Abstract
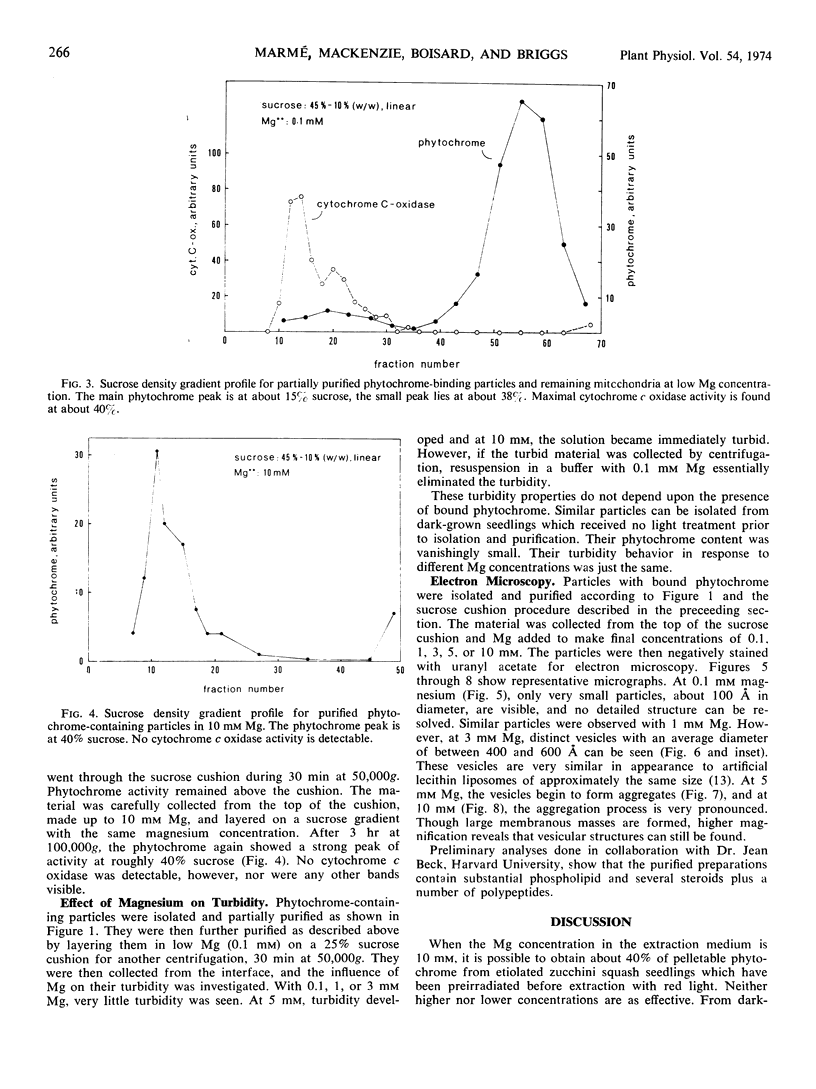
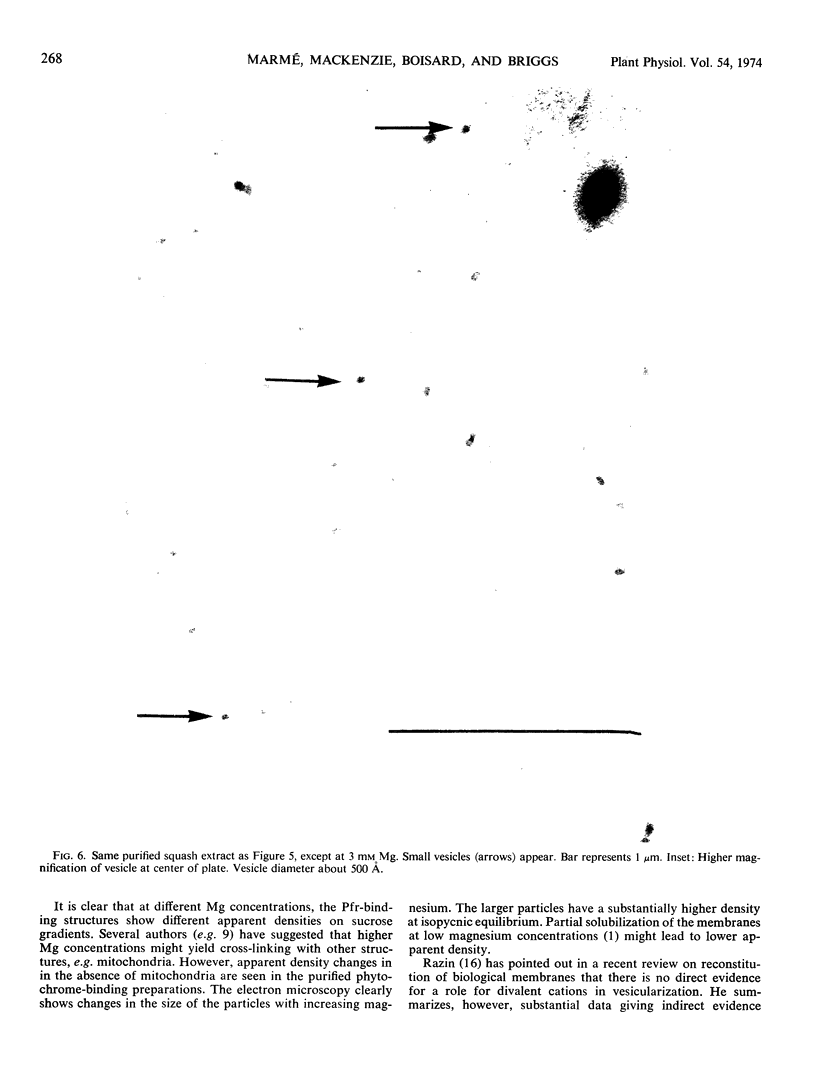
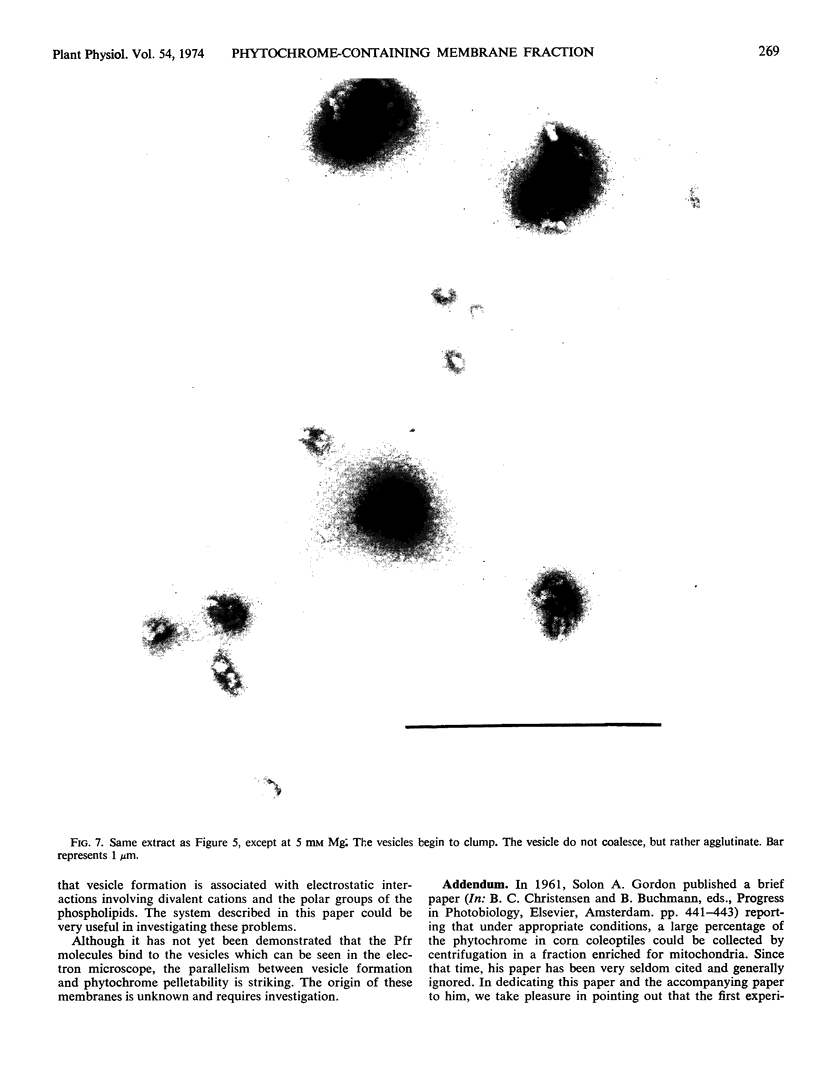
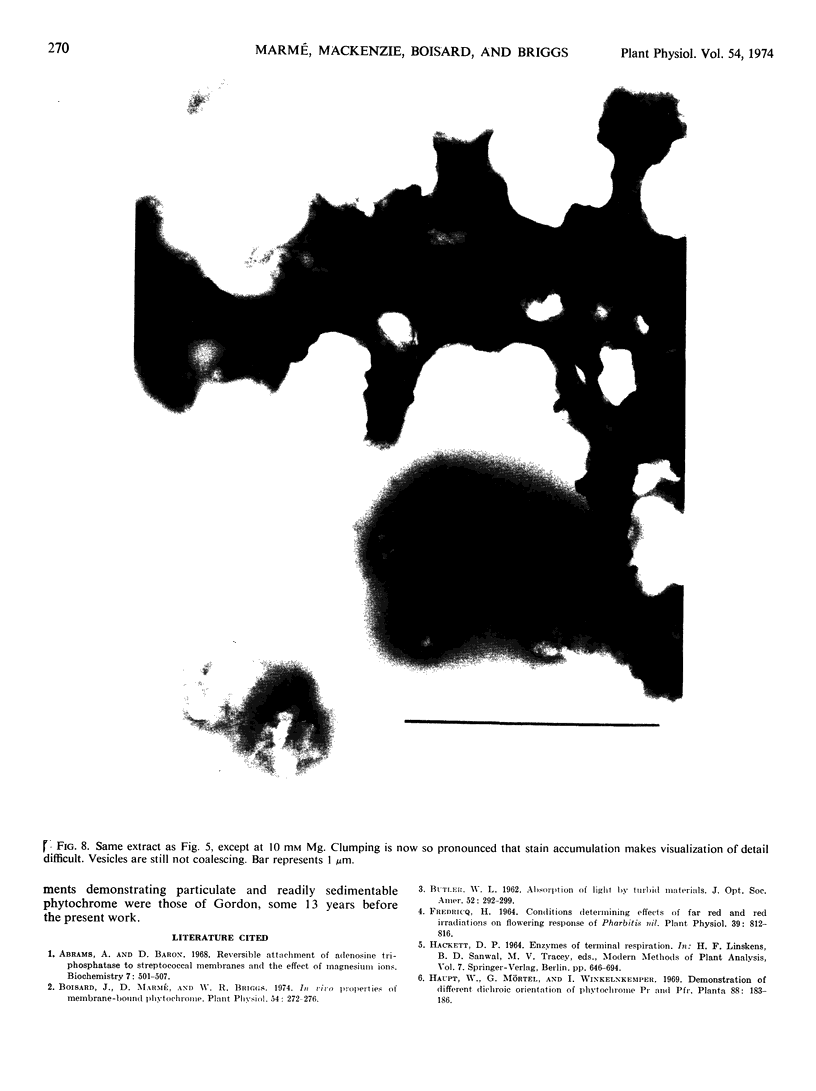
If 4-day-old dark-grown zucchini squash seedlings (Cucurbita pepo L. cv. Black Beauty) are exposed briefly to red light, subsequent cell fractionation yields about 40% of the total extractable phytochrome in the far red-absorbing form bound to a particulate fraction. The amount of far red-absorbing phytochrome in the pellet is strongly dependent on the Mg concentration in the extraction medium. The apparent density of the Pfr-containing particles following sedimentation on sucrose gradients corresponds to 15% (w/w) sucrose with 0.1 mm Mg and 40% sucrose with 10 mm Mg. This particulate fraction could be readily separated from mitochondria and other particulate material by taking advantage of these apparent density changes with changes in Mg concentration. Electron microscopy of negatively stained preparations shows that with 1 mm Mg only minute particles are present. These were too small to