal: No Hepatic: Yes Delavirdine: Renal: N/E Hepatic: N/E Etravirine: Renal: No Hepatic: No Nevirapine: Renal: No (if CrCL >20 mL/min) Hepatic: Yes Rilpivirine: Renal: No Hepatic: No |
ABCB1, ABCG2, ABCC1, ABCC2, ABCC10 |
Efavirenz: 99.5–99.75% (mostly albumin) Delavirdine: 98% (mostly albumin) Etravirine: 99.6 % albumin 97.66–99.02% AAG Nevirapine: 60% Rilpivirine: 99.7% |
| NRTI (abacavir) (zidovudine) (tenofovir) (emtricitabine) (lamivudine) (didanosine) (stavudine) |
Abacavir: None Zidovudine: None Tenofovir: None Emtricitabine: None Lamivudine: None Didanosine: ↓ with food Stavudine: None |
Abacavir: 1.2% Zidovudine: 29% Tenofovir: 75% Emtricitabine: 86% Lamivudine: 71% Didanosine: 42% Stavudine: 74% |
UGT2B7 ADH |
Abacavir: Renal: No Hepatic: Yes Zidovudine: Renal: Yes Hepatic: Insufficient data Tenofovir: Renal: Yes Hepatic: No Emtricitabine: Renal: Yes Hepatic: No Lamivudine: Renal: Yes Hepatic: No Didanosine: Renal: Yes Hepatic: No Stavudine: Renal: Yes Hepatic: No |
ABCB1, ABCC1, ABCC2, ABCC4, ABCC10, ABCG2, OCT1-3, OAT1-3 |
Abacavir: 50% Zidovudine: <38% Tenofovir: <0.7% Emtricitabine: <4% Lamivudine: <36% Didanosine: <5% Stavudine: Negligible |
| Protease inhibitors (atazanavir) (indinavir) (darunavir) (tipranavir) (lopinavir) (saquinavir) (fosamprenavir) (ritonavir) (nelfinavir) |
Atazanavir: ↑ with food, ↓ with antacid Indinavir: ↓ with food Darunavir: ↑ with food Tipranavir: None Lopinavir: Formulation dependentg Saquinavir: ↑ with food Fosamprenavir: None Ritonavir: ↓ with foodf Nelfinavir: ↑ with food |
Atazanavir: 7% Indinavir: 19% Darunavir: 8% Tipranavir: 0.5% Lopinavir: 2% Saquinavir: 1% Fosamprena vir: 1% Ritonavir: 3.5% Nelfinavir: 1.5% |
CYP3A, CYP2C19 |
Atazanavir: Renal: No Hepatic: Yes Indinavir: Renal: No Hepatic: Yes Darunavir: Renal: No Hepatic: No Tipranavir: Renal: No Hepatic: Yes Lopinavir: Renal: No Hepatic: No Saquinavir: Renal: No Hepatic: No Fosamprenavir: Renal: No Hepatic: Yes Ritonavir: Renal: No Hepatic: No Nelfinavir: Renal: No Hepatic: Yes |
ABCB1, ABCC1, ABCC2, OATP1A2, OATP1A3, OATP1B1 |
Atazanavir: 86% albumin 89% AAG Indinavir: 60% Darunavir: 95% (predominantly AAG) Tipranavir: >99.9% Lopinavir: 98.5% (predominantly AAG) Saquinavir: 98% Fos(amprena vir): 90% Ritonavir: 98.5% Nelfinavir: >98% |
N/A=not applicable; N/E=not evaluated; AAG=alpha-1-acid glycoprotein; ADH=alcohol dehydrogenase; CYP=cytochrome P450; ABC=ATP binding cassette transporter; OATP=organic anion-transporting polypeptide; OAT= organic anion-transporter; OCT=organic cation-transporter; other letters/numbers refer to specific isozymes.
The effect of food on absorption was not indicated unless the impact was significant enough to require specific administration recommendations with respect to food. This same approach was applied to concomitant antacid use.
Most ARV drugs have not been evaluated in patients with severe hepatic impairment. “No” refers to patients with mild to moderate (Child Pugh A or B) hepatic impairment. “Yes” indicates that there may be dosing adjustments or that use in moderate to severe (Child Pugh B or C) is not recommended
Where specific distributions in protein binding for albumin and AAG were available, these were indicated. Where not available, the % shown indicates the overall plasma protein binding.
Cobicistat and elvitegravir are co-formulated with tenofovir and emtricitabine, which require dose adjustments with renal dysfunction. Due to increased absorption of elvitegravir with food, Stribild® should be taken with food
Enfuvirtide is cleared via protein catabolism
Efavirenz is recommended on an empty stomach and ritonavir with a meal to reduce adverse effects.
The absorption of the tablet formulation is not significantly altered by food, whereas absorption of the solution formulation is increased in the presence of food
2.3 Unique pharmacological characteristics for antiretroviral drugs
Antiretroviral drugs are always used in combination for optimal effectiveness, and recommended regimens consist of three active agents from at least two drug classes.[*19] Combination antiretroviral therapy increases the risk of drug-drug interactions. The protease inhibitors and elvitegravir (the newest integrase inhibitor) exhibit poor bioavailability and/or rapid systemic clearance predominantly through CYP3A, necessitating pharmacokinetic enhancing with ritonavir or cobicistat, CYP3A inhibitors. This raises the potential for drug-drug interaction with other therapies used in older patients. In the Swiss cohort study, older HIV infected persons (≥ 50 years) had 1.45-fold more potential drug-drug interactions compared with younger HIV infected persons (< 50 years), most notably for CNS and cardiovascular drugs.[23] Some of the most powerful CYP3A-mediated drug interactions are between antiretroviral drugs and non-antiretroviral drugs, such as a 30-fold elevation in the area under the concentration time curve (AUC) observed for simvastatin when combined with ritonavir-saquinavir and a 49-fold elevation in AUC observed for vardenafil when combined with ritonavir.[24, 25] The general consensus is that older age does not impact the magnitude of drug inhibition interactions, but this has not been rigorously examined for antiretroviral drug interactions.[*26] One study evaluated the effect of chronic viral hepatitis (reduced hepatic reserve) on the magnitude of ritonavir inhibition of midazolam clearance as a surrogate for CYP3A activity. The subjects with chronic viral hepatitis had no difference in CYP3A activity compared with normal volunteers, but when receiving ritonavir, those with chronic viral hepatitis had half the CYP3A activity versus normal volunteers.[27] Thus, the inhibition effect of ritonavir was exaggerated when hepatic reserve was compromised by presence of chronic viral hepatitis. An analogous effect is possible with reduced hepatic reserve in older persons such as those with frailty phenotype.
Additional drug interaction potential arises from metabolic induction through activation of nuclear receptors such as pregnane X receptor (PXR) and constitutive androstane receptor (CAR) by protease inhibitors including ritonavir (inducers of CYP3A, 2B6 and UGT) and NNRTIs including efavirenz, etravirine, and nevirapine (inducers of CYP3A and/or 2B6).[28, 29] Some studies suggest that metabolic induction is blunted in older adults, although this finding is inconsistent.[*26, 30] One analysis of efavirenz autoinduction in 129 HIV infected persons from Tanzania, aged 39.6 ± 9.1 years, found no association between age and efavirenz autoinduction (efavirenz/M-8 metabolite ratio). [31] More information is needed for the effect of aging on antiretroviral drug induction.
Antiretroviral drugs also exhibit well-established pharmacokinetic-pharmacogenomic relationships that could manifest differently in older adults. For instance, in younger adults, CYP3A5 expressor-status (defined as those with at least one *1 allele versus only *3, *6, or *7 alleles) increases protease inhibitor oral clearance by approximately 30% in the absence of ritonavir boosting.[32–34] This CYP3A5 effect on atazanavir was retained in one study with ritonavir-boosting, but not in another study.[32, 35] CYP2B6 polymorphisms (e.g. 516 G>T and 983T>C) are associated with a significant loss of function and 3-fold elevations in efavirenz AUC.[36, 37] The bilirubin conjugating enzyme UGT1A1 metabolizes raltegravir and is inhibited by atazanavir.[38, 39] Homozygous UGT1A1*28 was associated with 40% higher raltegravir AUC, and several-fold greater bilirubin increases during atazanavir therapy.[38, 39] An evolving area in pharmacogenomics is the effect of polymorphisms in certain transport proteins in the gut, liver, and kidneys, such as ABCB1, ABCC2, ABCC4, SLCO1B1, etc, which may also impact clearance and distribution of antiretroviral drugs.[*40] Most studies of non-antiretroviral drugs suggest that pharmacogenetic changes are preserved in the elderly.[14, *26] This suggests that aging decrements in hepatic or renal function may add to decrements from pharmacogenetics, resulting in additive effects on clearance for antiretroviral drugs. However, this has not been adequately studied.
