Abstract
BACKGROUND
The focal adhesion kinase (FAK) is a non-receptor tyrosine kinase linked to tumor growth, invasion, and metastasis. FAK is overexpressed and associated with prognosis in many cancers, but its prognostic value in smallcell lung carcinoma (SCLC) is unknown and was the focus of this study.
METHODS
Total FAK expression was analyzed via immunohistochemistry in tissue microarrays consisting of formalin-fixed, paraffin-embedded SCLC specimens from 85 patients. FAK staining scores were tested for correlations with pathological characteristics and clinical outcomes. Phospho-paxillin was also tested in 35 of the 85 specimens to evaluate whether FAK expression was associated with downstream signaling.
RESULTS
Specific FAK expression was localized to the cytoplasm of 78/85 (92%) SCLCs. FAK expression was scored low in 11 (13%), moderate in 17 (20%), and high in 50 (59%) SCLCs. FAK staining scores treated as continuous variables did not correlate with SCLC disease stage, response to therapy, recurrence/ progression-free survival, or overall survival. Moreover, total FAK expression did not correlate with phospho-paxillin Tyr118 expression.
CONCLUSIONS
Total FAK is strongly expressed in a majority of SCLC tumors. However, the expression evaluated via immunohistochemistry is not a prognostic factor in patients with SCLC.
Keywords: FAK, immunohistochemistry, phospho-paxillin, prognosis, SCLC
INTRODUCTION
Small-cell lung carcinoma (SCLC) represents approximately 15% of annual lung cancer cases1 and is the most aggressive subtype in its clinical behavior, with early development of widespread metastasis.2 Despite a good initial response to chemotherapy and/or radiation therapy,3–5 most patients will recur and progress with a median overall survival (OS) of 24 months in limited stage disease,6,7 and 7–10 months in extensive stage disease,8 and with a 5-year OS as low as 5%.1 To date, no targeted therapy is available for SCLC. New biomarkers and therapeutic targets are needed to improve the detection, treatment, and prognosis of this aggressive cancer.
Focal Adhesion Kinase (FAK) is a 125 kDa non-receptor tyrosine kinase that has recently been suggested as a potential cancer therapy target. FAK is widely expressed in various tissues and cell types. It is localized to focal adhesions, or contact points, between actin cytoskeleton and extracellular matrix. Once activated by integrins, G protein–coupled receptor ligands, or growth factors, FAK is autophosphorylated at Tyr397, then binds and activates proteins such as Src, p130CAS, paxillin, and PI3KR2.9,10 Through this signaling pathway, FAK plays a central role in cell adhesion, migration, invasion, survival, and anchorage-independent growth.9–12 Based on its common overexpression in cancer and known roles in promoting cell survival and migration/invasion, FAK has been suggested to play a role in cancer initiation and progression.12–15 Therefore, small-molecule inhibitors targeting the FAK kinase domain (NVP-TAE226; PF-573,228; PF-562,271) have been developed for use as potential cancer therapies.16–18 These inhibitors have been shown to decrease FAK phosphorylation at Tyr397 and inhibit migration in various cancer cells. Preclinical studies with PF-562,271 have shown tumor stasis or regression in mouse models of various cancer types, including non–small-cell lung cancer (NSCLC).16 PF-562,271 is currently being tested in phase 1 clinical trials, and preliminary results have shown that it is well tolerated and allows cancer patients to exhibit prolonged stable disease.19 Therefore, these inhibitors are promising anticancer targeted therapies and support a role of FAK in carcinogenesis.
Using different methods such as immunohistochemistry (IHC), western blotting (WB), northern blotting, and quantitative real-time polymerase chain reaction (qRT-PCR), FAK has been shown to be overexpressed in several human cancers,13,20–25 and its expression has been correlated to patient prognosis in many of them, including NSCLC.20,21,23,26–33 In a study evaluating total FAK expression via IHC, WB, and qRT-PCR in NSCLC and matching normal lung tissues, the authors found FAK overexpression in cancer and a significant correlation between FAK overexpression and higher disease stages.20 In another study, patients with lung adenocarcinoma had a better OS when FAK was not expressed.34 Similarly, in a study of 381 NSCLCs evaluated via IHC, WB, and qRT-PCR, FAK was found to be overexpressed in 27% of the NSCLCs, and patients without FAK expression had a better OS.21 However, the prognostic value of total FAK has never been reported in SCLC.
In a recent study, we reported FAK copy number gain in 50% of 46 SCLC tissues analyzed by array comparative genomic hybridization.35 We also observed FAK activation in SCLC cell lines and showed that the inhibition of FAK phosphorylation at Tyr397 with PF-573,228 decreased cell adhesion on laminin-332, suggesting a role of FAK in SCLC progression and its potential as a SCLC therapy target.35
Based on our recent findings and the literature, we hypothesized that FAK expression in SCLC correlates with disease stage, response to therapy (RTT), recurrence/progression-free survival (R/PFS), and OS. To test our hypothesis, we assessed FAK expression via IHC in SCLC tissues coming from 85 patients and tested FAK staining scores for correlations with pathological and clinical outcomes. We also indirectly evaluated whether total FAK expression correlated with its biological activity by testing the expression of phospho-paxillin Tyr118, a downstream effector of FAK, in tissues coming from 35 of the 85 patients tested for total FAK.
