Abstract
Similar to other highly self-renewing tissues, the intestinal epithelium contains both slowly and rapidly cycling progenitor/stem cells, though their relationship has been largely unexplored. Two recent reports in Nature (Tian et al., 2011) and Science (Takeda et al., 2011) shed new light on their dynamic interplay.
The small intestinal epithelium has enormous capacity for self-renewal, replacing itself every 3 to 5 days. The cellular basis for this regenerative potential has long been accepted to reside in multipotent intestinal stem cells (ISCs) (Cheng and Leblond, 1974). Based on the hypothesis that ISCs would be slowly cycling, Potten and colleagues initially employed DNA label retention models to identify these cells. These studies led to the discovery and characterization of putative ISCs located in the “+4 crypt position” (Fig 1) (Potten et al., 1974). While this finding subsequently gave rise to the discovery of a number of additional markers based on co-localization with label retention, functionally validated ISC markers remained elusive for over 3 decades (reviewed in Montgomery and Breault, 2008).
FIGURE 1.
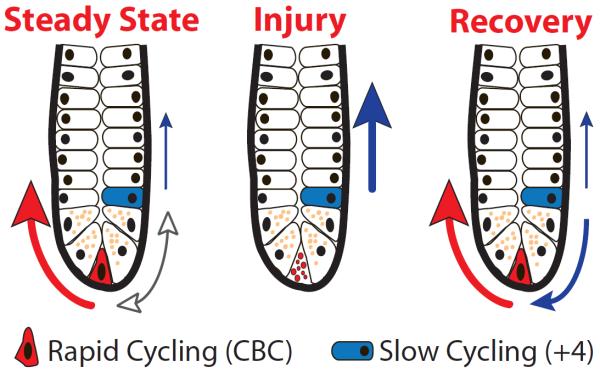
(a) Intestinal crypt under steady state conditions showing inter-conversion of slowly and rapidly cycling stem cells (bidirectional arrow) as well as the dominant contribution of rapidly cycling stem cells (bold red arrow) and minor contribution of slowly cycling stem cells in the +4 position (thin blue arrow). (b) Upon injury, rapidly cycling stem cells undergo apoptosis forcing slowly cycling stem cells to assume a dominant role (blue arrow). (c) During recovery from injury, slowly cycling stem cells give rise to the rapidly cycling population restoring homeostasis. Paneth cells, marked by dense granules, define the niche boundaries.
The first functionally validated ISC marker to be identified was Lgr5, a downstream target of canonical Wnt signaling (Barker et al., 2007). In contrast to Potten's original observation, Lgr5 expression corresponded to crypt base columnar cells, located between Paneth cells at the crypt base (Fig. 1), a site previously suggested to contain ISCs (Bjerknes and Cheng, 1999). Surprisingly, and in stark contrast to the label-retaining population, the majority of Lgr5-expressing cells were shown to be rapidly cycling, raising doubt whether bona fide slowly cycling ISCs were also present in this highly self-renewing tissue. On the other hand, as with other rapidly cycling cells, this population has been shown to be highly sensitive to the effects of intestinal damage (Barker et al., 2007), strongly suggesting an alternative system would be required to restore homeostasis following injury. Moreover, how such a rapidly cycling population could be maintained as the solitary stem cell for the life of the organism, without accumulating deleterious mutations, has been the subject of much debate.
In support of Potten's initial hypothesis, the intestinal stem cell field has recently witnessed the emergence of functional evidence establishing that slowly cycling, label-retaining ISCs exist within the intestinal crypt and are distinct from Lgr5-expressing cells. These cells, marked by Bmi1 (Sangiorgi and Capecchi, 2008) and mTert expression (Montgomery et al., 2011) are largely quiescent (or presumed to be in the case of Bmi1-expressing cells) and are located predominately in the “+4 crypt position.” Under steady state conditions, slowly cycling stem cells contribute to intestinal lineage development predominantly through an Lgr5 cell-dependent pathway (Fig 1a, bold red arrow). In addition, a second lineage pathway, that is Lgr5 cell-independent, also mediates cell fate decisions, albeit less frequently (Fig 1a, thin blue arrow) (Montgomery et al., 2011). These discoveries raise important questions regarding the cellular plasticity of daughter cells and the mechanisms regulating their fate decisions. Intriguingly, slowly cycling stem cells are highly resistant to intestinal injury and play an important role during intestinal regeneration (Fig 1b, c) (Montgomery et al., 2011), perhaps by restoring the rapidly cycling ISC population. Together, these observations suggest that regulation of intestinal homeostasis may be similar to other highly self-renewing tissues, e.g., blood (Wilson et al., 2008) and skin (Fuchs, 2009), which are maintained by slowly cycling stem cell populations capable of restoring homeostasis following loss of rapidly cycling progenitor cells. Definitive evidence to support this claim, however, has been lacking.