3. Pharmacokinetic-relevant physiological changes with age
Physiological declines associated with older age impact pharmacokinetics in multiple ways. A number of excellent reviews are available that summarize these aging-pharmacokinetic relationships.[*9, 14, *26, *41–43] Table 2 presents the general findings from these reviews in terms of aging effects on absorption, distribution, metabolism, and excretion. The potential influence on antiretroviral drugs, based on the pharmacology described in Table 1, is also provided.
Table 2.
Potential Impact of Age Related Changes on Pharmacokinetics
| PK Parameter | Age Related Change affected PK parameter | PK Impact | Predicted overall PK effect | Examples of potential HIV drugs affected |
|---|---|---|---|---|
| Absorption (ka, F) | ↑ gastric pH ↓ gastric emptying ↓ splanchnic blood flow ↓ intestinal CYP3A4 ↓ intestinal P-gp |
↓ F ↔ F, ↓ Ka ↔ F, ↓ Ka ↑ F ↑ F |
↓ F or ↑ F ↓ Ka |
Atazanavir Rilpivirine Other PIs Maraviroc |
| Distribution (Vd) | ↓ albumin ↑ α-1-acid-glycoprotein ↑ body fat composition ↓ lean muscle and total body water ↓ transport protein activity |
↑ Vd ↓ Vd ↑ Vda ↑ Vd |
↑ Vd | NNRTI PIs Maraviroc |
| Metabolism (CL) | ↓ albumin ↑ α-1-acid-glycoprotein ↓ liver mass (↓ Vmax) ↓ hepatic blood flow |
↑ CL/F ↓ CL/F ↓ CL/F ↔ CL/F |
↓ CL/F | PIs NNRTIs Maraviroc InSTI |
| Excretion (CL) | ↓ renal function ↓ transport processes |
↓ CL/F ↓ CL/F |
↓ CL/F | NRTI |
Ka = absorption rate constant, F = bioavailability, Vd = volume of distribution, CL/F = apparent oral clearance, PI = protease inhibitors, InSTI = integrase strand transfer inhibitor
An increase in Vd would be expected for lipophilic drugs; lipophilicity is assumed for hepatically metabolized medications.
Aging is associated with gastric changes such as increased pH, delayed gastric emptying time, decreased splanchnic blood flow, decreased gastrointestinal motility, and decreased absorption surface. Additionally, potential reduced intestinal enzymes and transport proteins, such as CYP3A4 and ABCB1 (P-gp) would be important for antiretroviral drugs.[*9, 44] Such changes could result in slowed absorption rate and either reduced or enhanced bioavailability for antiretroviral drugs. Increased gastric pH may decrease absorption of antiretroviral drugs requiring acid for dissolution such as atazanavir and rilpivirine.[45] Conversely, lower CYP3A and Pgp content or function would theoretically increase bioavailability for substrates such as protease inhibitors, maraviroc, and some NNRTIs. Finally, as food intake changes in older persons (usually declining), dosing recommendations relative to meals could be affected.[46]
Aging is also associated with increased adiposity, lower lean body mass and total body water, decreased albumin, increased alpha-1-acid-glycoprotein, and potentially reduced drug transporter function, all of which can impact volume of distribution. [42, 44] Volume of distribution (Vd) determines loading doses (not relevant for antiretroviral drugs), half-life, and the shape of the concentration-time profile (peak and trough). The shape of the concentration time curve is important for antiretroviral drugs because target concentrations are typically troughs.[*19] Antiretroviral drugs, except NRTIs, have lipophilic characteristics, suggesting an increase in adipose tissue may increase Vd. In general, drugs with protein binding greater than 70% are considered sensitive to changes in protein binding (see Table 1). As albumin levels typically decline with age, whereas alpha1-acid glycoprotein levels are unchanged or increased, it would be expected that drugs bound to albumin could exhibit an increased Vd in the elderly, while drugs bound to alpha1-acid glycoprotein may show a decrease or no change in Vd. Little is known about the effects of age on drug transporter proteins. Small studies have found higher brain penetration of P-gp (ABCB1) substrate drugs in older persons, suggesting reduced P-gp activity at the level of the blood brain barrier.[47, 48] Increased antiretroviral drug penetration into the brain could be beneficial for patients suffering from HIV-associated neurocognitive disorders, which is an important co-morbidity in older persons.[49] Conversely, higher brain penetration could also be detrimental if associated with higher CNS toxicities, which are potential concerns for efavirenz and rilpivirine.[*19] Additional studies are needed to understand the influence of age on transporters and antiretroviral drug distribution into the brain and other important tissues such as the gut, kidney, and liver.
Aging is associated with reduced hepatic function, and potentially impaired drug clearance, which is the most important pharmacokinetic parameter because it determines average steady-state concentrations. The liver is the chief drug metabolizing organ, although the intestines, kidneys, and other tissues contribute as well. Reduced liver mass, decreased hepatic blood flow, and changes in plasma protein binding impact hepatic clearance in the elderly.[*9, *26] Protein binding changes impact the availability of drug for metabolism, depending on the rate limiting process for clearance (i.e. blood flow or metabolic activity). Oral plasma clearance (CL/F) for both flow and metabolism limited drugs is rate-limited by intrinsic clearance (CLint) and the fraction unbound in plasma (Fu), that is CL/F~CLint*Fu.[*41, 50] Both CLint and Fu can change in older people, sometimes in opposite, offsetting directions (↓CLint and ↑Fu), especially for drugs bound to albumin.[*41] This could result in relatively unchanged oral clearance (CL/F ~ ↓CLint * ↑Fu) based on total drug concentrations, but slower CLint and significantly higher unbound, pharmacologically-active concentrations.[*41] Put another way, changes in protein binding in older people may mask slower CLint. This has been proposed to explain why some studies do not find age associated changes in CL/F.[*41] When studies have accounted for protein binding changes in older people, the unbound clearance (CLint) for both phase I and phase II metabolized drugs were consistently diminished in older people by 30%-50%.[*41]
Intrinsic clearance (CLint) depends upon maximal metabolic enzymatic capacity (Vmax) and drug affinity for the metabolizing enzyme (Km). CLint for first order metabolism is approximately, Vmax/Km. Vmax is proportional to liver size, which is decreased up to ~30% with older age.[*9, 51, 52] A smaller liver and reduced Vmax would decrease intrinsic clearance for oral hepatically metabolized drugs in older persons. General CYP450 clearance has been shown to diminish by up to 30% after age 65 as measured by antipyrine clearance, a broad spectrum CYP450 probe. [53] Most evidence supports similar reductions in CL/F in the older adults for CYP3A substrates, and 20% lower clearance has also been reported for buproprion, a 2B6 substrate, in older adults.[54, 55] These findings suggest that antitretroviral drugs will be similarly affected by older age given the importance of CYP3A and 2B6 on their clearance (Table 2).