MATERIALS AND METHODS
Patients and SCLC Tissue Samples
Two tissue microarrays (TMAs) made of SCLC specimens were prepared from formalin-fixed, paraffin-embedded (FFPE) tissue blocks in accordance with reported methods.36,37 Pathology blocks were retrieved from the archives of the Department of Pathology at Vanderbilt University Medical Center, Nashville VA Medical Center, and St. Thomas Hospital in Nashville, Tennessee. The tissue blocks were obtained between 1996 and 2008 from 85 patients who underwent surgery or bronchoscopy before medical treatment. SCLC diagnosis was confirmed on hematoxylin and eosin–stained sections by an experienced lung cancer pathologist (A.L.G.). Treatment was administered on an individual basis according to disease stage and patient performance status as per standard of care (chemotherapy and radiotherapy). All patients were followed with chart review until death or until data analysis of the manuscript. Clinical data were obtained from tumor registry and hospital charts. Patient characteristics are summarized in Table 1. The study was approved by the institutional review board at each medical center.
Table 1.
Patient Clinical and Pathological Characteristics and Association With Total FAK and Phospho-paxillin Staining Scores Treated as Continuous Variables on Univariable Analysis
| Characteristic | Total FAK | Phospho-paxillin | ||
|---|---|---|---|---|
| Overall (n = 85) | P | Overall (n = 35) | P | |
| Sex, no. (%) | .311 | .310 | ||
| Men | 58 (68) | 19 (54) | ||
| Women | 27 (32) | 16 (46) | ||
| Race, no. (%) | .550 | .765 | ||
| Caucasian | 80 (94) | 33 (94) | ||
| African American | 5 (6) | 2 (6) | ||
| Smoking status, no. (%) | .956 | .111 | ||
| Current smoker | 36 (42) | 17 (48.5) | ||
| Ex-smoker | 40 (47) | 17 (48.5) | ||
| Never smoker | 1 (1) | 0 (0) | ||
| Unknown | 8 (9) | 1 (3) | ||
| Pack years, median (IQR) | 60 (40–80) | .383 | 50 (40–60) | .342 |
| Age at time of diagnosis | ||||
| Median, y (IQR) | 63 (54–69) | .84 | 64 (55.5–69) | .512 |
| £60 y, no. (%) | 33 (39) | .949 | 16 (46) | .903 |
| >60 y, no. (%) | 52 (61) | 19 (54) | ||
| SCLC disease stage, no. (%) | .134 | .794 | ||
| Limited | 53 (62) | 11 (31) | ||
| Extensive | 32 (38) | 24 (69) | ||
| Response to therapy, no. (%) | .098 | .411 | ||
| Complete response | 27 (32) | 6 (17) | ||
| Partial response | 24 (28) | 13 (37) | ||
| Stable disease | 3 (4) | 1 (3) | ||
| Progressive disease | 4 (5) | 2 (6) | ||
| Unknown | 27 (32) | 13 (37) | ||
| Recurrence/progression after treatment, no. (%) | ||||
| No | 11 (13) | 4 (11) | ||
| Yes | 47 (55) | 14 (40) | ||
| Unknown | 27 (32) | 17 (49) | ||
| Recurrence/progression-free survivala | .171 | .106 | ||
| Median, d (95% CI) | 293 (175–499) | 215 (161–NA) | ||
| Recurrence/progression-free at 3 y, no. (%) | 12 (24) | 4 (27) | ||
| Overall survival | .207 | .360 | ||
| Median, d (95% CI) | 362 (284–585) | 407 (267–858) | ||
CI, confidence interval; FAK, focal adhesion kinase; IQR, interquartile range; NA, not applicable.
Recurrence/progression dates were available for 41 of the 47 patients who recurred/progressed among those tested for total FAK and for 12 of the 14 patients who recurred/progressed among those tested for phospho-paxillin Tyr118.
Immunohistochemistry
Total FAK
After deparaffinization and rehydration, tissue sections were placed in Reveal Retrieval Reagent (Biocare Medical, Concord, CA) and heated in a Decloaking Chamber (Biocare Medical). Endogenous peroxidase was neutralized with 0.03% hydrogen peroxide (Fischer Scientific, Fair Lawn, NJ). Tissues were treated with Background Sniper block (Biocare Medical) and incubated for 60 minutes with rabbit anti-FAK antibody (sc-557) (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), specifically recognizing total FAK on FFPE sections. The MACH3 detection system (Biocare Medical) and DAB+ (Dakocytomation, Carpinteria, CA) was used to produce visible staining.
