Abstract
Rationale
Individuals with a family history of alcoholism (family history positive [FHP]) show higher alcoholism rates and are more impulsive than those without such a family history (family history negative [FHN]), possibly due to altered N-methyl-D-aspartate (NMDA) receptor function.
Objectives
We investigated whether memantine, an NMDA receptor antagonist, differentially influences impulsivity measures and Go/No-Go behavior and fMRI activity in matched FHP and FHN individuals.
Methods
On separate days, participants received a single dose of 40 mg memantine or identical-appearing placebo.
Results
No group performance differences were observed on placebo for Go correct hit or No-Go false alarm reaction time on the Go/No-Go task. During fMRI, right cingulate activation differed for FHP vs. FHN subjects during No-Go correct rejects. Memantine had attenuated effects in FHP vs. FHN subjects: For No-Go false alarms, memantine was associated with limited reduction in subcortical, cingulate, and temporal regions in FHP subjects and reduced activity in fronto-striatal–parietal networks in FHN subjects. For No-Go correct rejects, memantine (relative to placebo) reduced activity in left cingulate and caudate in FHP but not FHN subjects.
Conclusions
Lower sensitivity to the effects of memantine in FHP subjects is consistent with greater NMDA receptor function in this group.
Keywords: Memantine, Genetic risk of alcoholism, Go/No-Go, NMDA receptor antagonist, fMRI, Response inhibition, Response execution
Introduction
A family history of alcoholism is an important risk factor for the development of alcoholism (Cloninger et al. 1981; Goodwin et al. 1973). Individuals with a family history of alcoholism (family history positive [FHP]) have reduced sensitivity to the sedative, dysphoric, and cognitive effects of acute alcohol intoxication relative to those without a family history (family history negative [FHN]; for a review, see Quinn and Fromme 2011), suggesting that an important alcohol-related negative feedback signal may be disrupted in FHP individuals (Krystal et al. 2003a, b). Evidence also suggests that nonalcoholic FHP individuals exhibit increased impulsiveness and sensation-seeking compared to FHN individuals (Knop 1985; Petry et al. 2002; Saunders et al. 2008; Sher 1991). FHP children who later go on to develop drinking problems show signs of behavioral disinhibition, novelty-seeking, and impulsivity (Cloninger 1987; Ernst et al. 2006).
Impulsivity forms a crucial component of addiction susceptibility (Oberlin and Grahame 2009). Genetic susceptibility to addiction is associated with increased impulsivity, specifically impaired response inhibition (Goldstein and Volkow 2002; Kreek et al. 2005). The Go/No-Go task is a classic response inhibition task where a prepotent bias towards fast responding to “Go” stimuli increases the difficulty of withholding a response to “No-Go” stimuli. Functional magnetic resonance imaging (fMRI) studies consistently demonstrate that a fronto-striatal-parietal network is activated during response inhibition in healthy populations (e.g., Rubia et al. 2001; Stevens et al. 2007). Clinical populations with problems of impulsivity have a reduced capacity to inhibit responses to No-Go stimuli (Chamberlain and Sahakian 2007). We recently found that alcohol influences error processing and associated fMRI response of No-Go false alarms (Anderson et al. 2011). fMRI evidence suggests that FHP individuals manifest impaired No-Go activity relative to FHN individuals in fronto-striatal regions (DeVito et al. 2010; Heitzeg et al. 2010; Schweinsburg et al. 2004). Among youth aged 16–22 years, No-Go response inhibition fMRI deactivations in the ventral striatum were present in FHN but not FHP individuals, regardless of whether the FHP individuals showed problem drinking behavior (Heitzeg et al. 2010). Failure to deactivate this region in FHP was related to more externalizing problems. In a preliminary study of a separate and larger sample, No-Go response inhibition activity positively correlated with behavioral impulsivity measures, indicating that deficient response inhibition may contribute to an overall impairment in impulsivity and behavioral disinhibition in FHP individuals (DeVito et al. 2010).
