Abstract
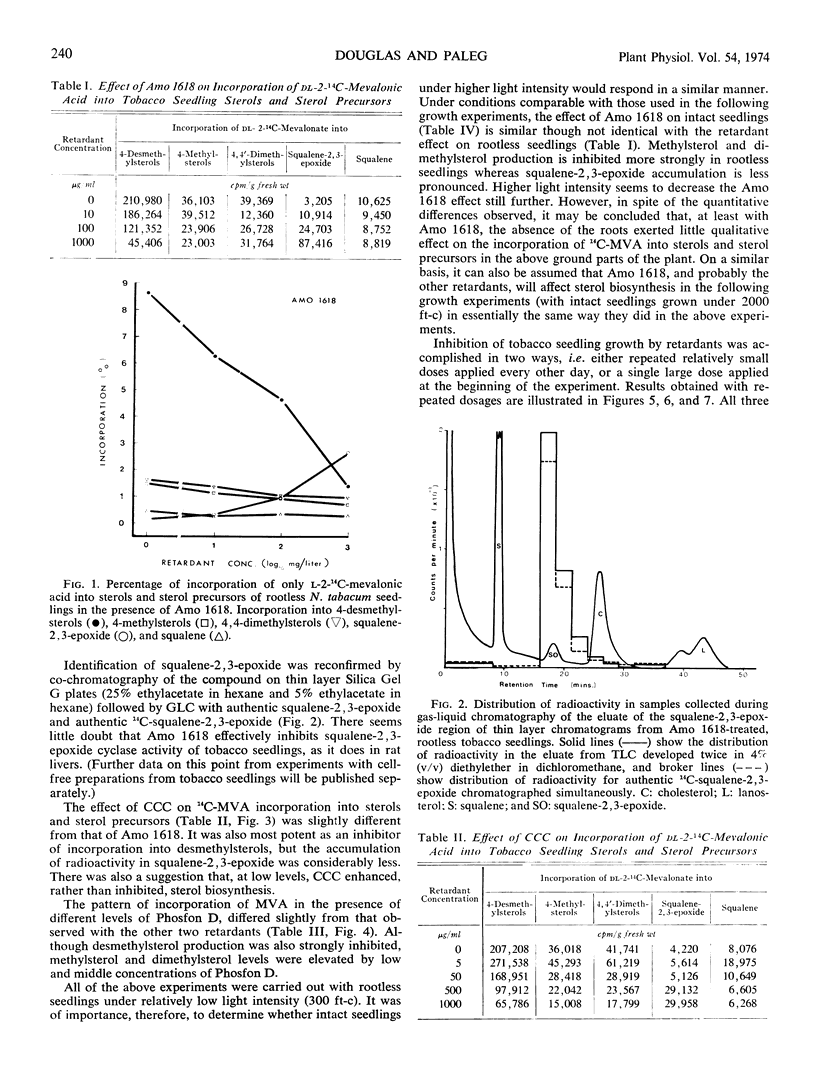
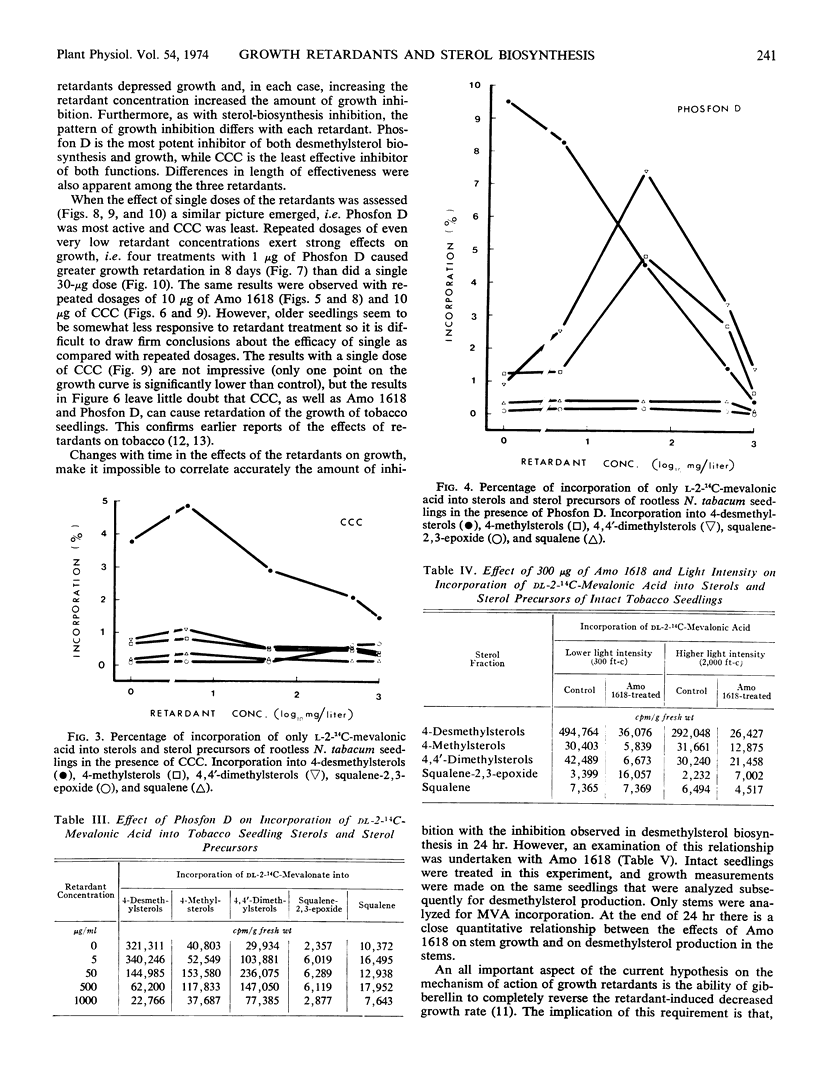
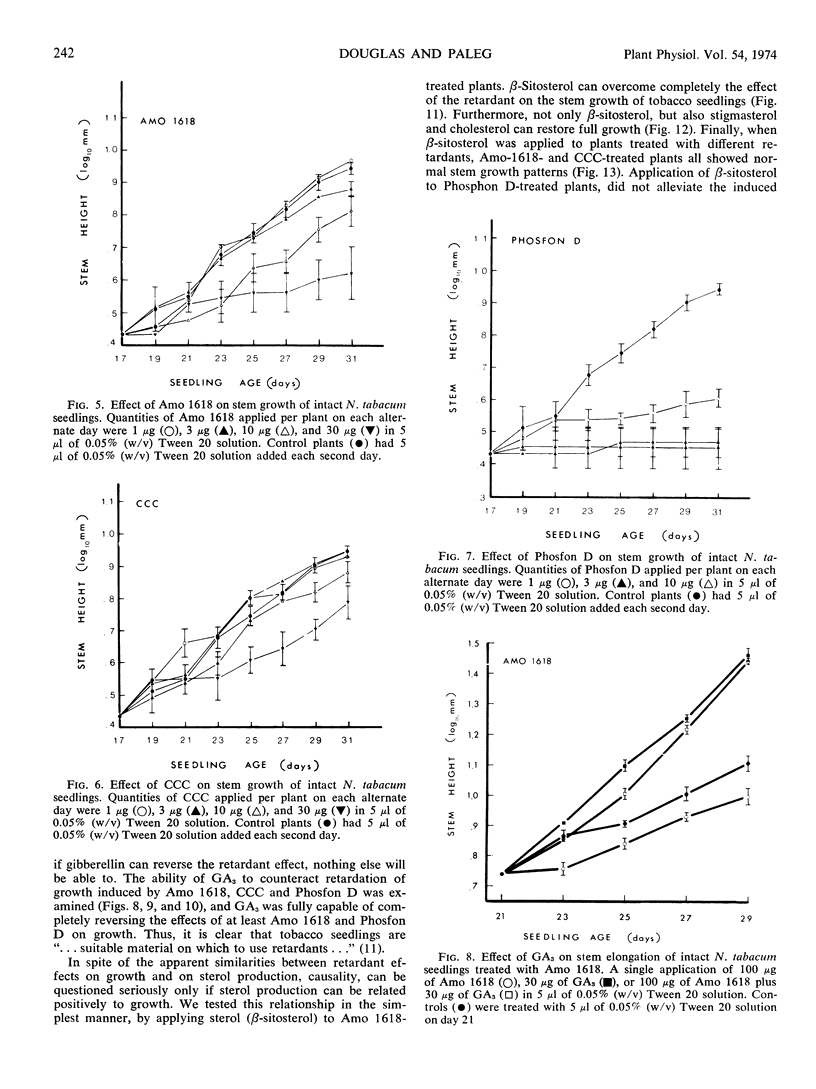
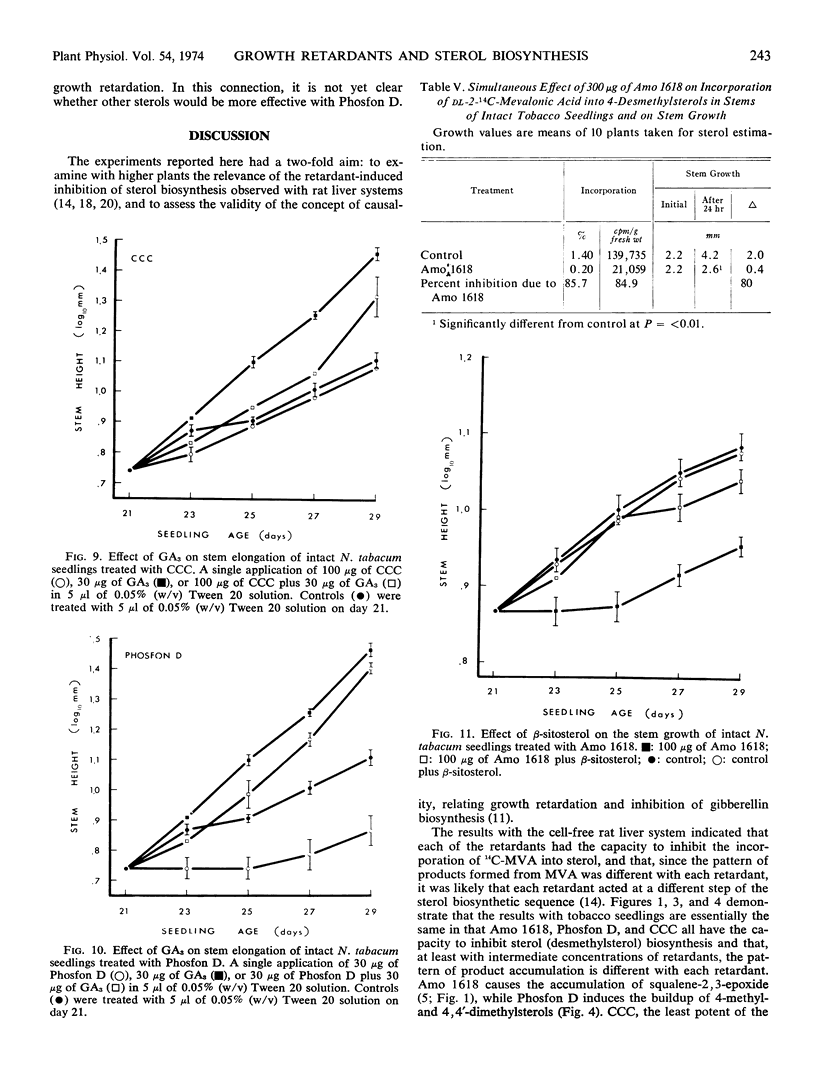
Three plant-growth retardants 2′-isopropy1-4′-(trimethylammonium chloride)-5-methylphenylpiperidine carboxylate (Amo 1618), β-chloroethyltrimethylammonium chloride, and tributyl-2, 4-dichlorobenzylphosphonium chloride were tested for their effects on sterol production in, and growth of tobacco (Nicotiana tabacum) seedlings. As the concentration of each retardant increased, there was an increased inhibition of the incorporation of dl-2-14C-mevalonic acid into sterol (particularly desmethylsterol) fractions and an increased retardation of stem growth. Growth retardation was observed with both single and repeated retardant treatments, and with Amo 1618, in particular, a close quantitative relationship between inhibition of sterol biosynthesis and stem growth was obtained. Gibberellic acid completely overcame retardant effects and application