Abstract
The Rhodobacter capsulatus response regulator CtrA controls the expression of 227 genes, some of which are upregulated by both the phosphorylated and unphosphorylated forms of CtrA. Therefore, CtrA concentration alone, regardless of phosphorylation state, may determine expression of downstream genes, yet little is known about the regulation of ctrA in R. capsulatus. In this study we used a ctrA : : lacZ fusion plasmid to study the effects of medium composition, growth conditions and growth phase on R. capsulatus ctrA gene expression. These experiments indicate that ctrA expression is higher when cultures are grown in phototrophic (anaerobic) conditions compared with chemotrophic (aerobic) conditions, and is higher when grown in a minimal medium compared with a rich medium. We used several mutants to investigate possible regulatory pathways, and found that in R. capsulatus ctrA is not autoregulated but is regulated by a quorum-sensing system. The expression of ctrA increased as cell cultures moved through exponential phase and into stationary phase, with high levels of expression persisting long after culture turbidity plateaued. Although this growth phase-dependent pattern of expression was also observed in a quorum-sensing mutant, the magnitude of ctrA expression was about 50 % of the wild-type strain at all phases. Furthermore, reduction of phosphate concentration in the growth medium decreased ctrA expression in a culture density-independent manner, whereas reduction of malic acid (carbon source) or ammonium (nitrogen source) concentration had no effect. The regulation of ctrA expression in R. capsulatus appears to require the coordination of multiple pathways involved in detecting a variety of environmental conditions.
INTRODUCTION
The ability to detect changing conditions and regulate gene expression accordingly is essential for the survival of all organisms. Bacteria use response regulator proteins in two-component and phosphorelay signalling systems for this purpose, and changes in the concentrations of response regulators affect the processes they control. The response regulator protein CtrA is widespread in the alphaproteo-bacteria, and has been found to be essential for cell viability in several species, best-documented in Caulobacter crescentus (Barnett et al., 2001; Hallez et al., 2004; Laub et al., 2000, 2002; Quon et al., 1996). In C. crescentus, the CtrA protein is a global regulator, and transcription of the ctrA gene is auto-regulated (Bowers et al., 2008).
In contrast with the well-studied C. crescentus and some other species of alphaproteobacteria, the CtrA protein of the nonsulfur purple photosynthetic bacterium Rhodobacter capsulatus is not essential for cell survival (Lang & Beatty, 2000). Two functions of the R. capsulatus CtrA are to induce flagellum biosynthesis and the production of an unusual virus-like particle called the gene transfer agent (RcGTA) (Lang & Beatty, 2000, 2002; Leung, 2010). A recent transcriptome study identified 227 genes in the R. capsulatus CtrA regulon by comparing gene expression of a ctrA mutant to the wild-type (WT) strain (Mercer et al., 2010). The majority of these genes (216) were upregulated by CtrA (i.e. increased expression in the WT strain compared with the ctrA mutant), by between twofold and more than 20-fold. In addition to the RcGTA and flagellar genes, a suite of potential signal transduction genes was identified as part of the CtrA regulon (Mercer et al., 2010).
Alignment of the C. crescentus and R. capsulatus CtrA protein sequence shows 71 % overall identity, and 100 % identity in the helix–turn–helix motif that is thought to specify recognition of consensus DNA sequences 5′-TTAA-N7-TTAAC-3′ and 5′-TTAACCAT-3′ (Lang & Beatty, 2000; Laub et al., 2002). However, many of the R. capsulatus CtrA-regulated genes lack a recognizable CtrA consensus sequence (Mercer et al., 2010), as in C. crescentus (Laub et al., 2002).
Many response regulators are regulated at the post-translational level by processes such as phosphorylation or binding of signalling molecules. However, although in C. crescentus the phosphorylated form of CtrA (CtrA~P) binds DNA with high affinity (Biondi et al., 2006; Domian et al., 1999), non-phosphorylated CtrA also binds to DNA to regulate gene expression (Spencer et al., 2009). Analogously, in R. capsulatus either the phosphorylated or non-phosphorylated form of CtrA induces RcGTA expression (Mercer et al., 2012). This implies that although the activation of RcGTA expression is dependent on the presence of CtrA, transcription of RcGTA genes occurs regardless of the CtrA phosphorylation state. In contrast, release of RcGTA particles and cell motility are induced by CtrA~P but not CtrA in R. capsulatus (Mercer et al., 2012).
Because of the similarities and curious differences in structural and phenotypic properties of the R. capsulatus and C. crescentus CtrA proteins, we investigated aspects of ctrA expression in R. capsulatus to improve the understanding of species-specific transcriptional regulation of this widespread alphaproteobacterial gene. Unlike C. crescentus, R. capsulatus does not undergo asymmetrical cell division, and synchronous cultures have not been obtained. Therefore we investigated ctrA expression in response to culture conditions and growth phase, and in several regulatory mutants.
A plasmid containing the R. capsulatus ctrA promoter region and the first eight codons fused translationally in-frame with the Escherichia coli lacZ coding sequence was used to monitor ctrA expression (transcription and translation). The reporter plasmid yielded data in accordance with independent measurements of ctrA mRNA amounts (Lang & Beatty, 2000), verifying both approaches, and indicating that regulation of transcription is the major process in the control of ctrA expression.
The effects of culture conditions on ctrA gene expression were studied, using cultures grown in a minimal or rich medium under illuminated phototrophic (anaerobic) or unilluminated chemotrophic (aerobic) conditions. The levels of ctrA expression as cultures progress through the exponential phase and long into the stationary phase of growth were also studied. Several mutant strains were evaluated for ctrA : : lacZ expression, revealing that the R. capsulatus ctrA gene is not autoregulated and that the gtaI gene is needed for maximal stationary phase expression of ctrA. The gtaI gene encodes an acylhomoserine lactone synthase (Schaefer et al., 2002) and functions in a quorum-sensing system (Leung et al., 2012). However, culture density alone does not control ctrA expression because cultures at a low culture density in stationary phase, due to carbon limitation, expressed ctrA : : lacZ at the same level as cultures grown to a high cell density in the replete culture medium.
A 5′ end of ctrA mRNA was mapped, and cis-active sequences in the 5′ regulatory region of the ctrA gene were found by measuring the activity of a series of deletions in a ctrA : : lacZ fusion plasmid. Promoter −10 and −35 regions were identified, and two inverted repeat sequences were found to be needed for maximal expression of ctrA.
