Abstract
Background
The sacroiliac joint is a common but under-recognized source of low back and gluteal pain. Patients with degenerative sacroiliitis or sacroiliac joint disruption resistant to nonsurgical treatments may undergo open surgery with sacroiliac joint arthrodesis, although outcomes are mixed and risks are significant. Minimally invasive sacroiliac joint arthrodesis was developed to minimize the risk of iatrogenic injury and to improve patient outcomes compared with open surgery.
Methods
Between April 2009 and January 2013, 5319 patients were treated with the iFuse SI Joint Fusion System® for conditions including sacroiliac joint disruption and degenerative sacroiliitis. A database was prospectively developed to record all complaints reported to the manufacturer in patients treated with the iFuse device. Complaints were collected through spontaneous reporting mechanisms in support of ongoing mandatory postmarket surveillance efforts.
Results
Complaints were reported in 204 (3.8%) patients treated with the iFuse system. Pain was the most commonly reported clinical complaint (n = 119, 2.2%), with nerve impingement (n = 48, 0.9%) and recurrent sacroiliac joint pain (n = 43, 0.8%) most frequently cited. All other clinical complaints were rare (≤0.2%). Ninety-six revision surgeries were performed in 94 (1.8%) patients at a median follow-up of four (range 0–30) months. Revisions were typically performed in the early postoperative period for treatment of a symptomatic malpositioned implant (n = 46, 0.9%) or to correct an improperly sized implant in an asymptomatic patient (n = 10, 0.2%). Revisions in the late postoperative period were performed to treat symptom recurrence (n = 34, 0.6%) or for continued pain of undetermined etiology (n = 6, 0.1%).
Conclusion
Analysis of a postmarket product complaints database demonstrates an overall low risk of complaints with the iFuse SI Joint Fusion System in patients with degenerative sacroiliitis or sacroiliac joint disruption.
Keywords: arthrodesis, iFuse, lumbar, minimally invasive, sacroiliac
Introduction
Low back pain is one of the most common reasons for physician visits and hospitalization in the US.1,2 The sacroiliac joint is a common but underappreciated source of low back pain, largely due to the challenges inherent in accurate differential diagnosis. Consequently the prevalence of sacroiliac joint-generated low back pain is not well characterized. The sacroiliac joint is the primary pain generator in approximately one in four cases of chronic low back pain.35 Pain-generating sacroiliac joint pathology may include joint degeneration secondary to degenerative sacroiliitis, osteoarthritis, or sacroiliac joint disruption.6 Up to 75% of patients develop radiographic evidence of sacroiliac joint degeneration after lumbar fusion surgery7 and failed back syndrome is often attributable to overlooking and undertreating the sacroiliac joint as the primary source of pain.8,9
Initial management strategies for sacroiliac joint pain utilize nonsurgical approaches such as analgesics, nonsteroidal anti-inflammatory drugs, and attempted correction of underlying biomechanical pathology via orthotics, chiropractic, or physical therapy. Resistant cases may be treated with intra-articular steroid injections or radiofrequency denervation, but pain relief is variable and often temporary (less than one year).10 A recent systematic review concluded that there was insufficient evidence to recommend commonly utilized nonsurgical treatment options for sacroiliac joint pain, including poor evidence to recommend intra-articular steroid injections, periarticular injections, botulinum toxin injection, conventional radiofrequency neurotomy, and pulsed radiofrequency, and only fair evidence to recommend cooled radiofrequency neurotomy10 Surgical sacroiliac joint arthrodesis is considered a last resort for cases recalcitrant to nonsurgical approaches. Limited evidence from small case series and retrospective studies suggests that the success rate with open surgical intervention is approximately 70%.11,12 Significant iatrogenic injury risks include blood loss, neurovascular injury, disruption of musculoligamentous structures, residual pain due to autograft harvesting, and extended hospitalization.6,13–16 Additionally, patients may need to avoid weight-bearing activity for several months following this invasive surgical procedure to allow healing.