Phospho-FAK Tyr397
After deparaffinization and rehydration, tissue sections were placed in heated Target Retrieval Solution (Labvision, Fremont, CA). Endogenous peroxidase was neutralized with 0.03% hydrogen peroxide followed by a casein-based protein block (DakoCytomation). Sections were incubated with anti–phospho-FAK Tyr397 antibody (Invitrogen, Carlsbad, CA). The Dako Envision+ HRP/DAB System (DakoCytomation) was used to produce visible staining.
Phospho-paxillin Tyr118
Sections were dried overnight and stained using a Leica Bond Max. They were incubated with an anti-phospho-paxillin Tyr118 antibody (1:100, Abcam, Cambridge, MA). Phospho-paxillin was visualized using the Leica Bond Polymer Refine Detection system (Leica Microsystems, Bannockburn, IL).
Scoring
The staining intensity of the SCLC cases represented in triplicates was evaluated by 2 independent observers (A.L.G., S.O.) using the following scoring system: 0, no staining; 1, weak; 2, moderate; and 3, strong. The staining intensity was then multiplied by stained tumor cell percentage to obtain the final staining score (range, 0–300).
Statistical Analysis
Wilcoxon rank-sum or Kruskal-Wallis tests were used to correlate maximum staining scores (from triplicates) with categorical outcomes, and Spearman’s rank correlation test was used for continuous outcomes. OS was calculated from date of diagnosis to date of death, or last date of contact for those alive at the time of analysis. R/PFS was calculated from date of diagnosis to date of recurrence/progression or last date of contact for those that did not recur/progress at the time of analysis. Score test was used to assess association between maximum staining scores and survival. Descriptive statistics, including median and interquartile ranges for continuous variables, and percentages or frequencies for categorical variables were reported. For time to event outcomes, multivariable analyses were performed using the Cox proportional hazard model to adjust for prespecified confounders (age, sex, smoking history, and disease stage). To optimize the use of partial information on a subject, missing values of pre-specified confounders were imputed using a multiple imputation method for the Cox proportional hazard model. For the binary outcome of response to therapy, logistic analyses were performed to determine whether FAK was an independent predictor after adjusting for disease stage. All tests of significance were 2-sided, and differences were considered statistically significant at P<.05. Analyses were performed using R version 2.9.2 software.38
RESULTS
Patient Characteristics