Our results show greater variability from the C. crescentus model for the functions of ctrA in the alphaproteobacteria than previously thought, and that in R. capsulatus there are unusual connections between ctrA expression, quorum-sensing, culture growth phase and nutrient availability.
METHODS
Bacterial strains and growth conditions
E. coli strain DH10B was used for cloning, and HB101(pRK2013) (Ditta et al., 1985) was used for conjugation of plasmids into R. capsulatus. The E. coli strains were grown at 37 °C in Luria broth supplemented with appropriate antibiotics for plasmid selection and maintenance at the following concentrations: kanamycin sulphate, 50 μg ml−1; tetracycline/HCl, 10 μg ml−1.
The R. capsulatus strains B10 (WT) (Marrs, 1974), BCKF (ctrA mutant), BKKR (cckA mutant) (Lang & Beatty, 2002), BLKI (gtaI mutant), BLKR (gtaR mutant) and BLKO (gtaR/gtaI double mutant) (Leung et al., 2012) have been described. Except when noted otherwise, R. capsulatus strains were grown in either rich YPS (Wall et al., 1975) or minimal RCV (Beatty & Gest, 1981) media under phototrophic (anaerobic) or chemotrophic (aerobic) conditions at ~30 °C. YPS medium contains 0.3 % yeast extract and 0.3 % peptone, whereas RCV medium contains malate as the sole carbon source, and ammonium as the sole nitrogen source (see below). Media were supplemented with tetracycline/HCl (0.5 μg ml−1) and kanamycin sulphate (10 μg ml−1) as required for plasmid maintenance and strain selection. The media used for nutrient-limited experiments were identical to RCV medium except for the following changes: the medium with decreased nitrogen concentration contained 3.8 mM (NH4)2SO4 instead of 7.6 mM [under these conditions cultures produced hydrogen gas, indicating nitrogen starvation (Hillmer & Gest, 1977)]; the carbon-limited medium contained 10 mM D,L-malic acid instead of 30 mM; the phosphate-limited media contained 1.9 mM, 15 μM or no phosphate buffer instead of 9.6 mM, and were supplemented with 9.6 mM 3-morpholinopropanesulfonic acid (MOPS).
Culture turbidity was used as a measure of the number of cells ml−1 and monitored by measuring light-scattering with a Klett–Summerson photometer (filter no. 66; red); 100 Klett units represents approximately 4×108 c.f.u. ml−1. We defined the early stationary phase time point as 4 h, after which culture turbidity changed less than 10 Klett units in a 4 h interval.
Recombinant plasmids
Standard DNA purification, restriction enzyme digestion and other modification techniques were used (Sambrook et al., 1989). Plasmids are listed in Table 1, and descriptions of primers used in the construction of ctrA : : lacZ gene fusion plasmids are given in Table 2. The plasmid pXCA601 contains a promoterless lacZ allele with a BamHI site in the eighth codon, with transcription from the plasmid 5′ region blocked by a transcription terminator (Adams et al., 1989). Full-length and deleted segments of the ctrA 5′ region were generated by PCR amplification using WT R. capsulatus genomic DNA as the template, and the downstream primer CEPS2 that annealed within the ctrA gene and introduced a BamHI site for cloning purposes. The upstream primers annealed from 150 bp to 1.5 kb 5′ of the CEPS2 primer and introduced a PstI site. Each amplicon was digested with BamHI and PstI, and ligated with BamHI/PstI-digested pXCA601, yielding in-frame fusions between the eighth codon of the ctrA and the lacZ genes.
Table 1.
Plasmids used in the ctrA : : lacZ fusion plasmid and 5′ RACE experiments
| Plasmid (use) | Description | Marker* | Reference or source |
|---|---|---|---|
| ctrA : : lacZ fusion studies | |||
| pXCA601 | Promoter probe vector, for construction of promoter : : lacZ fusions | TcR | Adams et al. (1989) |
| p601-5 | 47 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-7 | 116 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-9 | 227 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-11.7 | 270 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-11 | 379 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-17 | 1463 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pXCA601 as a PstI to BamHI fragment | TcR | This study |
| p601-17x | p601-17 with a deletion of the predicted 210 and 235 sites, and surrounding sequences | TcR | This study |
| p601-SIR | p601-17 with a deletion of the SIR inverted repeat | TcR | This study |
| p601-IRD | p601-17 with a deletion of the IRD inverted repeat | TcR | This study |
| pUC19 | Cloning Vector, lacZa (Invitrogen) | ApR | Yanisch-Perron et al. (1985) |
| pUC19-17 | 1463 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pUC19 as a PstI to BamHI fragment | ApR | This study |
| pUC5x-17 | 47 bp 59 of ctrA start codon and the first 9 ctrA codons cloned into pUC19 as a XbaI to BamHI fragment | ApR | This study |
| pUC17x | Subclone for the PstI to BamHI fragment cloned into p601-17x | ApR | This study |
| 5′ RACE | |||
| pCR 4-TOPO | Plasmid used for TA cloning of PCR products | ApR | Invitrogen |
Tc, Tetracycline/HCl; Ap, ampicillin.
Table 2. Oligonucleotides used in the construction of ctrA : : lacZ fusion plasmids and mRNA 5′ end-mapping experiments.
