Abstract
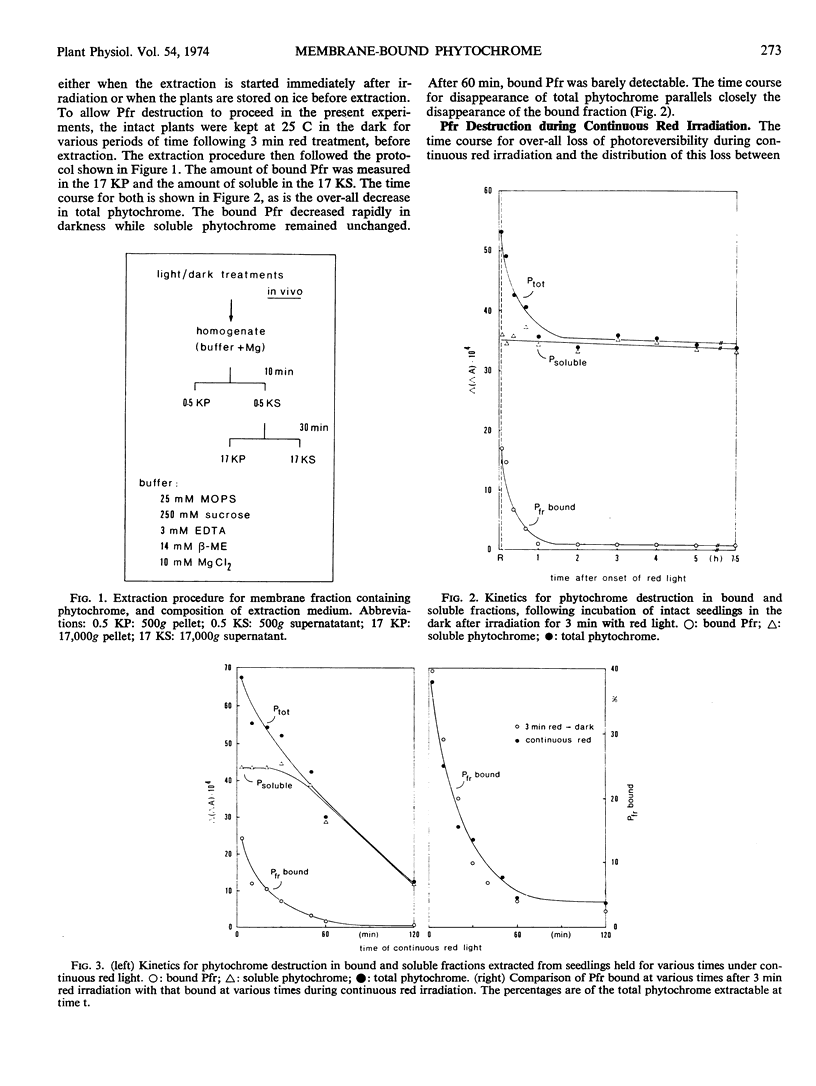
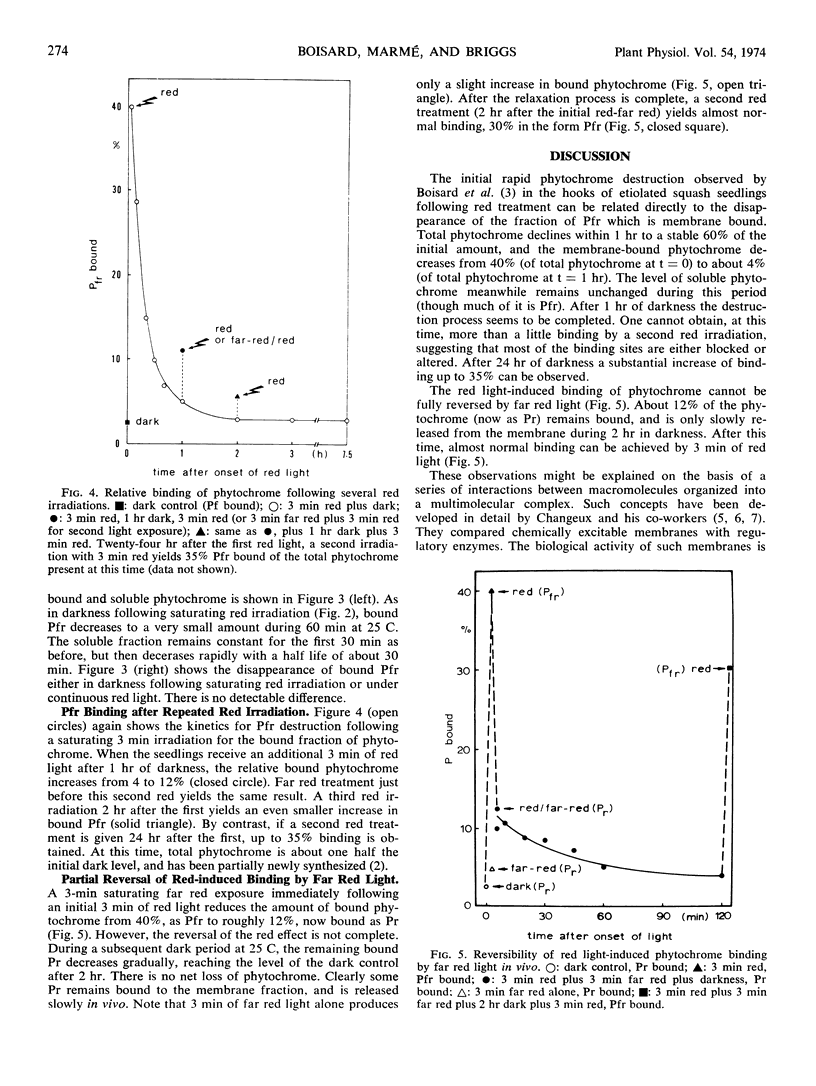
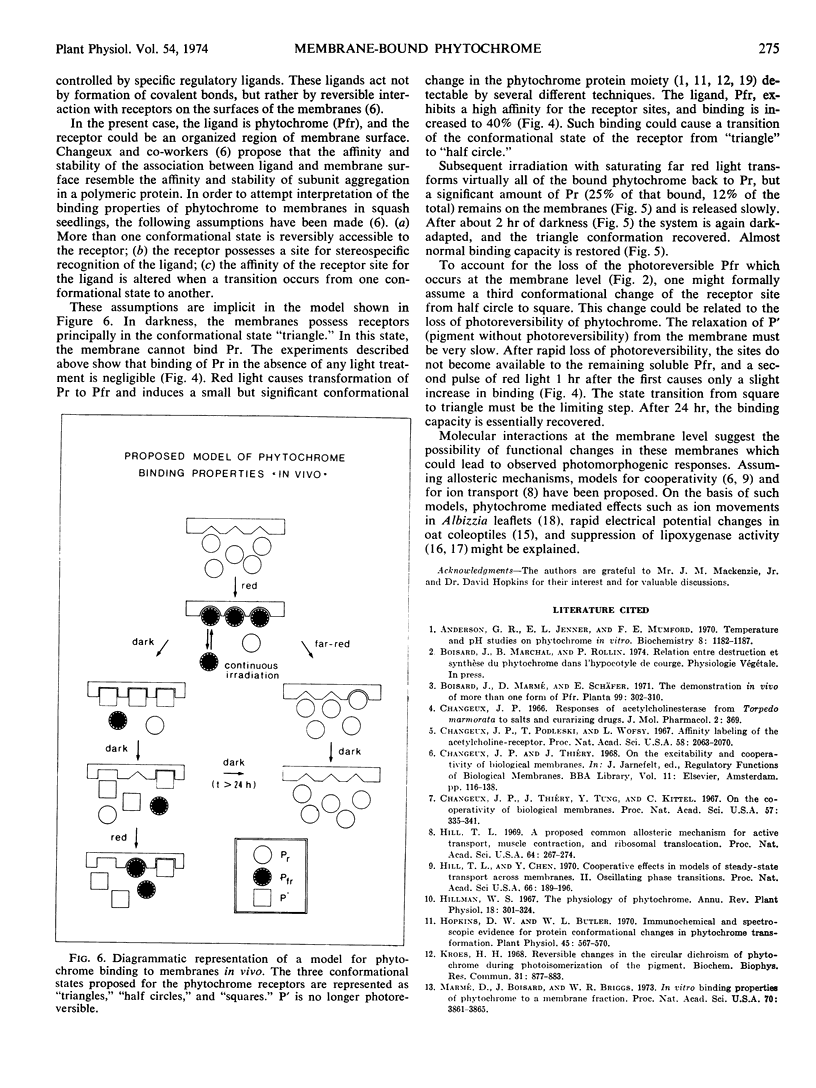
After a 3-minute irradiation with red light, which saturates the phototransformation from the red light-absorbing form of phytochrome to the far red light absorbing form of phytochrome, about 40% of the phytochrome extractable from hooks of etiolated squash seedlings (Cucurbita pepo L. cv. Black Beauty) can be pelleted as Pfr at 17,000g after 30 minutes. Dark controls yield only 2 to 4% pelletable phytochrome in the form Pr. If a dark period intervenes between red irradiation and extraction, the bound Pfr gradually loses its photoreversibility. The time course for this destruction parallels the time course for phytochrome destruction in vivo following saturating red irradiation. The soluble fraction of phytochrome remains constant. These results suggest that in squash seedlings phytochrome destruction is related exclusively to the fraction which becomes membrane-bound. The induction of phytochrome binding by red light is not completely reversible by far red. In plants given saturating red followed immediately by saturating far red light, 12% of the phytochrome is found in the bound fraction as Pr if the phytochrome extraction is immediate. If