A total of 85 patients diagnosed with SCLC were included in the study based on the availability of archival pathology specimens. Patient characteristics are described in Table 1. Of the 85 patients, 58 (68%) were men and 27 (32%) were women; 80 (94%) were Caucasian and 5 (6%) were African American. One patient had never smoked, but all of the other patients were current or ex-smokers with a median pack-year history of 60 (interquartile range, 40–80). The median age at diagnosis was 63 years (interquartile range, 54–69). Fifty-three (62%) patients were diagnosed with limited stage disease and 32 (38%) were diagnosed with extensive stage disease. All patients received medical treatment according to disease stage and performance status as per standard of care therapy. RTT was assessed according to Response Evaluation Criteria in Solid Tumors guidelines and was available for 58 patients, 27 (32%) of whom had complete response and 31 (37%) of whom had non–complete response. Of these 85 patients, 11 (13%) were disease-free at the last date known alive, whereas 47 (55%) suffered recurrence/progression after treatment. The recurrence/progression information was missing for 27 patients. The recurrence/progression dates were available for 41 of the 47 patients who recurred or progressed, revealing a median R/PFS of 293 days (95% confidence interval, 175–499), with 12 patients (24%) disease-free 3 years after diagnosis. The median OS was 362 days (95% confidence interval, 284–585), and 19 (22%) patients were alive 3 years after SCLC diagnosis. All but 9 (11%) patients were dead at the time of data analysis.
FAK Is Strongly Expressed in the Majority of SCLCs
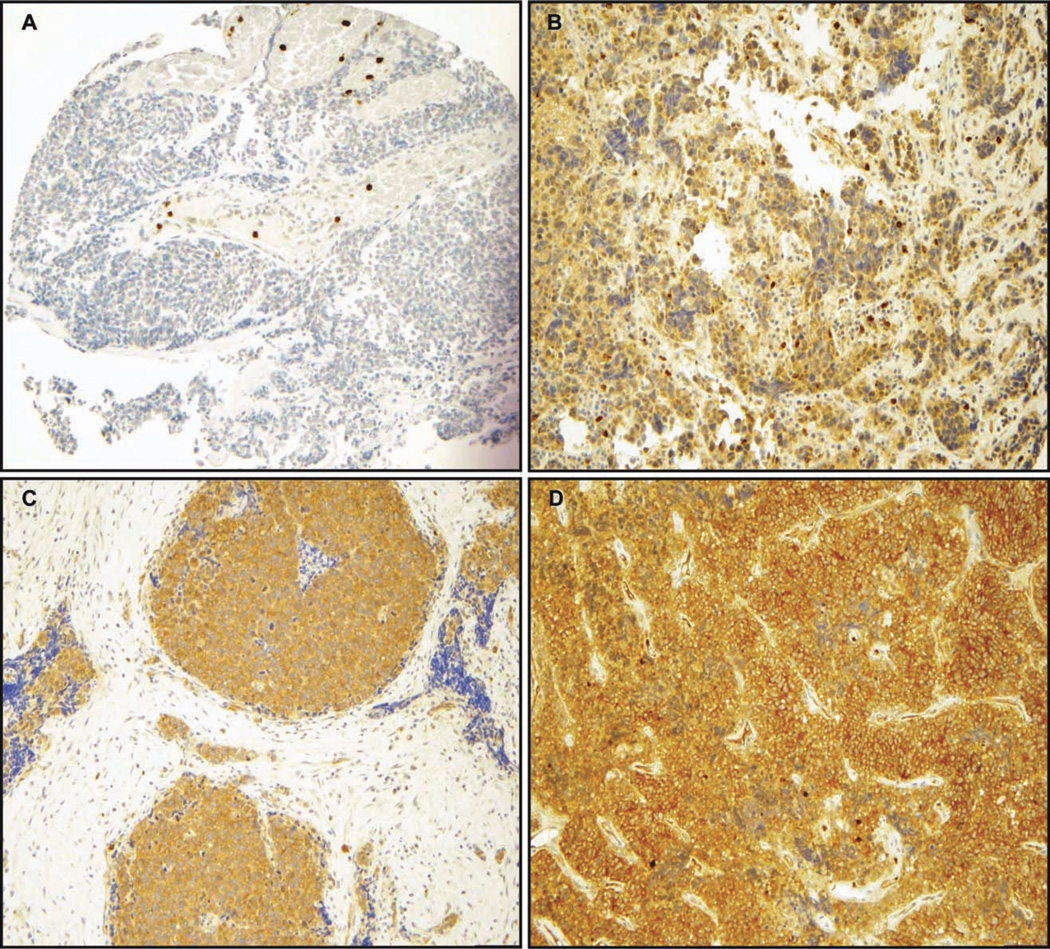
FAK was expressed in the cytoplasm of 78/85 (92%) SCLCs. The staining pattern was homogeneous, with either all the cells staining for FAK or none at all, although the staining intensity was clearly different between tumor samples. The FAK staining score was 0 in 7 (8%) samples, 100 (low) in 11 (13%) samples, 200 (moderate) in 17 (20%) samples, and 300 (strong) in 50 (59%) samples. Representative images of SCLCs with absent, low, moderate, and strong FAK staining scores are shown in Figure 1.We also attempted to stain for phospho-FAK Tyr397 via IHC, but the staining was nonspecific; therefore, we did not pursue analysis of these data.
Figure 1.
Total FAK expression was evaluated via immunohistochemistry. Two tissue microarrays consisting of small-cell lung carcinoma (SCLC) tissue samples from 85 patients were incubated with an antibody against total FAK (A-17). FAK displayed a cytoplasmic staining. The staining intensity of the SCLC cases was evaluated by 2 independent observers (A.L.G., S.O.) using the following scoring system: 0, no staining; 1, weak; 2, moderate; and 3, strong. The staining intensity was then multiplied by stained tumor cell percentage to obtain the final staining score (range, 0–300). Representative images are shown for SCLC tumors with (A) absent, (B) weak, (C) moderate, and (D) strong total FAK expression. (Original magnification,×200.)
Phospho-paxillin Tyr118 Is Expressed Only in a Subset of SCLC Tissues
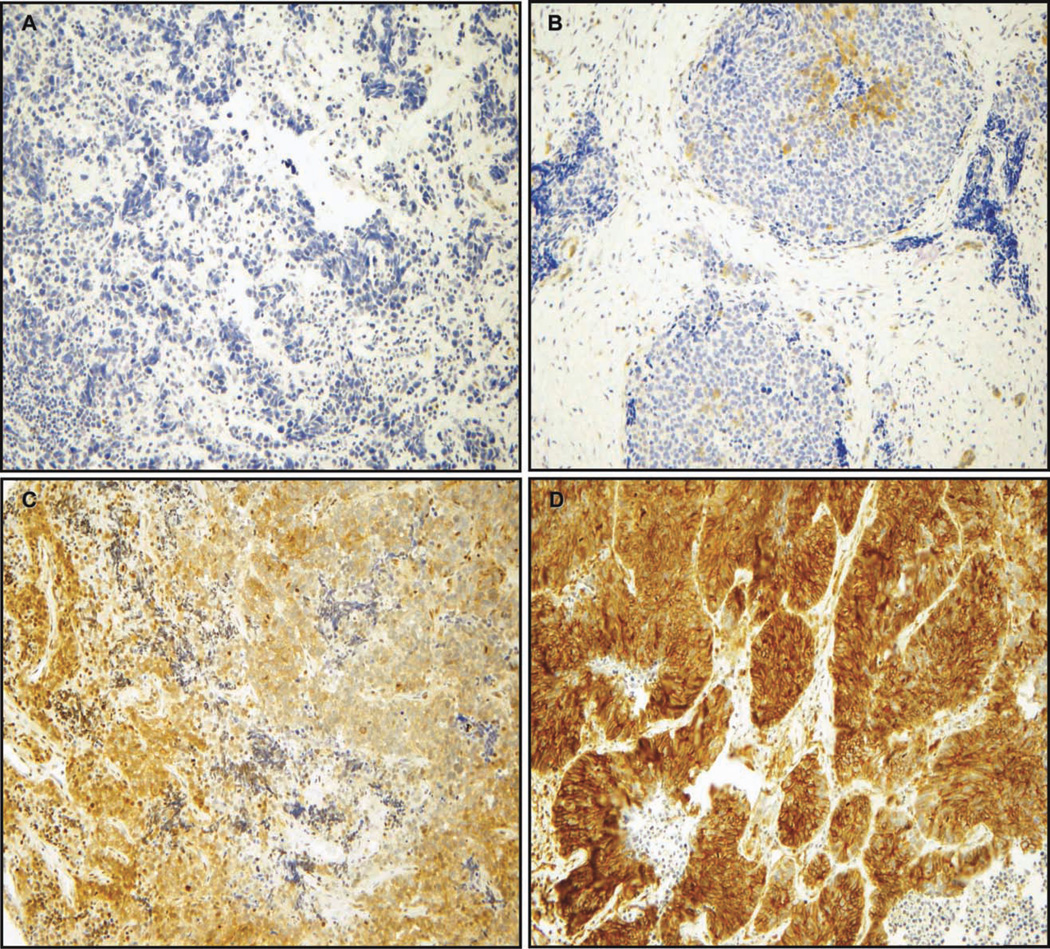