Two recent reports provide essential new insight into the inter-dependence of slowly and rapidly cycling ISC populations. Tian and colleagues employed lineage tracing and cell ablation studies using two newly generated mouse strains targeting the Lgr5 locus (Lgr5-dsRED-IRES-CreERT2 and Lgr5-EGFP-DTR). In a series of elegant studies, they report that elimination of the rapidly cycling ISC population had no effect on intestinal homeostasis, leading them to conclude that Lgr5-expressing cells are dispensable. They go on to demonstrate that Bmi1-expressing cells are dramatically increased in number following Lgr5 cell ablation and function as a reserve stem cell pool contributing to intestinal lineage development via the Lgr5 cell-independent pathway (Fig 1b). Of note, upon removal of the ablation signal, Bmi1-expressing cells rapidly give rise to Lgr5-expressing cells, thereby restoring the Lgr5 cell-dependent lineage pathway (Fig 1c). Finally, they demonstrate that like mTert (Montgomery et al., 2011) Bmi1-expressing cells can contribute to Lgr5-expressing cells under steady state conditions, further supporting the concept that slowly cycling ISCs restore rapidly cycling cells (Fig 1a).
Extending these observations, Takeda and colleagues identified Hopx gene expression as another marker of slowly cycling ISCs at the “+4 crypt position.” In contrast to the rare nature of Bmi1-expressing ISCs and the rarer mTert population, Hopx-expressing cells appear to be present in virtually every crypt, raising the possibility that functionally distinct sub-populations of slowly cycling ISCs exist. Through a series of elegant lineage-tracing studies using a newly generated mouse strain targeting the Hopx locus (Hopx-CreER), they demonstrate that slowly cycling Hopx-expressing ISCs give rise to Lgr5-expressing ISCs, consistent with Bmi1 and mTert ISCs. Importantly, however, they demonstrate for the first time that Lgr5-expressing cells can contribute to Hopx-expressing cells, data which further underscores that a dynamic relationship exists between distinct ISC populations under steady state conditions (Fig 1a). Whether a similar relationship exists with regenerative pressure remains unclear. However, given that Lgr5-expressing cells are highly sensitive to injury (Barker et al., 2007) and that slowly cycling ISCs utilize an Lgr5-independent pathway following injury to regenerate the crypt, (Fig. 1b) it is unlikely this interchange is functionally important under all conditions. Although Takeda et al. show that inter-conversion occurs between slowly and rapidly cycling ISCs, the precise mechanisms underlying this cellular plasticity remain to be determined.
Taken together, these two reports provide fundamental new insight into the dynamic role stem cells play in the intestinal crypt. These investigators demonstrate that rapidly and slowly cycling ISC populations are inter-dependent and work cooperatively to maintain intestinal homeostasis under both steady state and regenerative conditions (Fig. 1). The development of these and other mouse model systems will ultimately give rise to a detailed understanding of the lineage relationships and hierarchy among the various ISC populations within the crypt. Along these lines, it will be interesting to determine whether the principles governing intestinal lineage development are analogous to those involved in other stem cell systems. For example, the hematopoietic system is maintained by stem cells with long and short-term regenerative potential, which are predominantly quiescent and resistant to injury, as well as committed multipotent, oligopotent and unipotent progenitors, which have increased proliferative potential. Additionally, understanding how the ISC niche, environmental and physiological inputs and signaling pathways regulate slowly and rapidly cycling ISCs will be essential to fully understand their independent and cooperative roles in normal homeostasis and in pathological states such as cancer and inflammatory bowel disease.
REFERENCES
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, Leboeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion Between Intestinal Stem Cell Populations in Distinct Niches. Science. 2011 doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]