All primers were generated for this study, except for AAP, AUAP, −21M13F and −21M13R, which were purchased from Invitrogen.
| Primer (use) | Primer sequence (5′–3′) |
|---|---|
| Constructing ctrA : : lacZ fusion plasmids | |
| CEPS2 | GAGAGGTCGTCGGATCCTCCTCCAC |
| CEPS 17 | GATCTGCAGCGAGACCTCCCGTTC |
| CEPS 15 | CCTTTTTCTGCAGGGCCGCGC |
| CEPS 13 | GCCCGACCTGCAGACGACCAC |
| CEPS 11 | GGTGAGCCCCTGCAGCTGAAAAAAG |
| CEPS11.7 | GCGTCGTTGGCTGCAGTCGCGCAAGG |
| CEPS 9 | CGCATGGTCTGCAGGGACGCTC |
| CEPS 7 | GATCGAGGGCTGCAGGCGGAGAAC |
| CEPS 5 | CGTGTTAACCATCTGCAGACAAGGTCG |
| CEPS5x | CGTGTTAACCATCTAGAGACAAGGTCGAACG |
| CEPS11.7x | CCTTGCGCGACTCTAGACAACGACG |
| IRDR | CGATGATCGCCTTGCGCGACTCTAGATCGGCCCGGGTCAGAAT |
| IRDF | ATTCTGACCCGGGCCGATCTAGAGTCGCGCAAGGCGATCATCG |
| SIRF | ATCTGAAAAAAGTGGATGGTCTAGACGATTCTGACCCGGGCCG |
| SIRR | CGGCCCGGGTCAGAATCGTCTAGACCATCCACTTTTTTCAGAT |
| mRNA 5′ end-mapping and sequencing | |
| AAP | GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG |
| AUAP | GGCCACGCGTCGACTAGTAC |
| Fustin | GTCCTCCACCAACAAAATCCGCATCC |
| Fustout | GATGCTGCGAGAGGTCGTCGGGTC |
| Fustnest | CGCATCCTGGGTTCTCCGCATTA |
| −21M13F | GTTTTCCCAGTCACGACGTTGTA |
| −21M13R | CAGGAAACAGCTATGACC |
The internal deletion of the ctrA promoter region in plasmid p601-17x was created as described above, using primers CEPS5x and CEPS2 to introduce an XbaI and BamHI site, respectively, into the amplicon downstream of the deletion, which was ligated into pUC19 as an XbaI–BamHI fragment, resulting in the plasmid pUC5x-17. The primers CEPS17 and CEPS11.7x were used to introduce a PstI and an XbaI site, respectively, to the upstream amplicon, which was ligated into pUC5x-17 as a PstI–XbaI fragment, resulting in the plasmid pUC17x. The PstI–BamHI fragment was excised from pUC17x and ligated into BamHI/PstI-digested pXCA601 resulting in the plasmid p601-17x.
Plasmids p601-SIR and p601-IRD, containing deletions of inverted repeats found in the ctrA gene regulatory region, were generated by site-directed mutagenesis. The ctrA upstream region was amplified using the oligonucleotide primers CEPS2 and CEPS17, and WT R. capsulatus genomic DNA as the template. This amplicon was ligated into pUC19 as a PstI–BamHI fragment, resulting in the plasmid pUC-c17. Site-directed mutagenesis of pUC-c17 using the mutagenic primers IRDR and IRDF to delete the inverted repeat IRD, or primers SIRR and SIRF to delete the inverted repeat SIR, resulting in the plasmids pUC-IRD and pUC-SIR, respectively. These plasmids were digested with PstI and BamHI, and the resulting fragments containing the deletions were ligated with BamHI/PstI-digested pXCA601, resulting in p601-IRD and p601-SIR, respectively.
Promoter sequence analysis, RNA 5′ end-mapping and β-galactosidase assays
Analysis of sequences for putative promoter −10 and −35 sites was done using the Softberry bacterial promoter prediction computer program BPROM (http://www.softberry.com/all.htm).
Total cellular RNA was isolated from WT R. capsulatus cultures grown under phototrophic (anaerobic) conditions in RCV medium and harvested at the early stationary phase using the RNeasy kit (Qiagen). Primer extension assays to map the 5′ end of the ctrA transcript were performed using the 5′ RACE kit (Invitrogen) and the oligonucleotide primers described in Table 2. Abridged anchor primer (AAP) and abridged universal amplification primer (AUAP) were provided in the 5′ RACE kit, and the oligonucleotide primers Fustin, Fustout and Fustnest were designed according to the 5′ RACE kit manufacturer’s specifications. The resultant amplicons were cloned into the pCR4-TOPO vector (Invitrogen) following the suggested protocol, and sequenced using the primers −21M13F and −21M13R.
β-Galactosidase specific activities of cells containing ctrA : : lacZ fusions were assayed as described previously (Leung, 2010) using sonication to break cells and the Lowry method (Peterson, 1983) with BSA as the standard to measure total protein, which was expressed as Miller units (mg total protein)−1 (MU mg−1).
RESULTS
Culture conditions and ctrA expression in WT cells
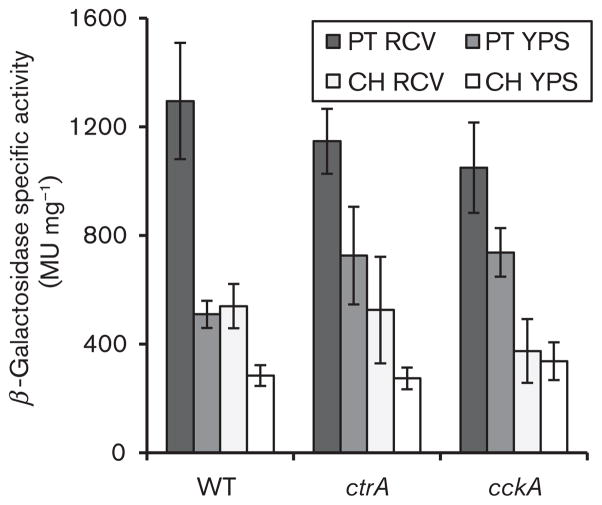
To begin to study the effects of culture medium nutrients and phototrophic versus chemotrophic energy generation on ctrA expression, WT cells containing the ctrA : : lacZ fusion plasmid p601-17 were collected at early stationary phase (see Methods) and assayed for β-galactosidase specific activity. The maximal growth rates (and cell numbers in the early stationary phase, given as Klett units in parentheses) of these cultures were: phototrophic YPS (513 Klett units) >phototrophic RCV (460 Klett units) >chemotrophic YPS (214 Klett units) >chemotrophic RCV (130 Klett units) (Fig. S1a, available with the online version of this paper). These experiments revealed that WT cells grown in RCV minimal medium under phototrophic (anaerobic) conditions had ~2.5 times more ctrA : : lacZ expression than cells grown in either YPS-rich medium under phototrophic conditions or cells grown in RCV medium under chemotrophic (aerobic) conditions, and almost five times more ctrA : : lacZ expression than cells grown in YPS medium under chemotrophic conditions (Fig. 1). These data show that the transcription and/or translation of ctrA is regulated in response to growth conditions, and that the mode of ATP synthesis (chemotrophy vs phototrophy) and the composition of the culture medium have additive effects on this regulation.