Uncertainty surrounds the impact of age on phase II metabolized drugs. Multiple review papers conclude that phase II metabolism is preserved in older persons, but other studies suggest declines in metabolism. In one comprehensive review, the AUCs for drugs that undergo glucuronidation were approximately 1.4 fold higher in older versus younger persons.[56] Further, the review of protein binding effects described above showed diminished phase II clearance in older people after accounting in changes in protein binding.[*41] This suggests that age could influence the clearance of several antiretroviral drugs that undergo substantial glucuronidation (Table 2).[57]
Perhaps the most important age-related change that impacts pharmacokinetics is reduced renal function. Physiological changes include decreased glomerular filtration rate (GFR), decreased kidney mass, decreased nephron size and number, decreased glomerular surface area, decreased tubular function, and decreased renal blood flow.[42] As a consequence, both renal secretion and GFR decline with age. Age is a critical variable in the Cockroft-Gault and Modified Diet in Renal Disease (MDRD) equations used to estimate GFR for drug dosing.[58] Renal function is particularly relevant for NRTIs, such as tenofovir and emtricitabine, which undergo substantial renal elimination, requiring dose adjustments with renal dysfunction.[59, 60]
4. Data specific to PK and ARV drugs in elderly
The most important consideration for drug dosing in the elderly is to assess potential drug-drug interactions, and hepatic and renal function, and to follow dosing recommendations based on these parameters (Table 1).[*19] In the absence of dosing guidance, clinicians should refer to pharmacokinetic data to guide therapy in the elderly. To date, few well-controlled prospective studies have assessed antiretroviral pharmacokinetics in the elderly. One prospective pharmacokinetic study in older adults evaluated a single dose of atazanavir in 60 HIV-negative adults stratified by gender and age. The mean (range) ages were approximately 70 years (65 to 81) versus 27 (19 to 39), balanced by gender. The maximum concentration (Cmax) and AUC were ~17% higher in older adults (90% CI −5% to 45%), which was partially contained in the 90% CI, but statistically inconclusive.[61] The study did not quantify the effect of ritonavir-boosting on atazanavir concentrations, protein binding, or steady-state conditions, which are all important considerations. Another prospective study measured lopinavir trough concentrations in antiretroviral naïve HIV infected adults aged 18 to 30 (n=37) versus aged 45 to 79 (n=40).[62] A population pharmacokinetic model predicted that lopinavir clearance would decrease 38% from 20 years to 80 years (P=0.025), after adjusting for adherence, which was higher in older versus younger participants. Again, protein binding was not evaluated in this study. A third prospective study compared lamivudine pharmacokinetics in 6 elderly males > 65 years versus 6 younger males (note, the original manuscript was not available to the authors).[63] The AUC was 1.4-fold higher in those > 65 years, which was attributed to reduced renal clearance.
In addition to these limited prospective studies, we evaluated 73 retrospective pharmacokinetic studies in adults that evaluated age as a covariate. These studies were population pharmacokinetic analyses or observational studies including either intensive sampling for AUC measurements, or single time points (e.g. peaks, midpoint concentrations, and/or troughs). Statistical analyses typically used standard regression approaches. Population pharmacokinetic studies typically compared objective functions from nested models with age as a covariate versus a base model with no covariates. Most studies used multivariate analyses. The studies reported various age distributions; most reported median (range). For those that reported mean (standard deviation) or median (interquartile range), we converted these to mean or median (± 2 standard deviations), assuming a normal distribution. In combination, these studies evaluated over 13,000 adult patients with a median (range) age of approximately 40 (22 to 62) years. Table S1 provides a summary of findings.[20, 64–136]
4.1 Cell entry and integrase inhibitors
No associations were observed between age and plasma maraviroc (n=538) or enfuvirtide (n=534).[65, 101, 108, 128] One study found a faster distribution rate for maraviroc in older persons, but this did not impact AUC.[65] Similarly, no associations were observed for age and integrase inhibitor pharmacokinetics including raltegravir (n=250) and elvitegravir (n=534).[73, 120, 129]
4.2 NNRTI
One population pharmacokinetic study of delavirdine (n=234) showed a correlation between age and intrinsic clearance, but the magnitude was not reported and the correlation did not explain a large portion of pharmacokinetic variability.[74] Twelve studies evaluated the association between age and efavirenz pharmacokinetics and no significant age effects were observed (n=1755). Two reported an age effect in the uni-variate analysis, but age was not retained in multi-variate models.[66, 75] One large population pharmacokinetic study of etravirine (n=577) found no influence of age on etravirine pharmacokinetics, whereas another (n=190) showed 5% higher AUC per decade of age.[70, 130] Twelve studies evaluated age as a covariate with nevirapine pharmacokinetics (n=1461). Four identified a relationship between age and clearance. One found an increase in clearance of 1.56% per year after 35 years of age (35 being the median age in the study).[97] Two studies found declines in clearance with age and one reported a correlation between age with nevirapine concentrations, without reporting the magnitude or direction.[20, 103, 124] One large population pharmacokinetic study of rilpivirine (n=679) did not find an association between age and rilpivirine pharmacokinetics.[131]
4.3 PIs
Nine studies evaluated the association of lopinavir pharmacokinetics with age (n=3065), eight studies found no associations in the final models. One study (n=30) found a predicted 100% increase in lopinavir AUC from 25 to 60 years.[85] Similarly, nine studies evaluated atazanavir pharmacokinetics (both with and without ritonavir) for associations with age, and eight found no assoications (n=1138). One small study of only unboosted atazanavir (n=31) found a slower atazanavir clearance with age over 30.[132]
Twenty-three studies evaluated the association between age with pharmacokinetics for amprenavir (2), darunavir (3), indinavir (6), nelfinavir (3), ritonavir (7), and saquinavir (2). Of these, one study with saquinavir showed slower clearance with age, but the magnitude was not reported.[94] One observational pharmacokinetic study with indinavir (n=46) showed a parabolic relationship between concentrations and age (concentrations increased from 40 to 50 years, then decreased from 50 to 60 years).[92] However, a population pharmacokinetic study with the same patient cohort found no age effect.[137] Another population pharmacokinetic study of indinavir (n=171) found increased volume in older persons, with no effect on clearance.[104] All the ritonavir studies were negative for age effects except one that showed a faster elimination rate in middle aged (40 years) versus younger adults (20 years).[109] Two of three darunavir population PK studies reported slightly higher AUC with age.[130, 134] Two of three nelfinavir studies showed reduced M8 metabolite concentrations in older adults. M8 is generated by CYP2C19 and cleared by CYP3A. Finally, two studies were not included in the table because age ranges were not reported: one showed no association between age and ritonavir-boosted fosamprenavir (n=61), and the other showed stable tipranavir troughs in older adults. [138, 139]
4.4 NRTI
Of the NRTIs, abacavir is predominantly metabolized in the liver. Two population pharmacokinetic studies found no age association with abacavir pharmacokinetics (n=229).[135, 136] All other NRTIs undergo renal excretion and require dose adjustments for reduced renal function (Table 1). Age, creatinine clearance, and/or serum creatinine correlated with tenofovir or lamivudine clearance in 6 of 7 studies.[68, 79, 80, 107, 117, 126] No studies with emtricitabine were identified, but its renal elimination is similar to lamivudine and tenofovir (~70% unchanged drug in urine). No relationships were identified between age and didanosine pharmacokinetics (2 studies, n=254) or stavudine pharmacokinetics (2 studies, n=120).[81, 87, 110, 111] Four studies evaluated zidovudine (n=348), and two identified relationships with age: one reported slower clearance in younger adults less than 30 years, the other reported decreased clearance in those over 50 years.[94, 111] No relationships were reported between active NRTI-triphosphate concentrations in peripheral blood mononuclear cells with age.[68, 79]
5. Other considerations for older HIV infected individuals
In general, older HIV infected individuals have better adherence to antiretroviral drugs, however an increase in cognitive impairment with aging could present problems with adherence.[*140, 141] Virologic responses are often better in older HIV infected persons even after controlling for adherence, suggesting that different pharmacokinetics may drive better antiviral responses.[*9, 44] Despite this better virologic response, older patients tend to have blunted CD4 responses, indicating impaired immunologic reserve.[*9] For this reason, guidelines recommend that older patients over 50 years of age should begin antiretroviral therapy as soon as possible regardless of CD4 count.[*19]
Older HIV-infected patients appear to experience more toxicities with antiretroviral drugs, including hematologic, lipid, and central nervous system, which might suggest higher drug concentrations, or a lower physiological reserve.[*1, *140, 142, 143] An increased risk of drug toxicities in older persons in the general population provides the impetus for the Beers criteria for “potentially improper medications” in the elderly.[*144] These criteria provide a list of medications to avoid in older adults, including those increasing the risk of orthostatic hypotension, QT-interval prolongation, and central nervous system side effects. Among antiretroviral therapies, these same side effects are important concerns for efavirenz (CNS side effects), saquinavir (QT-interval prolongation), and maraviroc (postural hypotension with renal dysfunction). These antiretroviral medications should be used cautiously in the elderly.