Phospho-paxillin Tyr118 expression was evaluated in 1 of the 2 TMAs tested for total FAK (the first one was no longer available), including samples from 35 SCLC patients. Phospho-paxillin Tyr118 was expressed in the cytoplasm of 18/35 (51%) samples. The expression was not homogeneous, because only a subset of cancer cells expressed it. The phospho-paxillin Tyr118 staining score was 0 in 17 (49%) samples, ≤100 in 6 (17%) samples, 101–200 in 3 (9%) samples, and 201–300 in 9 (26%) samples. Representative images of SCLC samples with absent, low, moderate, and strong phospho-paxillin Tyr118 staining scores are shown in Figure 2.
Figure 2.
Phospho-paxillin Tyr118 expression was evaluated via immunohistochemistry. A tissue microarray consisting of SCLC tissue samples from 35 patients was incubated with an antibody against phospho-paxillin Tyr118. Phospho-paxillin Tyr118 displayed a cytoplasmic staining. The staining intensity of the SCLC cases was evaluated by 2 independent observers (A.L.G., S.O.) using the following scoring system: 0, no staining; 1, weak; 2, moderate; and 3, strong. The staining intensity was then multiplied by stained tumor cell percentage to obtain the final staining score (range, 0–300). Representative images are shown for SCLC tumors with (A) absent, (B) weak, (C) moderate, and (D) strong phospho-paxillin expression. (Original magnification, ×200.)
FAK and Phospho-paxillin Tyr118 Staining Scores Do Not Correlate With Disease Stage, RTT, R/PFS, or OS
Samples represented in the TMAs were linked to a clinical database, allowing correlation analyses between various pathological and clinical characteristics and FAK or phospho-paxillin Tyr118 staining scores.
First, we treated staining scores as continuous variables in a univariable analysis. Patients’ pathological and clinical characteristics and their correlation with FAK and phospho-paxillin Tyr118 staining scores treated as continuous variables in a univariable analysis are summarized in Table 1. We found that FAK and phospho-paxillin Tyr118 staining scores did not significantly correlate with sex, race, smoking status, age at time of SCLC diagnosis, disease stage, RTT, R/PFS, or OS. Moreover, there was no statistically significant correlation between FAK and phospho-paxillin Tyr118 expression. In a multivariable analysis including FAK staining scores, disease stage, age, sex, and smoking status, no significant association between FAK staining scores and RTT (Table 2), R/PFS (Table 3), or OS (Table 4) was found, even after stratifying for disease stage (data not shown). However, limited disease stage was an independent predictor of better RTT (P = .001), better R/PFS (P = .0002), and better OS (P = .002). Younger age was also an independent predictor of better R/PFS (P = .032) and better OS (P = .017).
Table 2.
Multivariable Logistic Regression Model for Response to Therapy (n = 85)
| Variable | FAK Staining Scores Treated as Continuous Variables |
|
|---|---|---|
| P | OR (95% CI) | |
| FAK staining score | .259 | 1.00 (1.00–1.01) |
| SCLC disease stage (limited vs extensive) | .001 | 12.00 (2.92–49.37) |
CI, confidence interval; FAK, focal adhesion kinase; OR, odds ratio; SCLC, small-cell lung carcinoma.
Table 3.