Fig. 1.
Growth conditions affect ctrA expression similarly in WT and regulatory mutant strains. WT, ctrA and cckA mutant strains containing the ctrA : : lacZ plasmid p601-17 were grown to early stationary phase under four different growth conditions, and β-galactosidase specific activity was determined as a measure of ctrA expression (n≥3). YPS, rich medium; RCV, minimal medium; PT, phototrophic (anaerobic, illuminated) culture conditions; CH, chemotrophic (aerobic, non-illuminated) culture conditions.
Expression of ctrA and nutrient limitation
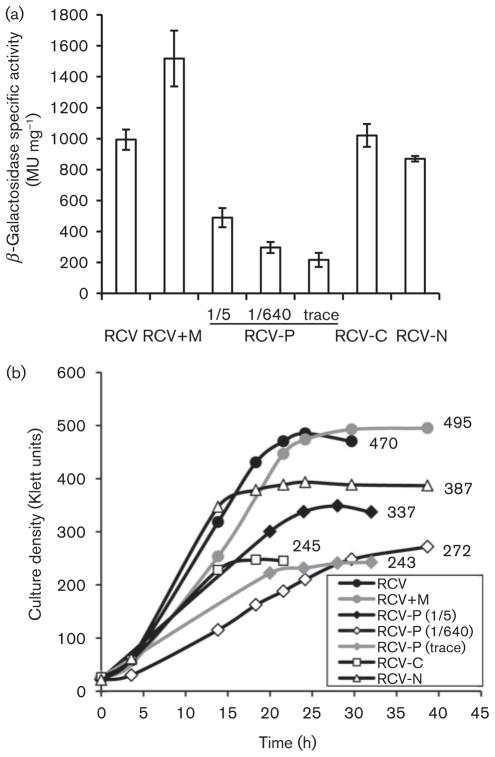
To further investigate the effects of growth conditions on the expression of ctrA, WT cells containing the plasmid p601-17 (ctrA : : lacZ fusion) were grown under phototrophic (anaerobic) conditions in minimal, defined, RCV-derived media that contained decreased concentrations of phosphate, nitrogen or carbon source (malic acid). These cultures were collected at early stationary phase and assayed for β-galactosidase specific activity. While decreased amounts of phosphate diminished ctrA : : lacZ expression to about 20–50 % compared with replete RCV medium, a decreased amount of nitrogen or carbon source had little effect on ctrA : : lacZ expression (Fig. 2a). Cultures grown in RCV supplemented with MOPS had increased ctrA : : lacZ expression, possibly due to increased buffering capability and greater cell concentrations. This indicates that the decreased expression seen in the phosphate-limited media (buffered with MOPS) is a phosphate effect and not a MOPS effect. One effect of phosphate-limited conditions is that cultures enter early stationary phase prior to the other conditions tested. Cultures grown in <1/5 phosphate-limited and carbon-limited media reached similar cell densities, about half that of cultures grown in replete RCV medium (Fig. 2b). Although these cultures plateaued at about the same culture density, cultures grown under carbon-limited conditions had ctrA : : lacZ expression levels similar to cultures grown in RCV medium, whereas cultures grown under all phosphate-limited conditions had significantly lower levels of expression (Fig. 2a). Thus, this nutrient-dependent control of ctrA : : lacZ expression is culture-density-independent, and instead relates to the type (phosphate vs carbon) of limitation.
Fig. 2.
Expression of ctrA is affected by phosphate limitation in a culture-density-independent manner. Cultures of WT cells containing ctrA : : lacZ plasmid p601-17 were grown under phototrophic (anaerobic, illuminated) conditions in RCV and nutrient-reduced RCV-derived media. The media contained: 9.6 mM phosphate (RCV); 9.6 mM MOPS in addition to phosphate (RCV+M); 1.9 mM phosphate (1/5); 15 μM phosphate (1/640); no phosphate (trace); 10 mM malic acid (RCV-C); 3.8 mM ammonium (RCV-N). The P-reduced medium was supplemented with MOPS buffer. (a) β-Galactosidase specific activities (n≥3). (b) Plot of culture cell density over time; the numbers in the graph give mean culture densities (in Klett units) at time of harvest, which varied by less than 20 % (n≥3).
Expression of ctrA in regulatory mutant strains
In C. crescentus, ctrA transcription is autoregulated by the CtrA protein and a cognate sensor kinase CckA. To determine whether R. capsulatus ctrA gene expression is regulated in a similar manner, the expression of the ctrA : : lacZ fusion in plasmid p601-17 was compared in ctrA mutant, cckA mutant and WT strains. After growth to the early stationary phase in minimal RCV medium, these three strains had essentially the same ctrA : : lacZ expression (Fig. 3). Other experiments comparing ctrA : : lacZ expression from p601-17 in the WT, ctrA and cckA knockout cultures grown under phototrophic or chemotrophic conditions in minimal RCV or rich YPS medium indicated that neither CtrA nor CckA affect ctrA expression under these conditions (Fig. 1). Furthermore, the growth rate and maximal growth density of the WT, ctrA and cckA mutant strains were similar when grown under the four growth conditions (Fig. S1).
Fig. 3.
Comparison of ctrA expression in the WT and ctrA, cckA, gtaI, gtaR and gtaRI mutant strains. Cells containing the ctrA : : lacZ plasmid p601-17 were grown to the early stationary phase in RCV medium under phototrophic (anaerobic, illuminated) conditions, and β-galactosidase specific activity was determined as a measure of ctrA expression (n≥3). The specific activity of WT cells containing the empty plasmid pXCA601 as a control is indicated (p601).