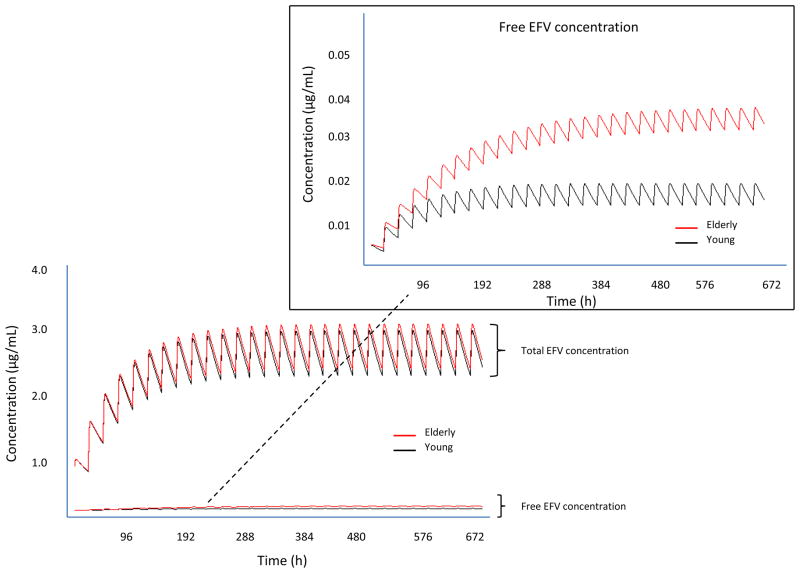
The future study of antiretroviral drugs in older persons must include more controlled prospective pharmacokinetic studies with attention to plasma protein-binding.[*41] This is particularly important for antiretroviral drugs with high binding to albumin, such as efavirenz. Although none of 12 studies reported in Table S1 identified an association between age and efavirenz concentrations, only total efavirenz concentrations were measured. To illustrate how protein binding can mask intrinsic clearance changes, we simulated concentration-time profiles for two theoretical populations, young versus older patients where intrinsic clearance was assumed to be ~50% in the older group, but binding to albumin was also decreased (fu increased by ~50%). These effects would offset the total CL/F (CL/F~↓CLint*↑fu) and result in similar total drug concentrations, but increased unbound drug concentrations, potentially leading to toxicity (Figure 1).
Figure 1.
Simulation of total and unbound (inset) efavirenz concentrations in young (light color) versus older adults (dark color) where intrinsic clearance in older individuals was assumed to be half that in younger adults, but unbound fraction was also assumed to be higher in older adults. Data for simulations were obtained from the product information.
Other antiretroviral drugs bind more avidly to alpha-1-acid-glycoprotein (Table 2), which may increase in older adults, especially in those with inflammatory conditions.[42,44] In the setting of increased binding, total clearance would decrease and total drug concentrations would increase (↓CL/F~CLint*↓fu). If CLint were decreased as well, total clearance would be reduced further, and total drug concentrations would be increased further (↓↓CL/F~↓CLint*↓fu). However, changes in total drug concentrations were not frequently observed among older adults in the studies described in Table S1, suggesting that this scenario was not playing out in the age ranges of these studies (~20 to 60 years).
6. Conclusion
Over the coming years, increasing numbers of older HIV infected people will be treated with antiretroviral medications, but there is little guidance on how to use antiretroviral therapy safely and effectively in this population. Aging is associated with numerous physiological changes that could impact antiretroviral drug pharmacokinetics. However, there is little evidence to date of clinically relevant changes in antiretroviral drug pharmacokinetics in HIV infected patients between ~ 20 and 60 years. Until more information is available in those > 60 years of age, the best course of action is to follow dosing guidelines based upon drug-drug interactions, and renal and hepatic function.
7. Expert opinion
Most pharmacokinetic data in older persons arise from retrospective observational analyses and population pharmacokinetic studies where patient ages ranged from ~20 to 60 years. These studies show that NRTIs, like other renally eliminated drugs, undergo pharmacokinetic changes as renal function declines. This is relevant for older adults, as approximately half of elderly adults have GFR < 60 mL/min per 1.73 m2.[145] The studies of hepatically cleared drugs do not support consistent, reproducible, substantial changes in pharmacokinetics in the participant age ranges of the studies (~20 to 60 years). Of note, these age ranges encompass the “older” age definition of 50 years for HIV infected individuals, suggesting that pharmacokinetics of liver metabolized antiretroviral drugs are minimally changed in HIV infected individuals at this “older” age definition. This includes drugs metabolized by CYP3A (protease inhibitors, rilpivirine), CYP2B6 (efavirenz, nevirapine), CYP2C9/2C19 (etravirine) UGT1A1 (raltegravir), and UGT2B7 (zidovudine). The most important limitations of these studies are the retrospective design, the limited number of subjects at the extremes of older age, probable exclusion of those with frailty or significant comorbidities, and the absence of protein binding measurements. Because of these limitations, these studies should not be the sole basis for decision-making when treating older HIV infected patients > 60 years of age. Additional studies are needed in more people > 60 years old, and in those with frailty and/or significant comorbidities. Future studies should also include an assessment of protein-binding, drug-drug interactions, and pharmacogenetics in older persons.
Supplementary Material
Highlights.
HIV infected individuals are reaching older ages in high numbers
Older HIV infected individuals experience frailty and co-morbidities at earlier ages and with higher incidences compared with non-HIV infected individuals.
Older age is associated with reduced renal and hepatic function, and polypharmacy, but few prospective pharmacokinetic studies have been conducted in older HIV infected individuals.
Retrospective studies do not support consistent, reproducible, substantial changes in pharmacokinetics of hepatically eliminated drugs in the age ranges of ~20 to 60 years.
Renal function should be assessed in older HIV infected individuals and dose adjustments made based upon renal function.
Additional studies are needed in more HIV infected individuals > 60 years old, and in those with frailty and/or significant comorbidities
Footnotes
Declaration of Interest
The authors are supported by a National Institutes of health grant (AI 84735).
Contributor Information
John C. Schoen, University of Colorado, Anschutz Medical Campus, Skaggs School of Pharmacy and Pharmaceutical Sciences
Kristine Mace Erlandson, University of Colorado, School of Medicine, Department of Medicine, Divisions of Infectious Diseases and Geriatric Medicine.
Peter L. Anderson, Email: peter.anderson@ucdenver.edu, University of Colorado, Anschutz Medical Campus, Skaggs School of Pharmacy and Pharmaceutical Sciences, Department of Pharmaceutical Sciences, Box C238, 12850 E. Montview Blvd., Aurora, CO 80045, O 303-724-6125, F 303-724-6135.