of sterols also restored normal growth. It is concluded that the concept of causality in the relationship between growth retardation and gibberellin biosynthesis is probably premature, since growth retardants have a more general inhibitory action on isoprenoid biosynthesis in plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt R. D., Benveniste P. Isolation and identification of sterols from subcellular fractions of bean leaves (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Sep 1;282(1):85–92. doi: 10.1016/0005-2736(72)90313-6. [DOI] [PubMed] [Google Scholar]
- Douglas T. J., Paleg L. G. Inhibition of Sterol Biosynthesis by 2-Isopropyl-4-dimethylamino-5-methylphenyl-1-piperidine Carboxylate Methyl Chloride in Tobacco and Rat Liver Preparations. Plant Physiol. 1972 Mar;49(3):417–420. doi: 10.1104/pp.49.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effect of sterols on the permeability of alcohol-treated red beet tissue. Plant Physiol. 1968 Apr;43(4):484–488. doi: 10.1104/pp.43.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971 Nov;48(5):653–655. doi: 10.1104/pp.48.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleg L. G. Differential inhibition of rat liver cholesterol biosynthesis by plant growth retardants. Nature. 1970 Mar 28;225(5239):1252–1253. doi: 10.1038/2251252a0. [DOI] [PubMed] [Google Scholar]
- Paleg L. G. Sites of action of plant growth retardants on cholesterol biosynthesis by cell-free rat liver preparations. Aust J Biol Sci. 1970 Dec;23(6):1115–1124. doi: 10.1071/bi9701115. [DOI] [PubMed] [Google Scholar]
- Paleg L., Kende H., Ninnemann H., Lang A. Physiological effects of gibberellic acid. 8. Growth retardants on barley endosperm. Plant Physiol. 1965 Jan;40(1):165–169. doi: 10.1104/pp.40.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleg L., Sabine J. R. Inhibition by plant growth retardants of cholesterol biosynthesis in slices of rat liver and hepatoma. Aust J Biol Sci. 1971 Dec;24(6):1125–1130. doi: 10.1071/bi9711125. [DOI] [PubMed] [Google Scholar]
- Robinson D. R., West C. A. Biosynthesis of cyclic diterpenes in extracts from seedlings of Ricinus communis L. II. Conversion of geranylgeranyl pyrophosphate into diterpene hydrocarbons and partial purification of the cyclization enzymes. Biochemistry. 1970 Jan 6;9(1):80–89. doi: 10.1021/bi00803a011. [DOI] [PubMed] [Google Scholar]
- Sabine J. R., Paleg L. G., Douglas T. J. The effects of a plant-growth retardant, Phosfon, on mammalian lipid metabolism in vivo. Aust J Biol Sci. 1973 Feb;26(1):113–122. [PubMed] [Google Scholar]
- Stowe B. B., Dotts M. A. Probing a membrane matrix regulating hormone action: I. The molecular length of effective lipids. Plant Physiol. 1971 Nov;48(5):559–565. doi: 10.1104/pp.48.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett J. D., Sharpless K. B., Lord K. E., van Tamelen E. E., Clayton R. B. Squalene-2,3oxide, an intermediate in the enzymatic conversion of squalene to lanosterol and cholesterol. J Biol Chem. 1967 Sep 25;242(18):4182–4191. [PubMed] [Google Scholar]