Minimally invasive sacroiliac joint fixation and fusion techniques have recently gained popularity and were developed in an effort to reduce iatrogenic complaints and improve patient outcomes compared with open sacroiliac joint arthrodesis.17,18 The iFuse SI Joint Fusion System® (iFuse system, SI-BONE, Inc., San Jose, CA, USA) utilizes a minimally invasive approach and novel implants that may overcome the limitations of traditional open sacroiliac joint arthrodesis. The iFuse system now represents the predominant surgical treatment in the US for patients with degenerative sacroiliitis and sacroiliac joint disruption, based on data from a 2012 International Society for the Advancement of Spine Surgery survey. Through January 2013, 5319 patients have been treated with iFuse implants. Isolated case series with the iFuse system suggest acceptable midterm clinical outcomes with few reported complaints.11,12 The purpose of the current study was to provide a detailed characterization of complaints reported with the iFuse system by performing an evaluation and analysis of the manufacturer’s postmarket complaints database.
Materials and methods
Description
The iFuse system received a 510(k) marketing clearance in November 2008 and CE Mark in November 2010. The iFuse system was originally intended for “fracture fixation of the large bones and large bone fragments of the pelvis”. A subsequent 510(k) marketing clearance in April 2011 clarified the intended use for “sacroiliac joint fusion”. The indication for the iFuse system has always included treatment of degenerative sacroiliitis and sacroiliac joint disruption.
Typically, three or four iFuse devices are implanted in a lateral-to-medial direction across the diseased sacroiliac joint via a minimally invasive incision. Each titanium implant is triangular in shape and utilizes an interference fit to minimize micromotion and rotation. The implant surface is covered with a porous plasma spray coating. Implants are available in either a 4.0 mm or 7.0 mm inscribed diameter and range in length from 30 mm to 70 mm, depending on patient anatomy (Figure 1). The final implanted construct is characterized by a large surface area, providing superior shear (3×) and bending (8×) strength compared with traditional threaded screws, based on the manufacturer’s preclinical device testing.
Figure 1.

The iFuse SI Joint Fusion System® (SI-BONE, Inc., San Jose, CA, USA)
Note: The rigid titanium implants are available in 4 mm and 7 mm diameters with lengths ranging from 30 mm to 70 mm.
Procedural details
Procedural steps with the iFuse system have been previously detailed.11,12 The procedure is performed under general anesthesia with the patient in the prone position. The entire procedure is monitored using lateral, inlet, and outlet views on fluoroscopy. The gluteal fascia is penetrated bluntly via a 3 cm incision and the muscle is split longitudinally to access the outer table of the ilium. A Steinmann pin is placed through the ilium across the sacroiliac joint and into the lateral portion of the sacrum (Figure 2A). The pin, the operative instruments, and the implants are placed in such a manner as to stay within the osseous envelope of the sacrum and to avoid the spinal canal and the neuroforamina (Figure 2B). A depth gauge is then used to determine implant length. Bone is prepared using a drill (Figure 2C) and triangular broach (Figure 2D) through a cannulated soft tissue protector. The first, or cephalad, implant is routinely placed at the level of S1 above the first neuroforamen (Figure 2E). A pin-guide system is used to facilitate placement of subsequent implants caudal to the first implant (Figure 2F). The second implant is generally located above or adjacent to the S1 foramen and the third is implanted between the S1 and S2 foramen (Figure 2G). Occasionally, patients may receive two or four implants, based on anatomical considerations (eg, short or tall height). The incision is then irrigated and the tissue layers are closed in a standard fashion. A typical postoperative radiograph demonstrating the iFuse implants is depicted in Figure 3.
Figure 2.

Intraoperative outlet views demonstrating the major sequences of iFuse implantation including initial (A) and final (B) pin placement, bone preparation using a drill (C) and triangular broach (D), first implant placement (E), use of a parallel pin guide for subsequent implants (F), and insertion of second and third iFuse implants (G).