References
- 1*.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clinical Infectious Diseases. 2008;47:542–553. doi: 10.1086/590150. comprehensive review of HIV and aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) HIV surveillance--United States, 1981–2008. MMWR Morb Mortal Wkly Rep. 2011;60:689–693. [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Pharmacokinetics and Drug Interactions in the Elderly. Pharmacokinetics and Drug Interactions in the Elderly and Special Issues in Elderly African-American Populations: Workshop Summary. [Accessed Jan 02, 2013];1997 The National Academies Collection: Reports funded by National Institutes of Health. Available at: http://www.nap.edu/catalog/5854.html. [PubMed]
- 5*.Guaraldi G, Orlando G, Zona S, et al. Premature Age-Related Comorbidities Among HIV-Infected Persons Compared With the General Population. Clinical Infectious Diseases. 2011;53:1120–1126. doi: 10.1093/cid/cir627. Evidence for earlier co-morbidities in HIV infected individuals. [DOI] [PubMed] [Google Scholar]
- 6*.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 Infection Is Associated With an Earlier Occurrence of a Phenotype Related to Frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62:1279–1286. doi: 10.1093/gerona/62.11.1279. Evidence for earlier frailty phenotype in HIV infected individuals. [DOI] [PubMed] [Google Scholar]
- 7.Guideline for Industry, Studies in Support of Special Populations: Geriatrics; Expert Working Group (Efficacy) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH); 1994. [Accessed Jan 02, 2013]. Available at: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM129519.pdf. [Google Scholar]
- 8.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Current Opinion in Infectious Diseases. 2013;26:17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- 9*.Rhee M, Greenblatt DJ. Pharmacologic consideration for the use of antriretroviral agents in the elderly. J Clin Pharmacol. 2008;48:1212–1225. doi: 10.1177/0091270008322177. Comprehensive review of clinical pharmacology for antiretroviral drugs in older HIV infected individuals. [DOI] [PubMed] [Google Scholar]
- 10.Branas F, Berenguer J, Sanchez-Conde M, et al. The eldest of older adults living with HIV: response and adherence to highly active antiretroviral therapy. Am J Med. 2008;121:820–824. doi: 10.1016/j.amjmed.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Negin J, Cumming RG. HIV infection in older adults in sub-Saharan Africa: extrapolating prevalence from existing data. Bull World Health Organ. 2010;88:847–853. doi: 10.2471/BLT.10.076349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hontelez JA, Lurie MN, Newell ML, et al. Ageing with HIV in South Africa. AIDS. 2011;25:1665–1667. doi: 10.1097/QAD.0b013e32834982ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther VP, Wilkin AM. HIV Infection in Older Adults. Clinics in geriatric medicine. 2007;23:567–583. doi: 10.1016/j.cger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 15.Önen N, Overton E, Seyfried W, et al. Aging and HIV Infection: A Comparison Between Older HIV-Infected Persons and the General Population. HIV Clin Trials. 2010;11:100–109. doi: 10.1310/hct1102-100. [DOI] [PubMed] [Google Scholar]
- 16.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and Aging in HIV-Infected Persons: The Swiss HIV Cohort Study. Clinical Infectious Diseases. 2011;53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 17.Bhavan KP, Kampalath VN, Overton ET. The aging of the HIV epidemic. Curr HIV/AIDS Rep. 2008;5:150–158. doi: 10.1007/s11904-008-0023-3. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19*.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed, Jan 02, 2013]. Updated March 27, 2012. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Excellent source of antiretroviral dosing informations for drug interactions and renal and hepatic impairment. [Google Scholar]
- 20*.Liptrott NJ, Pushpakom S, Wyen C, et al. Association of ABCC10 polymorphisms with nevirapine plasma concentrations in the German Competence Network for HIV/AIDS. Pharmacogenetics and genomics. 2012;22:10–19. doi: 10.1097/FPC.0b013e32834dd82e. Example of emerging information on drug transporters and antiretroviral pharmacokinetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pushpakom SP, Liptrott NJ, Rodríguez-Nóvoa S, et al. Genetic Variants of ABCC10, a Novel Tenofovir Transporter, Are Associated With Kidney Tubular Dysfunction. Journal of Infectious Diseases. 2011;204:145–153. doi: 10.1093/infdis/jir215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estudante M, Morais JG, Soveral G, Benet LZ. Intestinal drug transporters: An overview. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 23.Marzolini C, Back D, Weber R, et al. Ageing with HIV: medication use and risk for potential drug-drug interactions. The Journal of antimicrobial chemotherapy. 2011;66:2107–2111. doi: 10.1093/jac/dkr248. [DOI] [PubMed] [Google Scholar]
- 24.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 25.Levitra® (vardenafil) product information. GlaxoSmithKline; Research Triangle Park, NC: 2012. [Google Scholar]
- 26*.Cusack B. Pharmacokinetics in Older Persons. Am J Geriatr Pharmacother. 2004;2:274–302. doi: 10.1016/j.amjopharm.2004.12.005. Particularly thoughtful and complete review of pharmacokinetics in older adults. [DOI] [PubMed] [Google Scholar]
- 27.Knox TA, Oleson L, von Moltke LL, et al. Ritonavir greatly impairs CYP3A activity in HIV infection with chronic viral hepatitis. J Acquir Immune Defic Syndr. 2008;49:358–368. doi: 10.1097/qai.0b013e31818c7efe. [DOI] [PubMed] [Google Scholar]
- 28.Kharasch ED, Whittington D, Ensign D, et al. Mechanism of Efavirenz Influence on Methadone Pharmacokinetics and Pharmacodynamics. Clinical pharmacology and therapeutics. 2012;91:673–684. doi: 10.1038/clpt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svärd J, Spiers JP, Mulcahy F, Hennessy M. Nuclear Receptor-Mediated Induction of CYP450 by Antiretrovirals: Functional Consequences of NR1I2 (PXR) Polymorphisms and Differential Prevalence in Whites and Sub-Saharan Africans. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2010;55:536–549. doi: 10.1097/QAI.0b013e3181f52f0c. [DOI] [PubMed] [Google Scholar]
- 30.Tang C, Lin JH, Lu AY. Metabolism-based drug-drug interactions: what determines individual variability in cytochrome P450 induction? Drug Metab Dispos. 2005;33:603–613. doi: 10.1124/dmd.104.003236. [DOI] [PubMed] [Google Scholar]
- 31.Ngaimisi E, Mugusi S, Minzi OM, et al. Long-term efavirenz autoinduction and its effect on plasma exposure in HIV patients. Clinical pharmacology and therapeutics. 2010;88:676–684. doi: 10.1038/clpt.2010.172. [DOI] [PubMed] [Google Scholar]
- 32.Anderson PL, Aquilante CL, Gardner EM, et al. Atazanavir pharmacokinetics in genetically determined CYP3A5 expressors versus non-expressors. The Journal of antimicrobial chemotherapy. 2009;64:1071–1079. doi: 10.1093/jac/dkp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Josephson F, Allqvist A, Janabi M, et al. CYP3A5 genotype has an impact on the metabolism of the HIV protease inhibitor saquinavir. Clinical pharmacology and therapeutics. 2007;81:708–712. doi: 10.1038/sj.clpt.6100117. [DOI] [PubMed] [Google Scholar]
- 34.Anderson PL, Lamba J, Aquilante CL, et al. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441–449. doi: 10.1097/01.qai.0000225013.53568.69. [DOI] [PubMed] [Google Scholar]
- 35.Savic RM, Barrail-Tran A, Duval X, et al. Effect of adherence as measured by MEMS, ritonavir boosting, and CYP3A5 genotype on atazanavir pharmacokinetics in treatment-naive HIV-infected patients. Clinical pharmacology and therapeutics. 2012;92:575–583. doi: 10.1038/clpt.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenetics and genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Wyen C, Hendra H, Vogel M, et al. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. The Journal of antimicrobial chemotherapy. 2008;61:914–918. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotger M, Taffe P, Bleiber G, et al. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. The Journal of infectious diseases. 2005;192:1381–1386. doi: 10.1086/466531. [DOI] [PubMed] [Google Scholar]
- 39.Wenning LA, Petry AS, Kost JT, et al. Pharmacokinetics of Raltegravir in Individuals With UGT1A1 Polymorphisms. Clinical pharmacology and therapeutics. 2009;85:623–627. doi: 10.1038/clpt.2009.12. [DOI] [PubMed] [Google Scholar]