Abnormal response to alcohol and impulsivity problems in FHP individuals may be related to abnormal N-methyl-d-aspartate (NMDA) glutamate receptor function. NMDA receptors are among the highest affinity targets for alcohol in the brain (Grant and Lovinger 1995). Alcohol weakly antagonizes NMDA receptor function and chronic intake of alcohol results in the upregulation of NMDA receptors and reduced responses to the NMDA receptor antagonist ketamine (Tsai et al. 1995; Krystal et al. 2003a, 2011). Enhanced NMDA receptor function is also present in FHP individuals who, like alcohol-dependent individuals, have blunted psychotic and dysphoric mood responses to ketamine (Petrakis et al. 2004). Clinically depressed FHP individuals display superior antidepressant responses to ketamine than do FHN patients (Phelps et al. 2009).
NMDA receptor antagonists reduce alcohol craving before the initiation of alcohol consumption in moderate drinkers (Bisaga and Evans 2004) and reduce cue-induced craving for alcohol in alcohol-dependent inpatients (Krupitsky et al. 2007). Thus, NMDA receptor antagonists might play a therapeutic role by reducing enhanced NMDA receptor function in circuitry underlying the motivation to drink (Krupitsky et al. 2007). The hypothesis that NMDA receptor antagonists differentially influence cognitive functions underlying impulse control in addicted populations could explain why ketamine impairs response inhibition in healthy individuals (Morgan et al. 2004) and memantine improves response inhibition in people with pathological gambling (Grant et al. 2010). Such findings raise questions about how the relationship between NMDA receptor function and response inhibition may vary in FHP and FHN individuals and how these relate to aspects of behavioral control such as risk-taking and choice impulsivity (Lejuez et al. 2010).
In the current study, we examined the effects of a single dose of memantine on behavioral measures of risk-taking and impulsivity, Go/No-Go task performance, and associated fMRI activation in matched FHP and FHN individuals in a double-blind placebo-controlled design. Memantine is a moderate affinity, uncompetitive NMDA receptor antagonist (Jarvis and Figgitt 2003) that has been used to examine the effects of NMDA receptor antagonism in FHP individuals (Krupitsky et al. 2007), in people with pathological gambling (Grant et al. 2010), and on tasks of impulse and cognitive control and cognitive flexibility (van Wageningen et al. 2009; Grant et al. 2010). To our knowledge, this is the first study to investigate the neural effects of NMDA receptor antagonism on Go/No-Go response inhibition as related to behavioral measures of risk-taking and impulsivity. On the basis of previous results, we hypothesized that FHP subjects would show performance decrements on behavioral tasks assessing risk-taking (the Balloon Analog Risk Task (BART; Lejuez et al. 2002)) and choice impulsivity (the Experiential Discounting Task (EDT; Reynolds and Schiffbauer 2004)). We also hypothesized that FHP individuals would show impaired No-Go responses relative to FHN during placebo and that FHP subjects would show impaired Go/No-Go-related fMRI activity during placebo in frontal, striatal, and possibly parietal regions compared to FHN subjects (DeVito et al. 2010; Schweinsburg et al. 2004). Furthermore, given the relationship between NMDA receptors and impulsivity (Grant et al. 2010; Morgan et al. 2004), we hypothesized that memantine would alter Go/No-Go performance and related brain activations and that these effects of memantine would differ between FH groups.
Methods
Participants
Fifteen FHP individuals and 15 FHN individuals participated in the current study (Table 1). Groups did not differ in age or gender and all were right-handed. All participants provided written informed consent approved by the Yale and Hartford Hospital Institutional Review Boards. To be defined as FHP, participants needed a biological father and one or more additional first- or second-degree biological relatives with history of alcoholism as indexed by the Family History Assessment Module (FHAM; Compton et al. 2002). Individuals with a biological mother with a history of alcoholism were excluded to rule out possible fetal alcohol effects. To be defined as FHN, participants needed to have no FHAM history of alcoholism in any first- or second-degree relatives.
Table 1.