Figure 3.
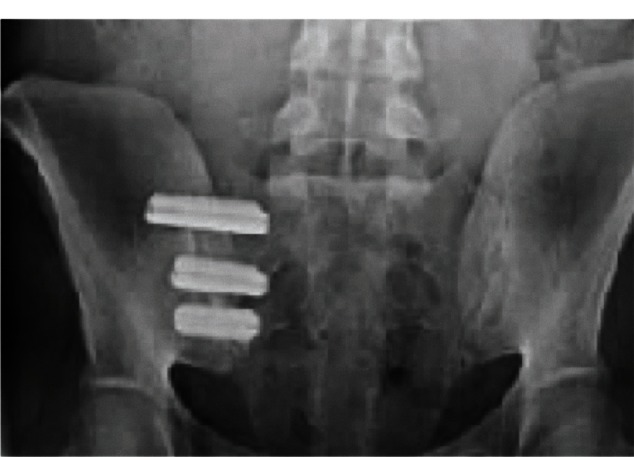
Postoperative radiograph demonstrating three iFuse implants in a female patient who presented with pregnancy-related chronic low back pain and right-sided sacroiliac joint disruption.
Complaints database construct and analysis
Between April 2009 and January 2013, 5319 patients in the US (n = 4962) and Europe (n = 357) were treated with approximately 16,000 iFuse implants based on product sales data. Prior to device commercialization, a database was developed to record all complaints reported to the manufacturer in patients treated with the iFuse system. Complaint data were collected through spontaneous complaint reporting mechanisms in support of ongoing postmarket surveillance efforts mandated by the US Food and Drug Administration (FDA). The FDA defines a complaint as “any written, electronic, or oral communication that alleged deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance” of the iFuse implant system. Sources of iFuse system complaints included physician users, patients, hospital staff, device manufacturer representatives, or any other person or group. The complaints that were recorded in the database included revision surgery, pain, device-related events, procedure-related events (eg, improper device placement, improper device size), and manufacturing-related events.
The device manufacturer employed multiple overlapping mechanisms to ensure thorough complaint reporting. A product specialist from the manufacturer was present during every case, and case details were recorded based on procedural observations and discussions with the treating physician. The postmarketing complaint reporting system is required by the FDA for all medical devices and all the manufacturer’s employees were trained to reporting requirements. Employees were mandated and nonemployees were encouraged to report any complaint to the manufacturer within 24 hours of first notification. In addition, relevant publications were reviewed and potential complaints were identified. Each complaint was investigated by a cross-functional team from the device manufacturer, including review of patient history, case details, and pretreatment and post-treatment imaging in an attempt to identify the root cause, which was determined by group consensus before entry into the database. The database used for this analysis was current as of January 2013.
The manufacturer of the iFuse system provided the authors with unrestricted access to their complaint reporting database. The authors generated multiple queries to the manufacturer to provide clarity on outstanding items. The authors take full responsibility for the integrity and analysis of the data.
Results
Complaints were reported in 204 (3.8%) of 5319 patients treated with the iFuse system (Table 1). The median time from the index operation to the complaint report was five months (range, intraoperative to 37 months). Overall, 43% of complaints were reported within 90 days of surgery, 30% between 90 days and one year, 21% between one and two years, and 6% beyond two years.
Table 1.