Because mutation of either ctrA or gtaI decreased RcGTA expression (Lang & Beatty, 2000; Schaefer et al., 2002), we were interested in determining whether ctrA expression is affected by mutation of the gtaI gene. GtaI is an acylhomoserine lactone synthase that is needed for quorum-sensing in R. capsulatus (Schaefer et al., 2002), which appears to involve the transcription regulator GtaR (Leung et al., 2012). As shown in Fig. 3, ctrA : : lacZ expression from the p601-17 plasmid in the gtaI mutant was less than 50 % of the WT level, indicating that the R. capsulatus GtaI protein is needed for maximal induction of ctrA transcription. Addition of exogenous C16-HSL to the gtaI mutant recovered ctrA expression to 77 % of the WT (data not shown). Further investigation into the regulatory role of the GtaI/GtaR quorum-sensing system showed that ctrA : : lacZ expression in gtaR and gtaRI mutant strains was similar to WT levels (Fig. 3). The expression profile of ctrA in these quorum-sensing mutants is similar to that of RcGTA (Leung et al., 2012), and indicates that GtaR functions as a negative regulator of ctrA in the absence of the acylhomoserine lactone synthesized by GtaI. Because CtrA is needed to induce transcription of RcGTA genes (Lang & Beatty, 2000) and our data show that GtaI is needed for maximal expression of the ctrA gene, it is possible that the reduced RcGTA expression in gtaI mutants is due to an indirect effect stemming from a decrease in ctrA expression.
Growth phase and ctrA expression
Previous RNA (Northern) blot and microarray experiments yielded a rough outline of ctrA expression over three time points in culture growth phases: very low expression in the mid-exponential phase, higher in the late-exponential, and highest in the early stationary phase of growth (Lang & Beatty, 2000, 2002; Mercer et al., 2010). To validate our use of ctrA : : lacZ expression as an index of ctrA transcription, and improve the understanding of the regulation of ctrA expression, a long-term experiment was done by measuring β-galactosidase specific activity in WT cells containing p601-17 at multiple time points, for 6 days after cultures entered the stationary phase (Fig. 4).
Fig. 4.
Effects of culture growth phase on ctrA expression. WT, ctrA and gtaI mutant cells containing the ctrA : : lacZ plasmid p601-17 were grown in RCV medium under phototrophic (anaerobic, illuminated) conditions. Samples were taken at the times indicated on the horizontal axis, and evaluated for β-galactosidase specific activity as a measure of ctrA expression (n≥3). Error bars show SD.
Focusing on the data obtained from the WT strain, the amount of ctrA : : lacZ expression increased from early- to late-exponential phases of growth, and even more in the early stationary phase. These data show that the results obtained with the plasmid-borne ctrA : : lacZ fusion mirror the results obtained using the Northern blot and transcriptome methods, and confirm that ctrA : : lacZ β-galactosidase specific activities parallel the amount of ctrA mRNA in WT cells. Interestingly, this long-term experiment also indicated that ctrA levels in WT cultures continue to increase well beyond early stationary phase (the 30 h time point) (Fig. 4). Therefore, although cultures are in the stationary phase of growth (i.e. no change in cell numbers as indicated by culture turbidity) after about 24 h under our standard conditions, the activity of the ctrA : : lacZ gene product increases to higher levels in successive days. After 144 h, the β-galactosidase specific activity eventually drops off (Fig. 4), presumably due to cell death.
Similar long-term experiments were done on the ctrA and gtaI mutant strains. As shown in Fig. 4, the pattern of the ctrA mutant is qualitatively almost identical to that of the WT strain. However, although the gtaI mutant followed a growth-phase-dependent pattern of ctrA : : lacZ expression similar to the WT strain, the β-galactosidase specific activity of the gtaI mutant was approximately 50 % of the WT value at each time point, with the exception of 168 h. These results confirm that ctrA expression can change independently of culture cell density, and indicate that ctrA expression is regulated by pathways both independent of and dependent on the GtaR/GtaI quorum-sensing system.
Identification of the ctrA promoter and cis-active regulatory sequences
The ctrA gene is flanked by ligA and sciP (Fig. 5a). In silico analysis of sequences 5′ of the ctrA gene identified a CtrA binding sequence, two inverted repeat sequences (SIR and IRD) and two sets of potential −10 and −35 sequences (Fig. 5b). An mRNA 5′ end of the ctrA transcript was mapped by the 5′ RACE method to the adenosine residue indicated by the asterisk in Fig. 5(b), confirming the predicted upstream −10 and −35 sequences (nt −208 to −178).
Fig. 5.
The ctrA gene, surrounding ORFs, and the sequence of the ctrA 5′ regulatory region. (a) Representation of ctrA and flanking orfs. Gene annotations are according to GenBank accession no. CP001312. From left to right: trmU is annotated as a tRNA (5-methylaminomethyl-2-thiouridylate)-methyltransferase; sciP encodes a transcription factor; ctrA encodes the CtrA response regulator homologue; and ligA is annotated as a NAD-dependent DNA ligase. (b) DNA sequence of the ctrA 5′ region, showing key features. Bent arrows show the ctrA and sciP start codons. Solid boxes and dashed boxes indicate the −35 and −10 sites predicted by the Softberry program BPROM (http://linux1.softberry.com/berry.phtml) and by visual inspection, respectively. Asterisk indicates the mRNA 5′ end mapped using 5′ RACE. Highlighted in grey is the stop codon of sciP. The black horizontal bars indicate the sequences of two inverted repeats, SIR and IRD, that were deleted in plasmids p601-SIR and p601-IRD, respectively. The underlined italicized sequence resembles a CtrA binding sequence. The vertical arrow labelled ‘2’ indicates the 3′ juncture of ctrA : : lacZ fusions. The vertical arrows labelled with odd numbers indicate the 5′ end of ctrA promoter region deletions. Each arrow is identified with a label that corresponds to the plasmid name (i.e., the arrow labelled ‘5’ corresponds to plasmid p601-5). Numbers on the right of each line indicate the number of bases before or after the ctrA start codon.
To characterize and confirm putative cis-active regulatory sequences in the ctrA promoter region, a series of progressively shorter segments of the 1.5 kb sequence 5′ of ctrA was used to create translationally in-frame fusions of the eighth codon of ctrA to the eighth codon of lacZ (as in p601-17) (Fig. 6). Each ctrA : : lacZ promoter deletion fusion plasmid (Table 1) was conjugated into the WT strain, cultures were grown in RCV medium under phototrophic (anaerobic) conditions, and cells were collected at early stationary phase and evaluated for β-galactosidase specific activity as a measure of ctrA expression. The longest 5′ fusion, p601-17, yielded a β-galactosidase specific activity of ~1400 MU mg−1, as did the deletion in plasmid p601-11 (Fig. 6), indicating that all cis-active elements necessary for full ctrA expression in cells grown under these conditions are found in the p601-11 plasmid. Plasmid p601-11 lacks all sequences 5′ of the ninth codon of the sciP gene, and so these results show that ctrA transcription is independent of possible sciP effects in cis, such as transcription read-through.