Demographic information for FHN and FHP groups
| Demographic | FHN | FHP | Statistic |
|---|---|---|---|
| n | 15 | 15 | |
| Age (years) | 18–30 | 18–27 | t(28)=−0.594, p = 0.56 |
| Mean 23.3 (SE 0.85) | Mean 22.7 (SE 0.72) | ||
| Gender | 8 males | 7 males | χ200.715, p=0.99 |
| 7 females | 8 females | ||
| Race | 12 White | 14 White | χ201.01, p=0.316 |
| 1 Black | 1 Black | ||
| 1 Hispanic | |||
| 1 other | |||
| Mean (SE) years of education | 14.5 (0.39) | 14.8 (0.53) | χ206.00, p00.306 |
| Mean (SE) number of standard drinks in last 30 days | 5.7 (1.96) | 15.92 (6.04) | t(27)=−1.604, p00.13 |
| Mean (SE) number of drinking days in past 30 days | 2.67 (1.07) | 3.43 (1.13) | t(27)=−0.489, p00.63 |
| Mean (SE) number of standard drinks in last 12 months | 3.2 (0.66) | 3.6 (0.64) | t(27)=−0.482, p00.63 |
| Number of cigarette smokers | 0 | 1 (<10/day) | |
| Number of subjects meeting criteria for past alcohol dependence | 0 | 1 | |
| Number of subjects meeting criteria for past cannabis dependence | 0 | 1 |
Groups did not differ in age, gender, race, years of education, or drinking behavior in the past 30 days or 12 months
SE standard error
Potential participants were screened to eliminate those with a central neurological disorder, a nonsubstance DSM-IV/ SCID-IV axis I disorder (First 2002), current abuse/dependence (as confirmed by urine toxicology) of substances other than nicotine dependence, and current alcohol intoxication based on breath alcohol at screening (Intoximeters, Inc., St. Louis, MO, USA) on each testing day. One participant in the FHP group currently smoked cigarettes (<10 per day); one FHP subject met the criteria for past alcohol dependence and one FHP subject met the criteria for past cannabis dependence. Since Heitzeg et al. (2010) showed fMRI activation differences between FHP individuals who did and did not have current problem drinking behavior, we thoroughly examined our data to detect any differences between these participants and the rest of the FHP group on impulsivity measures and Go/No-Go behavior and fMRI activity. These participants were not outliers on any measure; therefore, they were included in analyses. All women had negative urine pregnancy tests.
Table 1 summarizes alcohol drinking behavior in the last 30 days for each group. Groups did not differ in the number of standard drinks consumed in the last 12 months or 30 days or in the number of drinking days in the past 30 days.
Procedure
Participants attended two sessions on two separate days. In each session, they were administered either a single dose of 40 mg memantine orally or an identical-appearing placebo. A single dose of memantine has been shown to affect cognition in both rats (Loskutova and Kostjunina 2009) and humans (Collins et al. 2007). Dose administration was randomized in a double-blind design. The number of participants who received memantine vs. placebo first was also counterbalanced within groups. Three hours postdrug administration, participants completed the computerized risk-taking and impulsivity tasks. Following this, approximately 4 h postdrug administration, participants underwent fMRI scanning while performing the Go/No-Go task (Anderson et al. 2011; Kiehl et al. 2000; Stevens et al. 2007). Participants completed a visual analog intoxication scale every 30 min and heart rate and blood pressure were checked hourly.
For the risk-taking and impulsivity measures, as described previously (Andrews et al. 2011; Meda et al. 2009), participants completed two computerized tests. In the BART (Lejuez et al. 2002), participants inflate a virtual balloon linked to increasing monetary reward that can either grow larger or explode. The adjusted average for the total number of pumps was used as the behavioral risk-taking measure. The EDT (Reynolds and Schiffbauer 2004) assesses real-time delay discounting, defined as preference for smaller immediate rewards over larger delayed rewards. The behavioral measure is the average area under the curve, with a smaller area reflecting steeper discounting and greater impulsivity.