Postmarket complaints with the minimally invasive iFuse SI Joint Fusion System® (SI-BONE, Inc., San Jose, CA, USA)
| Complaints | All patients (n = 5319) |
|---|---|
| Patients with complaints (n, %) | 204 (3.8) |
| Clinical events, (n, %) | |
| Any pain | 119 (2.2) |
| Nerve impingement | 48 |
| Recurrent sacroiliac joint | 43 |
| Unknown cause | 18 |
| Neuropathic pain | 13 |
| Inadequate pain relief | 12 |
| Malalignment | 11 |
| Piriformis syndrome | 7 |
| Local soft tissue pain | 5 |
| Hematoma/excessive bleeding | 11 (0.2) |
| Iliac fracture | 4 (<0.1) |
| Superficial wound infection | 3 (<0.1) |
| Deep venous thrombosis | 2 (<0.1) |
| Deep wound infection | 1 (<0.1) |
| Pulmonary embolism | 0 |
| Vascular injury | 0 |
| Gastrointestinal injury | 0 |
| Genitourinary injury | 0 |
| Sacral fracture | 0 |
| Death | 0 |
| Device-related events, (n, %) | |
| Pin bind/bend/break | 43 (0.8) |
| Pin advancement | 14 (0.3) |
| Radiographic halo | 13 (0.2) |
| Migration | 4 (<0.1) |
| Procedure-related events, (n, %) | |
| Any improper device placement | 72 (1.4) |
| Medial | 20 |
| Anterior | 18 |
| Dorsal | 14 |
| Cephalad | 12 |
| Proud | 8 |
| Inferior | 2 |
| Other malposition | 2 |
| Any improper device size | 36 (0.7) |
| Too short | 30 |
| Too long | 7 |
Note: Sum of subgroups may be greater than the total due to multiple events in the same patient.
Pain was the most commonly reported clinical complaint (n = 119, 2.2%), with nerve impingement (n = 48, 0.9%) and recurrent sacroiliac joint pain (n = 43, 0.8%) frequently cited. The time course of pain complaints was 14% within 30 days of the procedure, 9% between 31 and 90 days, 43% between 91 and 365 days, and 34% beyond 365 days. Significant operative bleeding or hematoma was noted in 11 (0.2%) patients. Other clinical complaints (eg, iliac fracture, deep venous thrombosis, wound infection) were rarely reported (<0.1%) with no reports of death, pulmonary embolism, vascular injury, gastrointestinal injury, or genitourinary injury.
Device-related events were predominantly related to issues with the Steinmann pin, including binding/bending/breakage and advancement difficulties. Improper implant placement was reported in 72 (1.4%) cases, with medial (20), anterior (18), dorsal (14), and cephalad (12) placement in relation to the correct anatomical location being most common. Improper device length was identified in 36 (0.7%) cases with most (30) implants deemed to be too short. Complaints related to manufacturing issues were rare (0.1%), most commonly related to the implant-tool interface and the implant coating.
Ninety-six revision surgeries were performed in 94 (1.8%) patients at a median follow-up of four (range 0–30) months (Table 2). Revisions were typically performed in the early postoperative period for treatment of a symptomatic malpositioned implant (n = 46, 0.9%) or to correct an improperly sized implant in an asymptomatic patient (n = 10, 0.2%). Revised malpositioned implants were most often placed too medial (20), anterior (15), or cephalad (10). Revised improperly sized implants were deemed to be too short in every case and were subsequently explanted. Early revisions were performed at a median 19 days postoperatively.
Table 2.
Revision causes and treatments with the minimally invasive iFuse SI Joint Fusion System® (SI-BONE, Inc., San Jose, CA, USA)
| Revision cause and treatment | All revisions (n = 96) |
|---|---|
| Early (n, %) | 56 (58) |
| Malposition, symptomatic | 46 (48) |
| Nerve impingement | 19 |
| Piriformis syndrome | 17 |
| Local soft tissue pain | 13 |
| Malposition, asymptomatic | 10 (10) |
| Nerve impingement | 9 |
| Piriformis syndrome | 2 |
| Local soft tissue pain | 1 |
| Late (n, %) | 40 (42) |
| Symptom recurrence | 34 (35) |
| Revision details | |
| Explant only | 15 |
| Additional implant only | 13 |
| Unknown | 6 |
| Device adjustment only | 3 |
| Explant and reimplant | 3 |
| Supplemental fixation | |
| Lateral screws | 15 |
| None | 14 |
| Posterior fixation | 3 |
| Anterior fixation | 2 |
| Supplemental bone grafting | |
| Local lateral | 17 |
| None | 9 |
| Open posterior | 6 |
| Open anterior | 2 |
| Other (n, %) | 6 (6) |
| Explant only | 3 |
| Explant and reimplant | 1 |
| Device adjustment only | 1 |
| Additional implant only | 1 |
| Unknown | 1 |
Note: Sum of subgroups may be greater than the total due to multiple events in the same patient.