Fig. 6.

Expression of ctrA promoter region deletions. On the left are representations of ctrA : : lacZ fusions. WT cells containing ctrA : : lacZ promoter deletion plasmids (named p601-11, p601-SIR, p601-IRD, p601-11.7, p601-9, p601-7, p601-5 and p601-17x) were grown to the early stationary phase in RCV medium under phototrophic (anaerobic, illuminated) conditions and assayed for β-galactosidase specific activity (n≥3), as shown on the right.
Independent deletion of either of the inverted repeats called SIR (in plasmid p601-SIR) or IRD (in p601-IRD) resulted in little decrease in ctrA : : lacZ expression (Fig. 6). This is in contrast with the drop to 35 % when both of these inverted repeats were deleted in plasmid p601-11.7. These results indicate that the SIR and IRD inverted repeat sequences have a concerted modulatory role in ctrA expression, because the loss of either inverted repeat did not greatly affect expression whereas the loss of both resulted in a great reduction of ctrA : : lacZ expression. Plasmid p601-9, the fusion lacking all sequences 5′ of the promoter (Fig. 5b), yielded the same β-galactosidase specific activities as p601-11.7 (Fig. 6). The similarity in expression between p601-9 and p601-11.7 indicates that the 44 bp sequence difference between these two plasmids (i.e. the sequence between the IRD and −35 sequences) has no role in ctrA transcription.
Plasmid p601-7 lacks the upstream confirmed −10 and −35 sites and all further 5′ sequences, but contains the possible CtrA binding sequence and the downstream predicted −10 and −35 sites. Plasmid p601-7 yielded β-galactosidase specific activities ~10 % of p601-17 in the WT strain (Fig. 6) and similar activity in the ctrA mutant (8.7 % of p601-17; data not shown). Plasmid p601-5 contains only 45 bp 5′ of the ctrA start codon, and yielded little activity in both the WT and ctrA mutant strain. The plasmid p601-17x is identical to the plasmid p601-17 except for the absence of 226 bp that include the predicted −10 and −35 sites (Fig. 5b), and yielded almost no expression (Fig. 6). These results support the location of the −10 and −35 sites in the ctrA promoter region as shown in Fig. 5(b), and indicate that a positive regulatory protein binds to the SIR/IRD region.
DISCUSSION
In contrast with other alphaproteobacteria such as C. crescentus, CtrA is not essential for viability in R. capsulatus, yet CtrA is an important response regulator that affects the expression of ~227 R. capsulatus genes (Mercer et al., 2010), including the RcGTA genes induced by CtrA regardless of phosphorylation state (Mercer et al., 2012). Therefore the concentration of the CtrA protein, as specified by the level of ctrA gene expression, may determine the degree of induction of downstream genes. Here, we describe the results of experiments on the regulation of ctrA gene expression, with a focus on culture conditions and connections with other regulatory genes.
We found that a combination of growth in a minimal medium and phototrophic (anaerobic) conditions resulted in the highest level of ctrA expression. Furthermore, this effect appears to be independent of cell culture density and growth rate. This is because under phototrophic (anaerobic) conditions, although cultures grown in the YPS-rich medium had the highest cell density in the stationary phase, the ctrA : : lacZ expression was lower than in cells grown in RCV minimal medium. Cells grown in YPS medium under chemotrophic (aerobic) conditions expressed ctrA : : lacZ at a level similar to that of cells grown under phototrophic conditions (Fig. 1), despite differences in early stationary phase cell culture density. Collectively, these data show that differences in ctrA expression in response to growth conditions do not necessarily correlate with a difference in cell culture density or growth rate. In other words, the growth condition-associated modulation of ctrA expression is independent of cell culture density and growth rate differences.
Our investigation into the effects of nutrient-reduced RCV-derived media revealed that growth in a medium containing a low concentration of phosphate resulted in a cell density-independent decrease in ctrA expression compared with the replete RCV medium, whereas growth in either the nitrogen- or carbon-reduced medium had little effect. The decrease in ctrA expression (to 1/2, 1/3 or 1/5 the level in replete RCV medium) paralleled decreases in the phosphate concentration (from 9.6 mM to 1.9 mM, 15 μM or absent from the medium, respectively) and so there is not a simple linear relationship between ctrA expression and phosphate concentration. Similarly, growth of R. capsulatus in an RCV-derived medium with a decreased concentration of phosphate resulted in altered RcGTA release compared with replete RCV medium (Taylor, 2004). CtrA was shown to regulate RcGTA expression (Lang & Beatty, 2000, 2002; Mercer et al., 2010) and so we suggest that either a system in common detects phosphate levels and regulates both ctrA expression and RcGTA release, or increased RcGTA release under low phosphate concentration stems from a decrease in ctrA expression. Mercer et al. (2012) proposed that the phosphorylated form of CtrA is needed for release of RcGTA, but we did not address the phosphorylation state of CtrA. It was reported that in C. crescentus guano-sine 3′,5′-bispyrophosphate (ppGpp) and poly-phosphate decrease the rate of CtrA degradation in response to carbon starvation, although phosphate starvation was not studied (Boutte et al., 2012).
In contrast with C. crescentus, the R. capsulatus CtrA protein does not regulate transcription of the ctrA gene. Rather, we found that ctrA expression is affected by the GtaI/GtaR quorum-sensing system in a growth phase-independent manner. The expression of ctrA : : lacZ in gtaI mutant cells (i.e. in the absence of acylhomoserine lactone) was decreased to and remained at approximately 50 % of the level in a WT culture throughout all phases of growth. Thus, in the gtaI mutant, ctrA expression increased as cultures progressed from log to early stationary phase (Fig. 4), albeit to a lower magnitude than in the WT strain. These observations led us to suggest that aspects of the GtaI-dependent and growth-phase-dependent ctrA expression are regulated additively by independent systems. GtaI-dependent and growth-phase-dependent expression patterns of RcGTA were previously observed (Lang & Beatty, 2000; Schaefer et al., 2002), and our findings may indicate a regulatory mechanism based on the regulation of RcGTA transcription by CtrA (Lang & Beatty, 2000). We speculate that GtaI- and growth-phase-dependent changes in ctrA expression, and thus changes in the intracellular concentration of the CtrA protein, may be the cause of increases in RcGTA expression as cultures progress from exponential to stationary phase.