reveal structural detail with this technique. With 3 mm Mg, separate membranous vesicles between 400 and 600 Ångstroms in diameter appear. At higher Mg concentrations, the vesicles aggregate, causing obvious turbity. The effect of Mg on vesicle formation and aggregation is completely reversible. Above 10 mm Mg, vesicle aggregation persists, but the percentage of bound Pfr decreases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Baron C. Reversible attachment of adenosine triphosphatase to streptococcal membranes and the effect of magnesium ions. Biochemistry. 1968 Feb;7(2):501–507. doi: 10.1021/bi00842a003. [DOI] [PubMed] [Google Scholar]
- Boisard J., Marmé D., Briggs W. R. In Vivo Properties of Membrane-bound Phytochrome. Plant Physiol. 1974 Sep;54(3):272–276. doi: 10.1104/pp.54.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericq H. Conditions Determining Effects of Far-Red and Red Irradiations on Flowering Response of Pharbitis nil. Plant Physiol. 1964 Sep;39(5):812–816. doi: 10.1104/pp.39.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks S. B., Borthwick H. A. The function of phytochrome in regulation of plant growth. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2125–2130. doi: 10.1073/pnas.58.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmé D., Boisard J., Briggs W. R. Binding properties in vitro of phytochrome to a membrane fraction. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3861–3865. doi: 10.1073/pnas.70.12.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Briggs W. R. Phytochrome-mediated Electric Potential Changes in Oat Seedlings. Plant Physiol. 1972 Dec;50(6):687–693. doi: 10.1104/pp.50.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Rubinstein B., Drury K. S., Park R. B. Evidence for bound phytochrome in oat seedlings. Plant Physiol. 1969 Jan;44(1):105–109. doi: 10.1104/pp.44.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada T. Substances essential for a red, far-red light reversible attachment of mung bean root tips to glass. Plant Physiol. 1968 Dec;43(12):2070–2071. doi: 10.1104/pp.43.12.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]