Revisions in the late postoperative period were performed to treat symptom recurrence (n = 34, 0.6%) or for continued pain of undetermined etiology (n = 6, 0.1%). The revisions for continued or recurring pain were performed at a median 279 days postoperatively. Adjunctive procedures utilized in the 34 revision cases for symptom recurrence included supplemental fixation in 20 patients (15 lateral screws, two anterior fixation, three posterior fixation) and bone grafting in 25 cases (17 local lateral, two open anterior, six open posterior).
Since product commercialization, 5319 patients have been treated with the iFuse system by 487 different physicians. The yearly number of iFuse cases in the US and Europe includes 31 in 2009, 273 in 2010, 1397 in 2011, and 3611 in 2012. The rate of complaints was 0% in 2009, 5.6% in 2010, 4.0% in 2011, and 3.5% in 2012. Complaint rates were similarly low in the US (4.0%) and Europe (1.4%). Revision surgery rates (Figure 4) were 0% in 2009 and 2010, 0.6% in 2011, and 2.4% in 2012, with similar rates in the US (1.9%) and Europe (0.8%).
Figure 4.
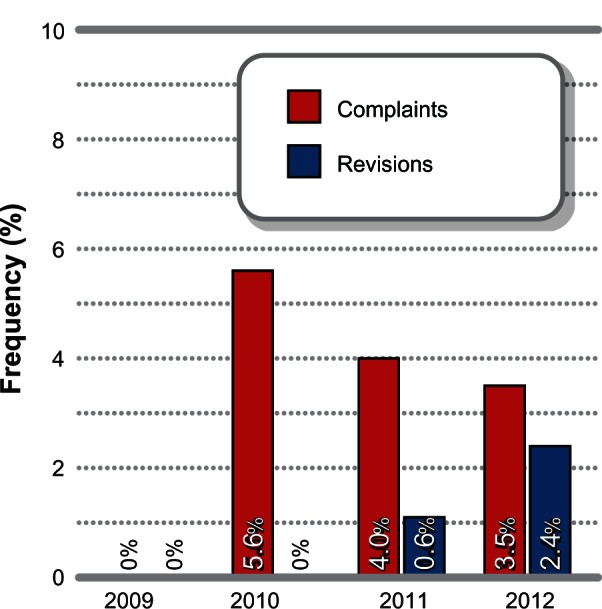
Time trends in complaints and revisions with the iFuse SI Joint Fusion System® (SI-BONE, Inc., San Jose, CA, USA) by index surgery date.
Discussion
Sacroiliac joint-generated low back pain remains an under-diagnosed and undertreated condition with no known therapeutic option offering excellent patient safety and acceptable long-term effectiveness outcomes.10 Traditional surgical options for chronic low back pain such as interbody fusion and decompression focus on the intervertebral disc or the vertebral column. The possible contribution of the sacroiliac joint to low back pain has been largely ignored, potentially leading to underdiagnosis, misdiagnosis, and misdirected surgical intervention on the lumbar spine. Even in patients with confirmed disc pathology on computed tomography or magnetic resonance imaging, sacroiliac joint provocation tests reveal positive findings in 60%–90% of cases.19 Several clinical trials have reported the strong relationship between failed back syndrome following lumbar or lumbosacral fusion and the sacroiliac joint, with possible contributing factors including increased mechanical load transfer, iliac crest bone graft harvesting, or original misdiagnosis of sacroiliac joint pathology.8,9 Consequently, a therapeutic shift emphasizing comprehensive assessment of possible sacroiliac joint involvement and proactive treatment in positive cases has recently gained momentum.