Using promoter prediction software, mRNA 5′ end-mapping and promoter deletion studies, we identified − 10 and −35 sequences of the major promoter of the ctrA gene. A second possible downstream promoter may contribute up to 10 % of the ctrA expression. Although a possible promoter sequence is located ~20 bp 3′ of a possible CtrA binding sequence, the expression from plasmids p601-5 and p601-7 relative to the full-length fusion in p601-17 was similar in the ctrA mutant and WT strains. This indicates that if there is a contribution to ctrA expression from this possible promoter, it is ~9–10 % of the total transcription initiated in the 1.5 kb region 5′ of the ctrA start codon, which we suggest is negligible. If this possible promoter had a measurable effect on ctrA transcription in WT cells, there would be a slight decrease in β-galactosidase activity encoded by plasmid p601-17 in the ctrA mutant relative to the WT strain, which was not seen (Fig. 3).
Little is known about R. capsulatus promoters and no consensus sequence has been published. One study of Rhodobacter promoters showed much variability in −10 and −35 sequences, with the predominant aspect being an increase in A/T residues relative to the average of 33.4 % for the entire genome (Swem et al., 2001). We suggest a consensus sequence (TTGAAC...N16...CAAAAT) using previously studied promoters, the ctrA and predicted gtaR and RcGTA promoters (Florizone, 2006). The ctrA major promoter −10 and −35 sequences share 6 of 6 bases and 4 of 6 bases, respectively, with the consensus sequence (Fig. 7). In contrast, the downstream possible promoter −35 and −10 sequences share only 3 of 6 and 2 of 6 bp, respectively, consistent with the low amount of transcription initiation associated with this region of ctrA 5′ sequences. The ctrA transcriptional and translational start sites are separated by 173 bp, and it is conceivable that these sequences are involved in post-transcriptional regulation, but we did not explore this possibility.
Fig. 7.

Alignment of R. capsulatus promoter −10 and −35 sequences. Promoters were identified in previous publications (Du et al., 1998; Dubbs et al., 2000; Elsen et al., 2000; Florizone, 2006; Karls et al., 1999; Leung, 2010; Swem et al., 2001; Vichivanives et al., 2000) and this study. The frequency of bases found at each position is indicated by the size of the coloured letters on the bottom, created by the Weblogo 3.0 software (Crooks et al., 2004; Schneider & Stephens, 1990).
We identified two inverted repeats (SIR and IRD) in the ctrA promoter region that are necessary for maximal expression of ctrA. To test the role of these two inverted repeats in growth-condition-modulated and quorum-sensing-regulated expression of ctrA, we measured the β-galactosidase activities from the fusions shown in Fig. 6 in WT cells grown under four different conditions, and in quorum-sensing mutants (gtaI, gtaR and gtaIR mutants). The results showed that these inverted repeats are needed for the growth-condition-modulated (Fig. S2) but not for quorum-sensing-regulated (Fig. S3) expression of ctrA. Specifically, two general growth-condition-modulated regulatory patterns disappeared when the inverted repeats were deleted: 1) the higher ctrA expression after growth under phototrophic (anaerobic) conditions than chemotrophic (aerobic) conditions; and 2) the higher ctrA expression after growth in RCV medium than YPS medium (Fig. S2). Because these inverted repeats are located 5′ of the −10 and −35 sequences, we suggest that a transcription factor(s) binds to these sequences to induce transcription initiation. The activity from the promoter deletion plasmids in the quorum-sensing gtaI mutant show a similar trend as in the WT strain, but at approximately threefold lower levels. This indicates that the SIR and IRD inverted repeats are not involved in quorum-sensing-mediated regulation of ctrA, which is not surprising because although we show that the GtaI/GtaR quorum-sensing system regulates CtrA, other work (Leung et al., 2012) showed that this quorum-sensing system indirectly regulates ctrA expression.
In conclusion, we studied the effects of growth conditions, growth phase and several regulatory proteins on the expression of the ctrA gene to better understand the role of the CtrA protein in R. capsulatus. As in other species, phosphorylation of CtrA is required for induction of downstream processes, which in R. capsulatus include RcGTA release and cell motility; however, CtrA concentration is also important because CtrA in both the phosphorylated and unphosphorylated states induces RcGTA expression (Mercer et al., 2012). It is possible that, as in other species, phosphorylation of the R. capsulatus CtrA protein changes the affinity for DNA sequences, and it is conceivable that the RcGTA gene cluster has two CtrA binding sites, one for CtrA~P and one for CtrA, both of which induce RcGTA expression. Because the CtrA-dependent induction of RcGTA genes is not affected by the phosphorylation of CtrA, the cellular levels of CtrA should be tightly controlled. Although the ctrA : : lacZ constructs reflect the frequency of ctrA transcription initiation, post-transcriptional regulation through 5′ mRNA sequences and translation initiation, our data do not address the possiblility of differential protein degradation rates on controlling CtrA concentration. Nevertheless, we discovered that expression of ctrA responds to multiple environmental conditions, and its regulation appears to involve multiple pathways and factors. Our work provides a foundation for future experiments to elucidate the details of these pathways and factors, and address the possiblity that CtrA degradation contributes to maintenance of biologically active CtrA levels.
Supplementary Material
Acknowledgments
This research was supported by grants from the Canadian Institutes for Health Research, and the Canada Council for the Arts (a Killam Research Fellowship to J. T. B.). We thank A. Lang (Dept. of Biology, Memorial University) for suggestions about and insights into R. capsulatus RcGTA and CtrA.
Abbreviations
- MOPS
3-morpholinopropanesulfonic acid
- MU mg−1
Miller units per mg
- WT
wild-type
Footnotes
Three supplementary figures are available with the online version of this paper.