- 40*.Haas D, Kuritzkes D, Ritchie M, et al. Pharmacogenomics of HIV Therapy: Summary of a Workshop Sponsored by the National Institute of Allergy and Infectious Diseases. HIV Clin Trials. 2011;12:277–285. doi: 10.1310/hct1205-277. Up to date review of well-established pharmacokinetic-pharmacogenetic relationships for antiretroviral drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Butler JM, Begg EJ. Free drug metabolic clearance in elderly people. Clinical Pharmacokinetics. 2008;47:297–321. doi: 10.2165/00003088-200847050-00002. Interesting review summarizing unbound drug clearances in older persons suggesting protein binding changes mask intrinsic clearance changes. [DOI] [PubMed] [Google Scholar]
- 42.Mayersohn M. Special Pharmacokinetic Considerations in the Elderly. In: Evans, Schentag, Jusko, editors. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring. 3. Applied Therapetuics, Inc; Vancouver, WA: 1992. [Google Scholar]
- 43.Hilmer SN. ADME-tox issues for the elderly. Expert opinion on drug metabolism & toxicology. 2008;4:1321–1331. doi: 10.1517/17425255.4.10.1321. [DOI] [PubMed] [Google Scholar]
- 44.Blanco JR, Caro AM, Perez-Cachafeiro S, et al. HIV infection and aging. AIDS Rev. 2010;12:218–230. [PubMed] [Google Scholar]
- 45.Falcon RW, Kakuda TN. Drug interactions between HIV protease inhibitors and acid-reducing agents. Clin Pharmacokinet. 2008;47:75–89. doi: 10.2165/00003088-200847020-00001. [DOI] [PubMed] [Google Scholar]
- 46.Morley JE. Undernutrition in older adults. Family Practice. 2012;29:i89–i93. doi: 10.1093/fampra/cmr054. [DOI] [PubMed] [Google Scholar]
- 47.Bauer M, Karch R, Neumann F, et al. Age dependency of cerebral P-gp function measured with (R)-[11C]verapamil and PET. European journal of clinical pharmacology. 2009;65:941–946. doi: 10.1007/s00228-009-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toornvliet R, van Berckel BN, Luurtsema G, et al. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clinical pharmacology and therapeutics. 2006;79:540–548. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8:54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowland M, Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. Wolters Kluwer Health, Lippincott, Williams and Wilkins; Baltimore MD: 1980, 1988, 1995, 2011. [Google Scholar]
- 51.Bach B, Hansen JM, Kampmann JP, et al. Disposition of Antipyrine and Phenytoin Correlated with Age and Liver Volume in Man. Clinical Pharmacokinetics. 1981;6:389–396. doi: 10.2165/00003088-198106050-00005. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez CE, Mahle DA, Gearhart JM, et al. Predicting Age-Appropriate Pharmacokinetics of Six Volatile Organic Compounds in the Rat Utilizing Physiologically Based Pharmacokinetic Modeling. Toxicological Sciences. 2007;98:43–56. doi: 10.1093/toxsci/kfm082. [DOI] [PubMed] [Google Scholar]
- 53.Sotaniemi E, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans; an analysis of 226 subjects with equal histopathic conditions. Clin Pharmacol Ther. 1997;61:331–339. doi: 10.1016/S0009-9236(97)90166-1. [DOI] [PubMed] [Google Scholar]
- 54.Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clinical Pharmacokinetics. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Sweet RA, Pollock BG, Kirshner M, et al. Pharmacokinetics of single- and multiple-dose bupropion in elderly patients with depression. Journal of clinical pharmacology. 1995;35:876–884. doi: 10.1002/j.1552-4604.1995.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 56.Dorne JL, Walton K, Renwick AG. Human variability in glucuronidation in relation to uncertainty factors for risk assessment. Food and chemical toxicology. 2001;39:1153–1173. doi: 10.1016/s0278-6915(01)00087-4. [DOI] [PubMed] [Google Scholar]
- 57.Tozzi V. Pharmacogenetics of antiretrovirals. Antiviral research. 2010;85:190–200. doi: 10.1016/j.antiviral.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Helou R. Should we continue to use the Cockcroft-Gault formula? Nephron Clinical practice. 2010;116:c172–185. doi: 10.1159/000317197. [DOI] [PubMed] [Google Scholar]
- 59.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43:595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 60.Frampton JE, Perry CM. Emtricitabine: a review of its use in the management of HIV infection. Drugs. 2005;65:1427–1448. doi: 10.2165/00003495-200565100-00008. [DOI] [PubMed] [Google Scholar]
- 61.O’mara E, Randall D, Stoltz RR, et al. BMS-232632: A Prospective Study of Age and Gender Effects on the Single-Dose Pharmacokinetics in Healthy Volunteers [Abstract 350]. 1st IAS Conference on HIV Pathogenesis and Treatment; July 7–11, 2001; Buenos Aires, Argentina. [Google Scholar]
- 62.Crawford KW, Spritzler J, Kalayjian RC, et al. Age-related changes in plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retroviruses. 2010;26:635–643. doi: 10.1089/aid.2009.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merle P, Trepo C, Zoulim F. Current management strategies for hepatitis B in the elderly. Drugs & aging. 2001;18:725–735. doi: 10.2165/00002512-200118100-00002. [DOI] [PubMed] [Google Scholar]
- 64.Mukonzo JK, Nanzigu S, Rekic D, et al. HIV/AIDS Patients Display Lower Relative Bioavailability of Efavirenz than Healthy Subjects. Clinical Pharmacokinetics. 2011;50:531–540. doi: 10.2165/11592660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.Chan PL, Weatherley B, McFadyen L. A population pharmacokinetic meta-analysis of maraviroc in healthy volunteers and asymptomatic HIV-infected subjects. British journal of clinical pharmacology. 2008;65 (Suppl 1):76–85. doi: 10.1111/j.1365-2125.2008.03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arab-Alameddine M, Di Iulio J, Buclin T, et al. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clinical pharmacology and therapeutics. 2009;85:485–494. doi: 10.1038/clpt.2008.271. [DOI] [PubMed] [Google Scholar]
- 67.Schipani A, Egan D, Dickinson L, et al. Estimation of the effect of SLCO1B1 polymorphisms on lopinavir plasma concentration in HIV-infected adults. Antivir Ther. 2012;17:861–868. doi: 10.3851/IMP2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bazzoli C, Benech H, Rey E, et al. Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients. Antimicrobial agents and chemotherapy. 2011;55:3423–3431. doi: 10.1128/AAC.01487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kityo C, Walker AS, Dickinson L, et al. Pharmacokinetics of lopinavir-ritonavir with and without nonnucleoside reverse transcriptase inhibitors in Ugandan HIV-infected adults. Antimicrobial agents and chemotherapy. 2010;54:2965–2973. doi: 10.1128/AAC.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakuda TN, Wade JR, Snoeck E, et al. Pharmacokinetics and pharmacodynamics of the non-nucleoside reverse-transcriptase inhibitor etravirine in treatment-experienced HIV-1-infected patients. Clinical pharmacology and therapeutics. 2010;88:695–703. doi: 10.1038/clpt.2010.181. [DOI] [PubMed] [Google Scholar]
- 71.Kwara A, Lartey M, Sagoe KW, et al. CYP2B6 (c. 516G→T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. British journal of clinical pharmacology. 2009;67:427–436. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schipani A, Siccardi M, D’Avolio A, et al. Population pharmacokinetic modeling of the association between 63396C->T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrobial agents and chemotherapy. 2010;54:5242–5250. doi: 10.1128/AAC.00781-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arab-Alameddine M, Fayet-Mello A, Lubomirov R, et al. Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrobial agents and chemotherapy. 2012;56:2959–2966. doi: 10.1128/AAC.05424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith PF, Dicenzo R, Forrest A, et al. Population pharmacokinetics of delavirdine and N-delavirdine in HIV-infected individuals. Clin Pharmacokinet. 2005;44:99–109. doi: 10.2165/00003088-200544010-00004. [DOI] [PubMed] [Google Scholar]
- 75.Sanchez A, Cabrera S, Santos D, et al. Population pharmacokinetic/pharmacogenetic model for optimization of efavirenz therapy in Caucasian HIV-infected patients. Antimicrobial agents and chemotherapy. 2011;55:5314–5324. doi: 10.1128/AAC.00194-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kappelhoff BS, Huitema AD, Yalvac Z, et al. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin Pharmacokinet. 2005;44:849–861. doi: 10.2165/00003088-200544080-00006. [DOI] [PubMed] [Google Scholar]
- 77.von Hentig N, Lotsch J. Cytochrome P450 3A inhibition by atazanavir and ritonavir, but not demography or drug formulation, influences saquinavir population pharmacokinetics in human immunodeficiency virus type 1-infected adults. Antimicrobial agents and chemotherapy. 2009;53:3524–3527. doi: 10.1128/AAC.00025-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez Aspiroz E, Santos Buelga D, Cabrera Figueroa S, et al. Population pharmacokinetics of lopinavir/ritonavir (Kaletra) in HIV-infected patients. Ther Drug Monit. 2011;33:573–582. doi: 10.1097/FTD.0b013e31822d578b. [DOI] [PubMed] [Google Scholar]