a dark period intervenes between red-far red treatment and extraction, the bound phytochrome is released within 2 hours. A model of the binding properties of phytochrome, based on molecular interaction at the membrane is proposed, and possible consequences for the mechanism of action of phytochrome are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Jenner E. L., Mumford F. E. Temperature and pH studies on phytochrome in vitro. Biochemistry. 1969 Mar;8(3):1182–1187. doi: 10.1021/bi00831a052. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R., Wofsy L. Affinity labeling of the acetylcholine-receptor. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2063–2070. doi: 10.1073/pnas.58.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. A proposed common allosteric mechanism for active transport, muscle contraction, and ribosomal translocation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):267–274. doi: 10.1073/pnas.64.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. Cooperative effects in models of steady-state transport across membranes. II. Oscillating phase transition. Proc Natl Acad Sci U S A. 1970 May;66(1):189–196. doi: 10.1073/pnas.66.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D. W., Butler W. L. Immunochemical and spectroscopic evidence for protein conformational changes in phytochrome transformations. Plant Physiol. 1970 May;45(5):567–570. doi: 10.1104/pp.45.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroes H. H. Reversible changes in the circular dichroism of phytochrome during photoisomerisation of the pigment. Biochem Biophys Res Commun. 1968 Jun 28;31(6):877–883. doi: 10.1016/0006-291x(68)90533-0. [DOI] [PubMed] [Google Scholar]
- Marmé D., Boisard J., Briggs W. R. Binding properties in vitro of phytochrome to a membrane fraction. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3861–3865. doi: 10.1073/pnas.70.12.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Briggs W. R. Phytochrome-mediated Electric Potential Changes in Oat Seedlings. Plant Physiol. 1972 Dec;50(6):687–693. doi: 10.1104/pp.50.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelze-Karow H., Schopfer P., Mohr H. Phytochrome-mediated repression of enzyme synthesis (lipoxygenase): a threshold phenomenon. Proc Natl Acad Sci U S A. 1970 Jan;65(1):51–57. doi: 10.1073/pnas.65.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M., Briggs W. R. Studies on the protein comformation of phytochrome. Photochem Photobiol. 1973 Dec;18(6):487–495. doi: 10.1111/j.1751-1097.1973.tb06454.x. [DOI] [PubMed] [Google Scholar]