Minimally invasive sacroiliac joint arthrodesis with the iFuse system is a commonly performed procedure that was developed specifically to fill the therapeutic gap between ineffective conservative care and invasive open surgery. An analysis of the iFuse system complaints database demonstrated an overall low risk of complaints with this minimally invasive treatment option for patients with degenerative sacroiliitis and sacroiliac joint disruption. Revision surgery was reported in 1.8% of cases and ongoing or recurring pain was reported in 2.2% of patients. These positive outcomes are congruent with the two small case series of the iFuse system that reported revision rates of 0%–8% and similarly low rates of late pain through 1–2 years post-treatment.11,12 Complication rates with iFuse also compare favorably with open surgical and other minimally invasive sacroiliac joint arthrodesis systems. Open surgery is associated with complication rates of 22%–65%, including reoperation (0%–65%), deep wound infection (0%–10%), iliac crest fracture (0%–6%), and pulmonary embolism (0%–2%).6,15,16,20,21 Minimally invasive sacroiliac joint arthrodesis techniques yield lower complication rates compared with open surgery, including overall complication rates of 8%–11%, reoperation (0%–11%), and deep wound infection (0%–11%).17,18,22 Additionally, the severity of the complaints with the iFuse system was relatively minor, given that no iatrogenic vascular, gastrointestinal, or genitourinary complaints were reported.
Ensuring proper implant selection and placement is critical to achieve ideal outcomes. The incidences of complaints related to improper device placement and improper device length in this analysis were 1.4% and 0.7%, respectively. Despite this low overall incidence, these complaints were commonly implicated in cases requiring revision surgery. Symptom recurrence was a rare (0.6%) complication. The iFuse implants were designed to resist rotational and pullout forces, with biomechanical testing confirming exponentially greater resistance to shear and bending forces versus traditional threaded screws used in percutaneous sacroiliac joint fixation procedures. Careful preoperative planning and careful interpretation of multiple fluoroscopic views (inlet/outlet/lateral/and oblique) during surgery can help the surgeon select the proper implant length. Implants must be positioned to engage the iliac and the sacral bones such that they are fully contained within the sacroiliac osseous envelope. The implants must be positioned to avoid violation or compromise of the nerve tunnels within the sacrum (spinal canal and the dorsal and ventral neuroforamina). The implants must also be positioned to avoid penetration of the outer cortex of the sacrum dorsally, cephalad, anterior, or inferior. Importantly, sacroiliac joint morphology is highly variable with respect to size, shape, and orientation, and up to 40% of patients present with a dysplastic sacrum.23,24 Preoperative computed tomography is mandatory to help the surgeon understand each patient’s unique sacroiliac joint anatomy, place the implants into the appropriate position to minimize implant malposition risk, and to ensure proper implant seating into the sacrum. Successful outcomes with minimally invasive sacroiliac joint arthrodesis using the iFuse system are dependent on several factors, including appropriate patient selection and meticulous surgical technique. Ideal candidates include patients with degenerative sacroiliitis or sacroiliac joint disruption that is refractory to nonsurgical care. Patients are selected for treatment based on positive concordant findings from medical history characterized by chronic low back or gluteal pain near the sacroiliac joint, clinical examination including a series of provocation maneuvers, imaging studies that rule out other potential pain-generating sources, and a notable reduction in pain immediately following properly performed diagnostic injection of the sacroiliac joint. This differential diagnosis methodology has been previously shown to reliably identify the sacroiliac joint as the primary pain generator.25,26
Although clinical effectiveness outcomes were not assessed in this paper, the initial reports are promising. Rudolf11 treated 50 patients with chronic degenerative sacroiliitis or sacroiliac joint disruption using iFuse implants and followed them for a minimum of two years. Pain severity decreased by 74%, the ability to engage in various activities of daily living was enhanced by 26%–46%, and patient satisfaction was 82%. Sachs and Capobianco12 followed 11 patients for one year following iFuse implantation for chronic degenerative sacroiliitis or sacroiliac joint disruption refractory to prolonged conservative treatment. Pain severity decreased by 71% and patient satisfaction was 100% following treatment. Large single-arm (SIFI: NCT01640353) and randomized controlled (INSITE: NCT01681004) trials have been initiated, and their results will further the characterization of safety and effectiveness outcomes of minimally invasive sacroiliac joint arthrodesis with the iFuse system.