Multivariate Cox Proportional Regression Analysis for the Association of Total FAK Staining Scores With Recurrence/Progression-Free Survival (n = 52)
| Variable | FAK Staining Scores Treated as Continuous Variables |
|
|---|---|---|
| P | HR (95% CI) | |
| FAK staining score | .196 | 0.998 (0.995–1.001) |
| SCLC disease stage (limited vs extensive) | .0002 | 0.219 (0.099–0.485) |
| Age | .032 | 1.053 (1.004–1.105) |
| Sex | .823 | 1.086 (0.526–2.241) |
| Smoking status (non-current vs current) | .045 | 0.412 (0.173–0.980) |
CI, confidence interval; FAK, focal adhesion kinase; HR, hazard ratio; SCLC, small-cell lung carcinoma.
Table 4.
Multivariate Cox Proportional Regression Analysis for the Association of Total FAK Staining Scores With Overall Survival (n = 85)
| Variable | FAK Staining Scores Treated as Continuous Variables |
|
|---|---|---|
| P | HR (95% CI) | |
| FAK staining score | .724 | 1.000 (0.997–1.002) |
| SCLC disease stage (limited vs extensive) | .002 | 0.441 (0.266–0.731) |
| Age | .017 | 1.036 (1.006–1.067) |
| Sex | .253 | 1.350 (0.807–2.260) |
| Smoking status (non-current vs current) | .539 | 0.842 (0.488–1.456) |
CI, confidence interval; FAK, focal adhesion kinase; HR, hazard ratio; SCLC, small-cell lung carcinoma.
DISCUSSION
Because FAK is known to promote cell survival and migration/invasion and is suggested to have a role in cancer initiation and progression,9,12,13,15 we investigated whether FAK expression in SCLCs correlated with patient prognosis.
Total FAK expression was analyzed via IHC in 2 TMAs made of SCLC specimens from 85 patients. Specific FAK expression was found in the cytoplasm (as predicted by its role as a non-receptor cytosolic tyrosine kinase) of 92% SCLC tissues, and the staining score was high or moderate in the majority of them. This was in accordance with the majority of published data in other cancers. Overexpression of FAK has been described using methods in various cancer types,13,23,25 and IHC studies have confirmed, in most cases, that FAK was overexpressed in cancer.
In our study, FAK staining scores did not independently correlate with RTT, R/PFS, or OS, whereas limited disease stage and younger age were independent predictors of better outcome as described in the literature.39 Therefore, we concluded that total FAK expression was not a prognostic factor in SCLC, though many studies in other cancers have reported prognostic significance.
FAK overexpression has been shown to correlate with invasion and to play a critical role in malignant and metastatic progression of human cancers.23,40 For instance, increased FAK expression has been associated with advanced disease stage in colon and breast cancers,41 and in metastatic prostate cancers compared with normal tissues.42 In NSCLC, a significant correlation was found between FAK overexpression and higher disease stage.20 Moreover, several IHC studies reported that FAK over-expression is a poor prognostic factor. In a study of 249 lung adenocarcinomas34 and another study with 381 NSCLCs,21 FAK overexpression was associated with poorer OS. In a study of 91 patients with esophageal squamous cell carcinoma, FAK overexpression was observed in 53% patients and linked with tumor invasiveness, lymph node metastasis, and higher disease stage; the survival was significantly lower in patients with FAK overexpression.30 Similarly, in a study of 64 patients with hepatocellular carcinoma, FAK overexpression was associated with a significantly poorer OS.29
In contrast, many other IHC studies correlating FAK expression with clinical outcomes did not find any prognostic association.26,27,33 In 1 of these studies, an analysis of 211 head and neck specimens revealed that FAK was overexpressed in the majority of head and neck squamous cell carcinomas and their lymph node metastases, but there was no correlation between FAK expression and tumor recurrence or OS.26 An analysis of 162 resected cervical cancer tissues via IHC even showed that weak FAK expression was correlated with pelvic lymph node metastases and recurrent disease.28 Additionally, in a study of colorectal adenocarcinomas, FAK expression evaluated via IHC was increased in primary tumors compared with normal epithelium, whereas the expression was decreased in liver metastases compared with matched primary tumors,43 suggesting a role of FAK in the premetastatic process. Reduced FAK expression was also observed via WB in highly malignant melanoma cells growing in suspension, suggesting that FAK is required in early/intermediate stage cancer cells and that further progression leads to a completely anchorage-independent phenotype where FAK no longer plays a role.44–46 Therefore, all these studies have suggested that FAK expression and its role may differ according to tissue type or tumor progression stage.