References
- Adams CW, Forrest ME, Cohen SN, Beatty JT. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989;171:473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MJ, Hung DY, Reisenauer A, Shapiro L, Long SR. A homolog of the CtrA cell cycle regulator is present and essential in Sinorhizobium meliloti. J Bacteriol. 2001;183:3204–3210. doi: 10.1128/JB.183.10.3204-3210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty JT, Gest H. Biosynthetic and bioenergetic functions of citric acid cycle reactions in Rhodopseudomonas capsulata. J Bacteriol. 1981;148:584–593. doi: 10.1128/jb.148.2.584-593.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, Laub MT. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- Boutte CC, Henry JT, Crosson S. ppGpp and polyphosphate modulate cell cycle progression in Caulobacter crescentus. J Bacteriol. 2012;194:28–35. doi: 10.1128/JB.05932-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers LM, Shapland EB, Ryan KR. Who’s in charge here? Regulating cell cycle regulators. Curr Opin Microbiol. 2008;11:547–552. doi: 10.1016/j.mib.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang XW, Finlay DR, Guiney D, Helinski DR. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Reisenauer A, Shapiro L. Feedback control of a master bacterial cell-cycle regulator. Proc Natl Acad Sci U S A. 1999;96:6648–6653. doi: 10.1073/pnas.96.12.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Bird TH, Bauer CE. DNA binding characteristics of RegA. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J Biol Chem. 1998;273:18509–18513. doi: 10.1074/jbc.273.29.18509. [DOI] [PubMed] [Google Scholar]
- Dubbs JM, Bird TH, Bauer CE, Tabita FR. Interaction of CbbR and RegA* transcription regulators with the Rhodobacter sphaeroides cbbI promoter-operator region. J Biol Chem. 2000;275:19224–19230. doi: 10.1074/jbc.M002125200. [DOI] [PubMed] [Google Scholar]
- Elsen S, Dischert W, Colbeau A, Bauer CE. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J Bacteriol. 2000;182:2831–2837. doi: 10.1128/jb.182.10.2831-2837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florizone SM. PhD thesis. Dept. of Microbiology and Immunology, University of British Columbia; 2006. Studies on the regulation of the Gene Transfer Agent (GTA) of Rhodobacter capsulatus. [Google Scholar]
- Hallez R, Bellefontaine AF, Letesson JJ, De Bolle X. Morphological and functional asymmetry in alpha-proteobacteria. Trends Microbiol. 2004;12:361–365. doi: 10.1016/j.tim.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hillmer P, Gest H. H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol. 1977;129:724–731. doi: 10.1128/jb.129.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karls RK, Wolf JR, Donohue TJ. Activation of the cycA P2 promoter for the Rhodobacter sphaeroides cytochrome c2 gene by the photosynthesis response regulator. Mol Microbiol. 1999;34:822–835. doi: 10.1046/j.1365-2958.1999.01649.x. [DOI] [PubMed] [Google Scholar]
- Lang AS, Beatty JT. Genetic analysis of a bacterial genetic exchange element: the gene transfer agent of Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 2000;97:859–864. doi: 10.1073/pnas.97.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AS, Beatty JT. A bacterial signal transduction system controls genetic exchange and motility. J Bacteriol. 2002;184:913–918. doi: 10.1128/jb.184.4.913-918.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, McAdams HH, Feldblyum T, Fraser CM, Shapiro L. Global analysis of the genetic network controlling a bacterial cell cycle. Science. 2000;290:2144–2148. doi: 10.1126/science.290.5499.2144. [DOI] [PubMed] [Google Scholar]
- Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci U S A. 2002;99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MM-L. PhD thesis. Dept. of Microbiology and Immunology, University of British Columbia; 2010. CtrA and GtaR: two systems that regulate the Gene Transfer Agent in Rhodobacter capsulatus. [Google Scholar]
- Leung MM, Brimacombe CA, Spiegelman GB, Beatty JT. The GtaR protein negatively regulates transcription of the gtaRI operon and modulates gene transfer agent (RcGTA) expression in Rhodobacter capsulatus. Mol Microbiol. 2012;83:759–774. doi: 10.1111/j.1365-2958.2011.07963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974;71:971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RG, Callister SJ, Lipton MS, Pasa-Tolic L, Strnad H, Paces V, Beatty JT, Lang AS. Loss of the response regulator CtrA causes pleiotropic effects on gene expression but does not affect growth phase regulation in Rhodobacter capsulatus. J Bacteriol. 2010;192:2701–2710. doi: 10.1128/JB.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RG, Quinlan M, Rose AR, Noll S, Beatty JT, Lang AS. Regulatory systems controlling motility and gene transfer agent production and release in Rhodobacter capsulatus. FEMS Microbiol Lett. 2012;331:53–62. doi: 10.1111/j.1574-6968.2012.02553.x. [DOI] [PubMed] [Google Scholar]
- Peterson GL. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schaefer AL, Taylor TA, Beatty JT, Greenberg EP. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J Bacteriol. 2002;184:6515–6521. doi: 10.1128/JB.184.23.6515-6521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider TD, Stephens RM. Sequence logos: a new way to display consensus sequences. Nucleic Acids Res. 1990;18:6097–6100. doi: 10.1093/nar/18.20.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer W, Siam R, Ouimet MC, Bastedo DP, Marczynski GT. CtrA, a global response regulator, uses a distinct second category of weak DNA binding sites for cell cycle transcription control in Caulobacter crescentus. J Bacteriol. 2009;191:5458–5470. doi: 10.1128/JB.00355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swem LR, Elsen S, Bird TH, Swem DL, Koch HG, Myllykallio H, Daldal F, Bauer CE. The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J Mol Biol. 2001;309:121–138. doi: 10.1006/jmbi.2001.4652. [DOI] [PubMed] [Google Scholar]
- Taylor TA. PhD thesis. Dept. of Microbiology and Immunology, University of British Columbia; 2004. Evolution and Regulation of the Gene Transfer Agent (GTA) of Rhodobacter capsulatus. [Google Scholar]
- Vichivanives P, Bird TH, Bauer CE, Tabita FR. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J Mol Biol. 2000;300:1079–1099. doi: 10.1006/jmbi.2000.3914. [DOI] [PubMed] [Google Scholar]
- Wall JD, Weaver PF, Gest H. Gene transfer agents, bacteriophages, and bacteriocins of Rhodopseudomonas capsulata. Arch Microbiol. 1975;105:217–224. doi: 10.1007/BF00447140. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.