- 79.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrobial agents and chemotherapy. 2011;55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benaboud S, Hirt D, Launay O, et al. Pregnancy-related effects on tenofovir pharmacokinetics: a population study with 186 women. Antimicrobial agents and chemotherapy. 2012;56:857–862. doi: 10.1128/AAC.05244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panhard X, Legrand M, Taburet AM, et al. Population pharmacokinetic analysis of lamivudine, stavudine and zidovudine in controlled HIV-infected patients on HAART. European journal of clinical pharmacology. 2007;63:1019–1029. doi: 10.1007/s00228-007-0337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schipani A, Wyen C, Mahungu T, et al. Integration of population pharmacokinetics and pharmacogenetics: an aid to optimal nevirapine dose selection in HIV-infected individuals. The Journal of antimicrobial chemotherapy. 2011;66:1332–1339. doi: 10.1093/jac/dkr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lubomirov R, di Iulio J, Fayet A, et al. ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenetics and genomics. 2010;20:217–230. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 84.Cressey TR, Urien S, Hirt D, et al. Influence of body weight on achieving indinavir concentrations within its therapeutic window in HIV-infected Thai patients receiving indinavir boosted with ritonavir. Ther Drug Monit. 2011;33:25–31. doi: 10.1097/FTD.0b013e3182057f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wyen C, Fuhr U, Frank D, et al. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clinical pharmacology and therapeutics. 2008;84:75–82. doi: 10.1038/sj.clpt.6100452. [DOI] [PubMed] [Google Scholar]
- 86.Molto J, Valle M, Miranda C, et al. Once- or twice-daily dosing of nevirapine in HIV-infected adults: a population pharmacokinetics approach. The Journal of antimicrobial chemotherapy. 2008;62:784–792. doi: 10.1093/jac/dkn268. [DOI] [PubMed] [Google Scholar]
- 87.Horton CM, Dudley MN, Kaul S, et al. Population pharmacokinetics of stavudine (d4T) in patients with AIDS or advanced AIDS-related complex. Antimicrobial agents and chemotherapy. 1995;39:2309–2315. doi: 10.1128/aac.39.10.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panhard X, Goujard C, Legrand M, et al. Population pharmacokinetic analysis for nelfinavir and its metabolite M8 in virologically controlled HIV-infected patients on HAART. British journal of clinical pharmacology. 2005;60:390–403. doi: 10.1111/j.1365-2125.2005.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kappelhoff BS, Huitema AD, Sankatsing SU, et al. Population pharmacokinetics of indinavir alone and in combination with ritonavir in HIV-1-infected patients. British journal of clinical pharmacology. 2005;60:276–286. doi: 10.1111/j.1365-2125.2005.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenetics and genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stohr W, Back D, Dunn D, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13:675–685. [PubMed] [Google Scholar]
- 92.Goujard C, Legrand M, Panhard X, et al. High variability of indinavir and nelfinavir pharmacokinetics in HIV-infected patients with a sustained virological response on highly active antiretroviral therapy. Clin Pharmacokinet. 2005;44:1267–1278. doi: 10.2165/00003088-200544120-00005. [DOI] [PubMed] [Google Scholar]
- 93.Chou M, Bertrand J, Segeral O, et al. Population pharmacokinetic-pharmacogenetic study of nevirapine in HIV-infected Cambodian patients. Antimicrobial agents and chemotherapy. 2010;54:4432–4439. doi: 10.1128/AAC.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vanhove GF, Kastrissios H, Gries JM, et al. Pharmacokinetics of saquinavir, zidovudine, and zalcitabine in combination therapy. Antimicrobial agents and chemotherapy. 1997;41:2428–2432. doi: 10.1128/aac.41.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kappelhoff BS, Huitema AD, Crommentuyn KM, et al. Development and validation of a population pharmacokinetic model for ritonavir used as a booster or as an antiviral agent in HIV-1-infected patients. British journal of clinical pharmacology. 2005;59:174–182. doi: 10.1111/j.1365-2125.2004.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. British journal of clinical pharmacology. 2005;60:378–389. doi: 10.1111/j.1365-2125.2005.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elsherbiny D, Cohen K, Jansson B, et al. Population pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV− and tuberculosis-infected South African patients. European journal of clinical pharmacology. 2009;65:71–80. doi: 10.1007/s00228-008-0481-y. [DOI] [PubMed] [Google Scholar]
- 98.Gandhi M, Benet LZ, Bacchetti P, et al. Nonnucleoside reverse transcriptase inhibitor pharmacokinetics in a large unselected cohort of HIV-infected women. J Acquir Immune Defic Syndr. 2009;50:482–491. doi: 10.1097/qai.0b013e31819c3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solas C, Gagnieu MC, Ravaux I, et al. Population pharmacokinetics of atazanavir in human immunodeficiency virus-infected patients. Ther Drug Monit. 2008;30:670–673. doi: 10.1097/FTD.0b013e3181897bff. [DOI] [PubMed] [Google Scholar]
- 100.Bertrand J, Treluyer JM, Panhard X, et al. Influence of pharmacogenetics on indinavir disposition and short-term response in HIV patients initiating HAART. European journal of clinical pharmacology. 2009;65:667–678. doi: 10.1007/s00228-009-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Siccardi M, D’Avolio A, Nozza S, et al. Maraviroc is a substrate for OATP1B1 in vitro and maraviroc plasma concentrations are influenced by SLCO1B1 521 T>C polymorphism. Pharmacogenetics and genomics. 2010;20:759–765. doi: 10.1097/FPC.0b013e3283402efb. [DOI] [PubMed] [Google Scholar]
- 102.Stöhr W, Back D, Dunn D, et al. Factors influencing lopinavir and atazanavir plasma concentration. Journal of Antimicrobial Chemotherapy. 2010;65:129–137. doi: 10.1093/jac/dkp408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dailly E, Billaud E, Reliquet V, et al. No relationship between high nevirapine plasma concentration and hepatotoxicity in HIV-1-infected patients naive of antiretroviral treatment or switched from protease inhibitors. European journal of clinical pharmacology. 2004;60:343–348. doi: 10.1007/s00228-004-0769-5. [DOI] [PubMed] [Google Scholar]
- 104.Zhou XJ, Havlir DV, Richman DD, et al. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14:2869–2876. doi: 10.1097/00002030-200012220-00008. [DOI] [PubMed] [Google Scholar]
- 105.van der Leur MR, Burger DM, la Porte CJL, Koopmans PP. A Retrospective TDM Database Analysis of Interpatient Variability in the Pharmacokinetics of Lopinavir in HIV-infected Adults. Therapeutic Drug Monitoring. 2006;28:650–653. doi: 10.1097/01.ftd.0000245681.12092.d6. [DOI] [PubMed] [Google Scholar]
- 106.Gengiah TN, Holford NH, Botha JH, et al. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. European journal of clinical pharmacology. 2012;68:689–695. doi: 10.1007/s00228-011-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gagnieu MC, Barkil ME, Livrozet JM, et al. Population pharmacokinetics of tenofovir in AIDS patients. J Clin Pharmacol. 2008;48:1282–1288. doi: 10.1177/0091270008322908. [DOI] [PubMed] [Google Scholar]
- 108.Okoli C, Siccardi M, Thomas-William S, et al. Once daily maraviroc 300 mg or 150 mg in combination with ritonavir-boosted darunavir 800/100 mg. The Journal of antimicrobial chemotherapy. 2012;67:671–674. doi: 10.1093/jac/dkr493. [DOI] [PubMed] [Google Scholar]
- 109.Sale M, Sadler BM, Stein DS. Pharmacokinetic modeling and simulations of interaction of amprenavir and ritonavir. Antimicrobial agents and chemotherapy. 2002;46:746–754. doi: 10.1128/AAC.46.3.746-754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Adams JM, Shelton MJ, Hewitt RG, et al. Relationship between didanosine exposure and surrogate marker response in human immunodeficiency virus-infected outpatients. Antimicrobial agents and chemotherapy. 1998;42:821–826. doi: 10.1128/aac.42.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou XJ, Sheiner LB, D’Aquila RT, et al. Population pharmacokinetics of nevirapine, zidovudine, and didanosine in human immunodeficiency virus-infected patients. The National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group Protocol 241 Investigators. Antimicrobial agents and chemotherapy. 1999;43:121–128. doi: 10.1128/aac.43.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clinical pharmacology and therapeutics. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 113.Heil SG, van der Ende ME, Schenk PW, et al. Associations between ABCB1, CYP2A6, CYP2B6, CYP2D6, and CYP3A5 alleles in relation to efavirenz and nevirapine pharmacokinetics in HIV-infected individuals. Ther Drug Monit. 2012;34:153–159. doi: 10.1097/FTD.0b013e31824868f3. [DOI] [PubMed] [Google Scholar]