Discordant results described in the literature may be related to differences in methodologies and antibodies used to evaluate FAK expression. They may also reflect an absence of correlation between total FAK expression and activity. Some authors reported that total FAK expression correlated well with FAK phosphorylation and therefore activity.11,30,31,47 However, in cervical carcinomas and melanoma cells analyzed via WB, it was reported that total FAK expression was not different between normal and cancer cell lines, whereas FAK phosphorylation was increased in cancer cell lines.46 Similarly, we recently reported that total FAK expression was similar in 5 SCLC and 3 immortalized normal bronchial epithelial cell lines, whereas FAK phosphorylation at Tyr397 (site of FAK autophoshorylation) was significantly increased in SCLC compared with normal cell lines, and that inhibition of FAK phosphorylation by a small-molecule inhibitor decreased SCLC cell adhesion on laminin-332.35 Therefore, total FAK expression is not necessarily correlated with FAK phosphorylation and activation. Unfortunately, there is currently no specific antibody to phosphorylation sites that would allow us to test this hypothesis in FFPE tissue sections. Nevertheless, specific phospho-FAK Tyr397 antibodies are available for WB, and some investigators have used them to assess the prognostic value of FAK activation in cancer. Increased FAK phosphorylation at Tyr397 in acute myeloid leukemia cells, evaluated by WB, was associated with decreased OS.31 In NSCLC, WB analysis of tissues coming from 41 patients showed that increased FAK phosphorylation was associated with nodal involvement and shorter R/PFS.22 In another study evaluating phospho-FAK expression by WB in 16 NSCLC tissues, the authors found a significant correlation between FAK overexpression and higher disease stages.20 In the present study, none of the SCLC tissues represented in the TMAs were available for WB evaluation of phospho-FAK Tyr397 expression, tissue availability often being limited to biopsy material in SCLC because the standard treatment is chemotherapy and/or radiation therapy and not surgery.
To evaluate whether total FAK expression was associated with activation of downstream effector, we tested the expression of phospho-paxillin Tyr118 in 1 of the SCLC TMAs used for total FAK staining. We did not find significant correlation between total FAK and phospho-paxillin Tyr118 expression, suggesting the absence of correlation between total FAK expression and its activity. Similarly, no correlation between phospho-paxillin Tyr118 expression and clinical outcomes was found.
In conclusion, the IHC analysis of 85 SCLCs revealed that total FAK is strongly and frequently expressed in these tumors. However, our results demonstrate that total FAK expression is not a prognostic biomarker in patients with SCLCand is not correlatedwith FAKactivity.
Acknowledgments
FUNDING SOURCES
This study was supported by a Merit Review grant from the Department of Veterans Affairs and grant CA90949. Sebahat Ocak was supported by an International Association for the Study of Lung Cancer Young Investigator Fellowship Award and a grant from Université Catholique de Louvain, Belgium.
We thank the Vanderbilt SPORE in Lung Cancer CA90949 and the Immunohistochemistry Core (supported by Cancer Center Support Grant 5P30 CA068485) at Vanderbilt University. We thank Kathy Taylor, Director of the Research Institute at St. Thomas Health Services, Nashville, Tennessee, for sharing archived tissue blocks.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 3.Cheng S, Evans WK, Stys-Norman D, Shepherd FA. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol. 2007;2:348–354. doi: 10.1097/01.JTO.0000263720.15062.51. [DOI] [PubMed] [Google Scholar]
- 4.Murray N, Turrisi AT., 3rd A review of first-line treatment for small-cell lung cancer. J Thorac Oncol. 2006;1:270–278. doi: 10.1016/s1556-0864(15)31579-3. [DOI] [PubMed] [Google Scholar]
- 5.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger DS, Berkey BA, Abrams RA, et al. Study of paclitaxel, etoposide, and cisplatin chemotherapy combined with twice-daily thoracic radiotherapy for patients with limited-stage small-cell lung cancer: a Radiation Therapy Oncology Group 9609 phase II study. J Clin Oncol. 2005;23:4991–4998. doi: 10.1200/JCO.2005.00.414. [DOI] [PubMed] [Google Scholar]
- 7.Kubota K, Nishiwaki Y, Sugiura T, et al. Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res. 2005;11:5534–5538. doi: 10.1158/1078-0432.CCR-04-1771. [DOI] [PubMed] [Google Scholar]
- 8.Murren J, Glatstein E, Pass H. Small Cell Lung Cancer. 6th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 9.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 11.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 12.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 13.Gabarra-Niecko V, Schaller MD, Dunty JM. FAK regulates biological processes important for the pathogenesis of cancer. Cancer Metastasis Rev. 2003;22:359–374. doi: 10.1023/a:1023725029589. [DOI] [PubMed] [Google Scholar]
- 14.Parsons JT, Slack-Davis J, Tilghman R, Roberts WG. Focal adhesion kinase: targeting adhesion signaling pathways for therapeutic intervention. Clin Cancer Res. 2008;14:627–632. doi: 10.1158/1078-0432.CCR-07-2220. [DOI] [PubMed] [Google Scholar]
- 15.Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol. 2008;18:45–52. doi: 10.1016/j.semcancer.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts WG, Ung E, Whalen P, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Hjelmeland AB, Keir ST, et al. A novel low-molecular weight inhibitor of focal adhesion kinase, TAE226, inhibits glioma growth. Mol Carcinog. 2007;46:488–496. doi: 10.1002/mc.20297. [DOI] [PubMed] [Google Scholar]