For the Go/No-Go task, as described previously (Anderson et al. 2011; Kiehl et al. 2000; Stevens et al. 2007), participants were instructed to respond by pressing a button with their right index finger as accurately and quickly as possible to Go stimuli (“X,” 85% probability, n=206) and to withhold a response to No-Go stimuli (“K,” 15% probability, n=40). Go and No-Go stimuli were presented for 50 ms with an interstimulus interval (ISI) of 750, 1,750, or 2,750 ms. The presentation of Go and No-Go stimuli was pseudorandomized with intervals between No-Go stimuli between 10 and 15 s. Trials were presented in 2 runs of 246 trials lasting 7 min 21 s. A break of approximately 1 min was given between runs. Given the high frequency of Go stimuli, the estimated response function was saturated, and it was not possible to extract a meaningful result to Go correct hit stimuli. Thus, results are presented for No-Go correct rejects (successful inhibitions) and No-Go false alarms (unsuccessful inhibitions) only.
Prior to scanning, participants completed 10 practice trials to ensure that instructions were understood. Reaction time (RT) and accuracy were equally emphasized in task instructions, and participants were encouraged to speed up or slow down to ensure they did not adopt an overly cautious or careless strategy. Participants were not given precise instructions to make a given number of errors nor provided with feedback during the task on the number of errors or speed of responses, information which could be used to adjust behavior. Participants were encouraged to perform at this level during both challenge sessions to ensure that within-subject performance differences were meaningful.
Data analysis
fMRI data from the memantine session for one participant from the FHP group were corrupt and removed from further analysis (FHP, n=14). Event timing data from one run for three participants from the FHN group were corrupt (run 1 for one subject, run 2 for two subjects); thus, a single intact run was analyzed for these participants.
Although FH groups did not differ in drinking behavior in the previous 12 months or 30 days, it is possible that statistically nonsignificant differences between groups were physiologically significant. Thus, in all analyses, number of standard drinks in the last 30 days was added as a covariate to control for potential differences in drinking behavior between groups.
Risk-taking and impulsivity data
BART (adjusted average pumps) and EDT (area under the curve) data were analyzed with two separate 2-dose (memantine, placebo)×2-group (FHP, FHN) mixed ANOVAs.
Go/No-Go behavioral data
RT data (in-scanner behavior) are presented for Go correct hit trials and No-Go false alarm trials. Although many Go/No-Go studies examine only Go correct hit RT, we also examined No-Go false alarm RT, since No-Go false alarms are believed to be primarily due to the failure to inhibit an impulsive response. RTs for Go correct hit and No-Go false alarms were analyzed with two separate 2-dose (memantine, placebo)×2-group (FHP, FHN) mixed ANOVAs. To further investigate effects of memantine on each group, additional 2-dose (memantine, placebo) repeated-measures ANOVAs were run for each group separately. In these analyses, we used an alpha level of α=0.05/200.025. Proportion error (i.e., false alarms) for No-Go trials was calculated as the number of errors on No-Go trials divided by the total number of No-Go trials and arcsine transformed to normalize the distribution. Proportion error was analyzed with a 2-dose (memantine,placebo)×2-group (FHP,FHN) mixed ANOVA.
fMRI data
Magnetic resonance images were acquired using a Siemens (Erlangen, Germany) Allegra 3T dedicated head scanner equipped with 40 mT/m gradients and a standard quadrature head coil at the Olin Neuropsychiatry Research Centre. Functional images were acquired using a T2*-weighted gradient-echo echo-planar imaging (EPI) protocol (ascending axial acquisition, 210 volumes, TR01.5 s, TE= 27 ms, FOV=24 cm, acquisition matrix=64×64, flip angle=70°, voxel size=3.75×3.75×4 mm, gap=1 mm, 29 slices). The first six images were discarded to account for T1 saturation effects.