- 114.Fabbiani M, Di Giambenedetto S, Ragazzoni E, et al. Mid-dosing interval concentration of atazanavir and virological outcome in patients treated for HIV-1 infection. HIV Med. 2010;11:326–333. doi: 10.1111/j.1468-1293.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 115.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrobial agents and chemotherapy. 2006;50:3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burger D, van der Heiden I, la Porte C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. British journal of clinical pharmacology. 2006;61:148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jullien V, Treluyer JM, Rey E, et al. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrobial agents and chemotherapy. 2005;49:3361–3366. doi: 10.1128/AAC.49.8.3361-3366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pfister M, Labbe L, Lu JF, et al. Effect of coadministration of nelfinavir, indinavir, and saquinavir on the pharmacokinetics of amprenavir. Clinical pharmacology and therapeutics. 2002;72:133–141. doi: 10.1067/mcp.2002.126183. [DOI] [PubMed] [Google Scholar]
- 119.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics of indinavir in patients infected with human immunodeficiency virus. Antimicrobial agents and chemotherapy. 2004;48:3226–3232. doi: 10.1128/AAC.48.9.3226-3232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siccardi M, D’Avolio A, Rodriguez-Novoa S, et al. Intrapatient and interpatient pharmacokinetic variability of raltegravir in the clinical setting. Ther Drug Monit. 2012;34:232–235. doi: 10.1097/FTD.0b013e31824aa50a. [DOI] [PubMed] [Google Scholar]
- 121.von Hentig N, Babacan E, Lennemann T, et al. The steady-state pharmacokinetics of atazanavir/ritonavir in HIV-1-infected adult outpatients is not affected by gender-related co-factors. The Journal of antimicrobial chemotherapy. 2008;62:579–582. doi: 10.1093/jac/dkn204. [DOI] [PubMed] [Google Scholar]
- 122.Barrail-Tran A, Mentre F, Cosson C, et al. Influence of alpha-1 glycoprotein acid concentrations and variants on atazanavir pharmacokinetics in HIV-infected patients included in the ANRS 107 trial. Antimicrobial agents and chemotherapy. 2010;54:614–619. doi: 10.1128/AAC.00797-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodriguez-Novoa S, Barreiro P, Rendon A, et al. Influence of 516G>T polymorphisms at the gene encoding the CYP450–2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clinical Infectious Diseases. 2005;40:1358–1361. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 124.Dailly E, Raffi F, Perre P, et al. Influence of darunavir coadministration on nevirapine pharmacokinetics in HIV-infected patients: a population approach. HIV Med. 2009;10:586–589. doi: 10.1111/j.1468-1293.2009.00721.x. [DOI] [PubMed] [Google Scholar]
- 125.Jackson KA, Rosenbaum SE, Kerr BM, et al. A population pharmacokinetic analysis of nelfinavir mesylate in human immunodeficiency virus-infected patients enrolled in a phase III clinical trial. Antimicrobial agents and chemotherapy. 2000;44:1832–1837. doi: 10.1128/aac.44.7.1832-1837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore KH, Yuen GJ, Hussey EK, et al. Population pharmacokinetics of lamivudine in adult human immunodeficiency virus-infected patients enrolled in two phase III clinical trials. Antimicrobial agents and chemotherapy. 1999;43:3025–3029. doi: 10.1128/aac.43.12.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Maat MM, Huitema AD, Mulder JW, et al. Population pharmacokinetics of nevirapine in an unselected cohort of HIV-1-infected individuals. British journal of clinical pharmacology. 2002;54:378–385. doi: 10.1046/j.1365-2125.2002.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mould DR, Zhang X, Nieforth K, et al. Population pharmacokinetics and exposure-response relationship of enfuvirtide in treatment-experienced human immunodeficiency virus type 1-infected patients. Clinical pharmacology and therapeutics. 2005;77:515–528. doi: 10.1016/j.clpt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Custodio J, Gordi T, Chang S, et al. Population pharmacokinetics of Cobicistat-boosted Elvitegravir in adult healthy subjects and HIV-infected patients (paper P-O2). 13th International Workshop on Clinical Pharmacology of HIV Therapy; April 16–18, 2012; Barcelona, Spain. [Google Scholar]
- 130.Kakuda T, Sekar V, Vis P, et al. Pharmacokinetics and Pharmacodynamics of Darunavir and Etravirine in HIV-1-Infected, Treatment-Experienced Patients in the Gender, Race, and Clinical Experience (GRACE) Trial. AIDS Res Treat. 2012;2012:186987. doi: 10.1155/2012/186987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Crauwels H, Van Schaik R, Van Heeswijk RP, et al. Effect of intrinsic and extrinsic factors on the pharmacokinetics of TMC278 in antiretroviral-nave, HIV-1-infected patients in ECHO and THRIVE (paper P186). 10th International Congress on Drug Therapy in HIV Infection; Nov 7–11, 2010; Glasgow, UK. [Google Scholar]
- 132.Kile DA, Mawhinney S, Aquilante CL, et al. A Population Pharmacokinetic-Pharmacogenetic Analysis of Atazanavir. AIDS Res Hum Retroviruses. 2012 Oct;28(10):1227–34. doi: 10.1089/aid.2011.0378. [DOI] [PubMed] [Google Scholar]
- 133.Molto J, Valle M, Xinarianos G, et al. Simultaneous Pharmacogenetics-based Population Pharmacokinetic Analysis of Darunavir and Ritonavir in HIV+ Individuals (paper 623); 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA. [Google Scholar]
- 134.Dickinson L, Jackson A, Garvey L, et al. Population Pharmacokinetic Modelling of Once Daily Ritonavir-boosted Darunavir in HIV-infected Patients (paper P184) Journal of the International AIDS Society. 2010;13(Suppl 4):P184. [Google Scholar]
- 135.Weller S, Radomski KM, Lou Y, Stein DS. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrobial agents and chemotherapy. 2000;44:2052–2060. doi: 10.1128/aac.44.8.2052-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jullien V, Treluyer JM, Chappuy H, et al. Weight related differences in the pharmacokinetics of abacavir in HIV-infected patients. British journal of clinical pharmacology. 2005;59:183–188. doi: 10.1111/j.1365-2125.2004.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brendel K, Legrand M, Taburet AM, et al. Population pharmacokinetic analysis of indinavir in HIV-infected patient treated with a stable antiretroviral therapy. Fundamental & clinical pharmacology. 2005;19:373–383. doi: 10.1111/j.1472-8206.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 138.King JR, Acosta EP. Tipranavir: a novel nonpeptidic protease inhibitor of HIV. Clin Pharmacokinet. 2006;45:665–682. doi: 10.2165/00003088-200645070-00003. [DOI] [PubMed] [Google Scholar]
- 139.Dailly E, Raffi F, Biron C, et al. Impact of nevirapine or efavirenz co-administration on ritonavir-boosted amprenavir pharmacokinetics in HIV-infected patients. Fundamental & clinical pharmacology. 2008;22:101–104. doi: 10.1111/j.1472-8206.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 140*.Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med. 2007;167:684–691. doi: 10.1001/archinte.167.7.684. comparison of antiretroviral drug response and tolerability by age in large patient cohort. [DOI] [PubMed] [Google Scholar]
- 141.Nachega JB, Hsu AJ, Uthman OA, et al. Antiretroviral therapy adherence and drug–drug interactions in the aging HIV population. AIDS. 2012;26:S39–S53. doi: 10.1097/QAD.0b013e32835584ea. [DOI] [PubMed] [Google Scholar]
- 142.Silverberg MJ, Jacobson LP, French AL, et al. Age and racial/ethnic differences in the prevalence of reported symptoms in human immunodeficiency virus-infected persons on antiretroviral therapy. J Pain Symptom Manage. 2009;38:197–207. doi: 10.1016/j.jpainsymman.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus-infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170:57–65. doi: 10.1001/archinternmed.2009.432. [DOI] [PubMed] [Google Scholar]
- 144*.American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. recent guidelines and rationale for “potentially inappropriate medications” in the elderly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schaeffner ES, Ebert N, Delanaye P, et al. Two Novel Equations to Estimate Kidney Function in Persons Aged 70 Years or Older. Annals of Internal Medicine. 2012;157:471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.