- 18.Slack-Davis JK, Martin KH, Tilghman RW, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 19.Siu L, Burris H, Mileshkin L, et al. Phase 1 study of a focal adhesion kinase (FAK) inhibitor PF-00562271 in patients (pts) with advanced solid tumors. 2007 ASCO Annual Meeting Proceedings Part I. Journal of Clinical Oncology. 2007;25(suppl):3527. [Google Scholar]
- 20.Carelli S, Zadra G, Vaira V, et al. Up-regulation of focal adhesion kinase in non-small cell lung cancer. Lung Cancer. 2006;53:263–271. doi: 10.1016/j.lungcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Hsu NY, Chen CY, Hsu CP, et al. Prognostic significance of expression of nm23-H1 and focal adhesion kinase in non-small cell lung cancer. Oncol Rep. 2007;18:81–85. [PubMed] [Google Scholar]
- 22.Imaizumi M, Nishimura M, Takeuchi S, Murase M, Hamaguchi M. Role of tyrosine specific phosphorylation of cellular proteins, especially EGF receptor and p125FAK in human lung cancer cells. Lung Cancer. 1997;17:69–84. doi: 10.1016/s0169-5002(97)00650-8. [DOI] [PubMed] [Google Scholar]
- 23.Owens LV, Xu L, Craven RJ, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- 24.Wang XY, Liu T, Zhu CZ, et al. Expression of KAI1, MRP-1, and FAK proteins in lung cancer detected by high-density tissue microarray. Ai Zheng. 2005;24:1091–1095. [PubMed] [Google Scholar]
- 25.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 26.Canel M, Secades P, Rodrigo JP, et al. Overexpression of focal adhesion kinase in head and neck squamous cell carcinoma is independent of fak gene copy number. Clin Cancer Res. 2006;12:3272–3279. doi: 10.1158/1078-0432.CCR-05-1583. [DOI] [PubMed] [Google Scholar]
- 27.Furuyama K, Doi R, Mori T, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006;30:219–226. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel B, zur Hausen A, Stickeler E, et al. Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. Clin Cancer Res. 2006;12:2476–2483. doi: 10.1158/1078-0432.CCR-05-1867. [DOI] [PubMed] [Google Scholar]
- 29.Itoh S, Maeda T, Shimada M, et al. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res. 2004;10:2812–2817. doi: 10.1158/1078-0432.ccr-1046-03. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki T, Kato H, Nakajima M, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89:140–145. doi: 10.1038/sj.bjc.6601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recher C, Ysebaert L, Beyne-Rauzy O, et al. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64:3191–3197. doi: 10.1158/0008-5472.can-03-3005. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz KJ, Grabellus F, Callies R, et al. High expression of focal adhesion kinase (p125FAK) in node-negative breast cancer is related to overexpression of HER-2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res. 2005;7:R194–R203. doi: 10.1186/bcr977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theocharis SE, Kouraklis GP, Kakisis JD, et al. Focal adhesion kinase expression is not a prognostic predictor in colon adenocarcinoma patients. Eur J Surg Oncol. 2003;29:571–574. doi: 10.1016/s0748-7983(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang C, Yang R, Yue D, Zhang Z. Expression of FAK and PTEN in bronchioloalveolar carcinoma and lung adenocarcinoma. Lung. 2009;187:104–109. doi: 10.1007/s00408-008-9130-6. [DOI] [PubMed] [Google Scholar]
- 35.Ocak S, Yamashita H, Udyavar AR, et al. DNA copy number aberrations in small-cell lung cancer reveal activation of the focal adhesion pathway. Oncogene. 2010;29:6331–6342. doi: 10.1038/onc.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 37.Massion PP, Kuo WL, Stokoe D, et al. Genomic copy number analysis of non-small cell lung cancer using array comparative genomic hybridization: implications of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2002;62:3636–3640. [PubMed] [Google Scholar]
- 38.Team RDC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 39.Ihde DC, Makuch RW, Carney DN, et al. Prognostic implications of stage of disease and sites of metastases in patients with small cell carcinoma of the lung treated with intensive combination chemotherapy. Am Rev Respir Dis. 1981;123:500–507. doi: 10.1164/arrd.1981.123.5.500. [DOI] [PubMed] [Google Scholar]
- 40.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 41.Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 42.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 43.Ayaki M, Komatsu K, Mukai M, et al. Reduced expression of focal adhesion kinase in liver metastases compared with matched primary human colorectal adenocarcinomas. Clin Cancer Res. 2001;7:3106–3112. [PubMed] [Google Scholar]
- 44.Kahana O, Micksche M, Witz IP, Yron I. The focal adhesion kinase (P125FAK) is constitutively active in human malignant melanoma. Oncogene. 2002;21:3969–3977. doi: 10.1038/sj.onc.1205472. [DOI] [PubMed] [Google Scholar]
- 45.Maung K, Easty DJ, Hill SP, Bennett DC. Requirement for focal adhesion kinase in tumor cell adhesion. Oncogene. 1999;18:6824–6828. doi: 10.1038/sj.onc.1203094. [DOI] [PubMed] [Google Scholar]
- 46.Moon HS, Park WI, Choi EA, Chung HW, Kim SC. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer. 2003;13:640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- 47.McCormack SJ, Brazinski SE, Moore JL, Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene. 1997;15:265–274. doi: 10.1038/sj.onc.1201186. [DOI] [PubMed] [Google Scholar]