Functional images were preprocessed using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). Differences in EPI slice acquisition timing were corrected using the central slice as reference. Motion was corrected using INRIAlign (Freire et al. 2002), and images then spatially normalized into Montreal Neurological Institute (MNI) space. Normalized EPIs were then smoothed with a 9-mm FWHM Gaussian kernel.
Prior to first-level analysis, events for each participant were categorized as correct hits to Go stimuli, correct rejects to No-Go stimuli, and false alarms to No-Go stimuli. For first-level analyses, a canonical hemodynamic response function and its temporal derivative (Josephs et al. 1997) were fitted to the onset of these three stimulus types for each session separately. Realignment parameters were included in the model as covariates of no interest.
For second-level analyses, contrast images for No-Go correct rejects >baseline (successful inhibition) and No-Go false alarms >baseline (unsuccessful inhibition) for each subject for placebo and memantine sessions were entered into a full factorial random effects analysis. The term “baseline” in this context refers to implicit baseline; i.e., all unmodeled variance in the data. The number of standard drinks in the previous 30 days was entered as a covariate to control for potential differences in drinking behavior between groups. Activity for each contrast across groups during placebo was analyzed first in order to provide a context in which to examine the effects of group and dose. These whole-brain analyses were voxel-wise thresholded at p<0.001 (uncorrected) with a minimum cluster size of k=30 voxels (cluster puncorr <0.05). As shown in the results, the networks of regions activated in these contrasts overlap very well with known response inhibition-related networks (e.g. Aron and Poldrack 2006; Jamadar et al. 2010; Rubia et al. 2001; Stevens et al. 2007). Region of interest (ROIs) analyses were conducted to examine the differences in activity between FH groups during placebo and the effects of memantine vs. placebo challenge for each group separately. ROIs