The primary strengths of this report include a robust analysis of a large number of patients as part of a comprehensive, FDA-mandated postmarket product surveillance program. However, a limitation of this report is that spontaneous complaint reporting may underestimate the true incidence of events.27 While the extent of possible complaint under-reporting is unknown, it is plausible that the true rate of complaints with the iFuse system is higher than that reported in the current study.
In conclusion, analysis of a product surveillance database suggests that the iFuse system is a safe alternative to open surgery for patients with degenerative sacroiliitis or sacroiliac joint disruption that is resistant to conservative care.
Acknowledgments
The authors thank Evan Rosenfield for database development and Randy Asher for graphical assistance.
Footnotes
Disclosure
This study was supported in part by SI-BONE, Inc. (San Jose, CA, USA). Authors declare no other conflicts of interest in this work.
References
- 1.Friedman BW, Chilstrom M, Bijur PE, Gallagher EJ. Diagnostic testing and treatment of low back pain in United States emergency departments: a national perspective. Spine (Phila Pa 1976) 2010;35(24):E1406–E1411. doi: 10.1097/BRS.0b013e3181d952a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JL, Browning R. Impact of national low back pain guidelines on clinical practice. South Med J. 2005;98(2):139–143. doi: 10.1097/01.SMJ.0000136261.21711.85. [DOI] [PubMed] [Google Scholar]
- 3.Bernard TN, Jr, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–280. [PubMed] [Google Scholar]
- 4.Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine (Phila Pa 1976) 1996;21(16):1889–1892. doi: 10.1097/00007632-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976) 1995;20(1):31–37. doi: 10.1097/00007632-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J. 2005;5(5):520–528. doi: 10.1016/j.spinee.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Ha KY, Lee JS, Kim KW. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over five-year follow-up. Spine (Phila Pa 1976) 2008;33(11):1192–1198. doi: 10.1097/BRS.0b013e318170fd35. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara H. Sacroiliac joint pain after lumbar/lumbosacral fusion: current knowledge. Eur Spine J. 2012;21(9):1788–1796. doi: 10.1007/s00586-012-2350-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePalma MJ, Ketchum JM, Saullo TR. Etiology of chronic low back pain in patients having undergone lumbar fusion. Pain Med. 2011;12(5):732–739. doi: 10.1111/j.1526-4637.2011.01098.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansen H, Manchikanti L, Simopoulos TT, et al. A systematic evaluation of the therapeutic effectiveness of sacroiliac joint interventions. Pain Physician. 2012;15(3):E247–E278. [PubMed] [Google Scholar]
- 11.Rudolf L. Sacroiliac joint arthrodesis-MIS technique with titanium implants: report of the first 50 patients and outcomes. Open Orthop J. 2012;6:495–502. doi: 10.2174/1874325001206010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs D, Capobianco R. One year successful outcomes for novel sacroiliac joint arthrodesis system. Ann Surg Innov Res. 2012;6(1):13. doi: 10.1186/1750-1164-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson LA, Waddell JP, Leighton RK, Kellam JF, Tile M. Anterior approach and stabilization of the disrupted sacroiliac joint. J Trauma. 1987;27(12):1332–1339. doi: 10.1097/00005373-198712000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Dabezies EJ, Millet CW, Murphy CP, Acker JH, Robicheaux RE, D’Ambrosia RD. Stabilization of sacroiliac joint disruption with threaded compression rods. Clin Orthop Relat Res. 1989;246:165–171. [PubMed] [Google Scholar]