were defined as a sphere with 10-mm radius around the coordinate of the peak of activity found in the contrasts across FH groups during placebo. To examine the effects of group and dose1 for No-Go correct rejects and No-Go false alarms, whole-brain maps for FHN < and > FHP, FHN memantine < and > placebo, and FHP memantine < and > placebo were created, voxel-wise thresholded at p<0.001 and cluster-levelthresholded at k=30 voxels, and then a small volume correction (SVC) was applied in each of the ROIs identified above. These were thresholded with an SVC p<0.05, family-wise error corrected.
Results
Risk-taking and impulsivity results
Groups did not differ on BART or EDT scores during placebo (F(1,26)=0.77, p=0.39; F(1,26)=0.13, p=0.73, respectively). There was no effect of group, dose, or group×dose interaction for either task (BART: all p>0.32; EDT all p>0.29)
Go/No-Go results
Go correct hits
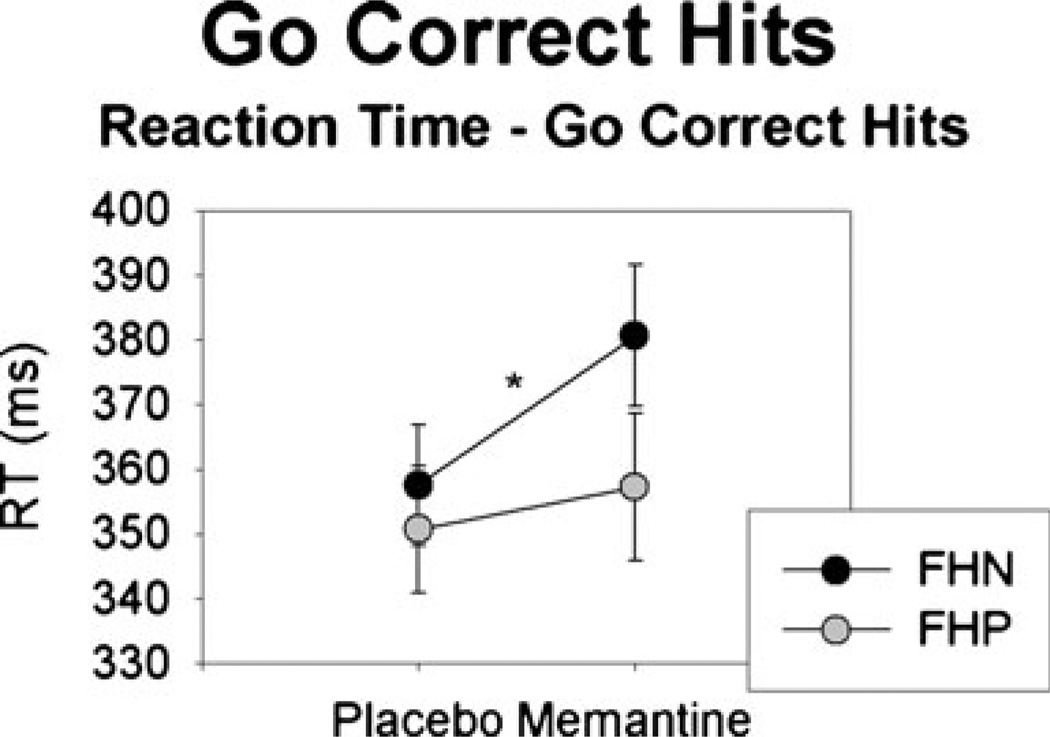
During placebo, there was no FH group difference for Go correct hit RTs (F(1,26)=0.022, p=0.88; Fig. 1). Following memantine, RTs for Go correct hit trials were increased relative to placebo (F(1,26)=5.83, p=0.023); this RTslowing was marginally larger in the FHN than the FHP group (F(1,26)= 3.66, p=0.07). When analyzing groups separately, the increased RT for memantine relative to placebo was significant in the FHN (F(1,13)=6.96, p=0.02) but not the FHP group (F (1,12)=0.27, p=0.61).
Fig. 1.
Mean (standard error) RT (in milliseconds) for Go correct hits after placebo or memantine in the FHN and FHP groups. The FHN (but not FHP) group exhibited a significant increase in RT in memantine vs. placebo sessions
No-Go correct rejects
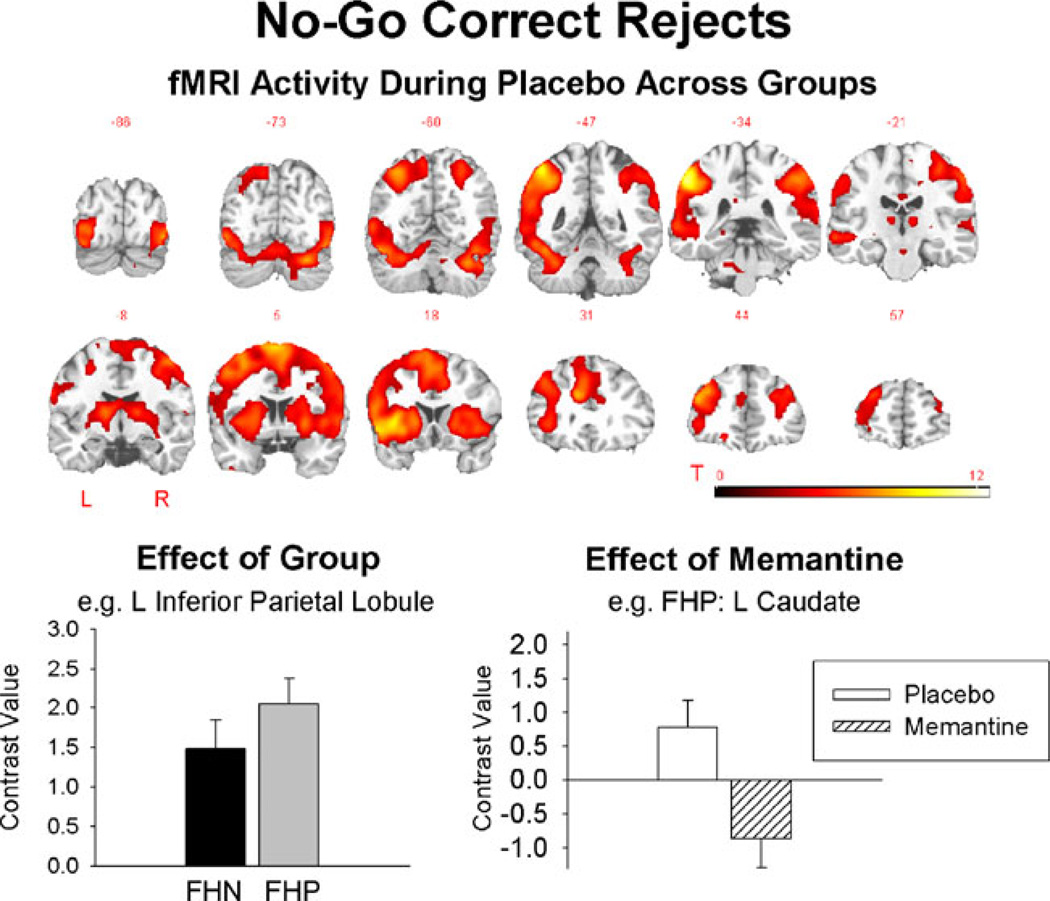
fMRI results for No-Go correct rejects vs. baseline are shown in Fig. 2 and Table 2. No-Go correct rejects during placebo activated a distributed cortical network that is commonly activated in tasks of response inhibition, including premotor, frontal, striatal, and parietal regions. During placebo, the FHP group showed increased activity in the right anterior cingulate and left inferior parietal lobule relative to the FHN group. Following memantine challenge, the FHN group showed reduced activity in the right cerebellar crus 1 relative to placebo. For the FHP group, memantine challenge reduced activity in left anterior cingulate and left caudate relative to placebo.