- 15.Waisbrod H, Krainick JU, Gerbershagen HU. Sacroiliac joint arthrodesis for chronic lower back pain. Arch Orthop Trauma Surg. 1987;106(4):238–240. doi: 10.1007/BF00450461. [DOI] [PubMed] [Google Scholar]
- 16.Schutz U, Grob D. Poor outcome following bilateral sacroiliac joint fusion for degenerative sacroiliac joint syndrome. Acta Orthop Belg. 2006;72(3):296–308. [PubMed] [Google Scholar]
- 17.Al-Khayer A, Hegarty J, Hahn D, Grevitt MP. Percutaneous sacroiliac joint arthrodesis: a novel technique. J Spinal Disord Tech. 2008;21(5):359–363. doi: 10.1097/BSD.0b013e318145ab96. [DOI] [PubMed] [Google Scholar]
- 18.Wise CL, Dall BE. Minimally invasive sacroiliac arthrodesis: outcomes of a new technique. J Spinal Disord Tech. 2008;21(8):579–584. doi: 10.1097/BSD.0b013e31815ecc4b. [DOI] [PubMed] [Google Scholar]
- 19.Weksler N, Velan GJ, Semionov M, et al. The role of sacroiliac joint dysfunction in the genesis of low back pain: the obvious is not always right. Arch Orthop Trauma Surg. 2007;127(10):885–888. doi: 10.1007/s00402-007-0420-x. [DOI] [PubMed] [Google Scholar]
- 20.Ebraheim NA, Ramineni SK, Alla SR, Ebraheim M. Sacroiliac joint fusion with fibular bone graft in patients with failed percutaneous iliosacral screw fixation. J Trauma. 2010;69(5):1226–1229. doi: 10.1097/TA.0b013e3181e4f3f8. [DOI] [PubMed] [Google Scholar]
- 21.Kibsgard TJ, Roise O, Sudmann E, Stuge B. Pelvic joint fusions in patients with chronic pelvic girdle pain: a 23-year follow-up. Eur Spine J. 2013;22(4):871–877. doi: 10.1007/s00586-012-2512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuire RA, Chen Z, Donahoe K. Dual fibular allograft dowel technique for sacroiliac joint arthrodesis. Evid Based Spine Care J. 2012;3(3):21–28. doi: 10.1055/s-0032-1327806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conflitti JM, Graves ML, Chip Routt ML., Jr Radiographic quantification and analysis of dysmorphic upper sacral osseous anatomy and associated iliosacral screw insertions. J Orthop Trauma. 2010;24(10):630–636. doi: 10.1097/BOT.0b013e3181dc50cd. [DOI] [PubMed] [Google Scholar]
- 24.Miller AN, Routt ML., Jr Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg. 2012;20(1):8–16. doi: 10.5435/JAAOS-20-01-008. [DOI] [PubMed] [Google Scholar]
- 25.Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354–368. doi: 10.1016/j.jpain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Liliang PC, Lu K, Liang CL, Tsai YD, Wang KW, Chen HJ. Sacroiliac joint pain after lumbar and lumbosacral fusion: findings using dual sacroiliac joint blocks. Pain Med. 2011;12(4):565–570. doi: 10.1111/j.1526-4637.2011.01087.x. [DOI] [PubMed] [Google Scholar]
- 27.McNeil JJ, Grabsch EA, McDonald MM. Postmarketing surveillance: strengths and limitations. The flucloxacillin-dicloxacillin story. Med J Aust. 1999;170(6):270–273. doi: 10.5694/j.1326-5377.1999.tb123612.x. [DOI] [PubMed] [Google Scholar]