Fig. 2.
Top fMRI activity during No-Go correct rejects during placebo averaged across groups (the groups did not differ). The groups differed on placebo sessions in several brain regions, exemplified by the bottom left graph showing higher contrast values in the FHP compared to the FHN group. Following memantine administration, fMRI activity reduced in cortical regions in FHP but not FHN as illustrated in the bottom right graph. As there were many ROIs showing a similar pattern of effects, examples are given here and full presentation of results across all ROIs is given in the Supplementary material (Supplementary Fig. 1). All error bars show the standard error
Table 2.
Regions activated for No-Go correct rejects compared to implicit baseline in participants with or without a family history of alcoholism and after either placebo or memantine
| Region (BA) | Left hemisphere |
FHP vs. FHN (placebo)a |
FHP—effect of memantineb |
Right hemisphere |
FHP vs. FHN (placebo)a |
FHN—effect of memantineb |
|---|---|---|---|---|---|---|
| Superior frontal gyrus (10) | −30, 48, 24 | 33, 51, 27 | ||||
| Supplementary motor area (6) | −15, 9, 69 | 9, 6, 69 | ||||
| Middle frontal gyrus (6/8) | −45, 3, 51 | 33, 3, 54 | ||||
| Inferior frontal gyrus (9/47) | −60, 6, 24 | 48, 18, −3 | ||||
| Anterior cingulate (32) | −15, 33, 21 | ↓ | 12, 30,24 | ↑ | ||
| Precentral gyrus (6) | −42, 0, 54 | 48, 6, 45 | ||||
| Insula | −39, 12, −6 | 39, 18, 0 | ||||
| Caudate | −6, 15, 12 | ↓ | − | |||
| Putamen | −24, −63, 51 | 12, −69, 51 | ||||
| Thalamus | −12, −18, 6 | 15, −12, 9 | ||||
| Pons | − | 9, −30, −39 | ||||
| Parahippocampal gyrus | − | 21, −33, −3 | ||||
| Posterior cingulate (23) | −3, −30, 24 | 9, −33, 27 | ||||
| Inferior parietal lobule (40) | −42, −39,48 | ↑ | 57, −39, 42 | |||
| Superior temporal gyrus (38) | −51, 12, −9 | 51, 18, −9 | ||||
| Middle temporal gyrus (37) | −51, −57, 3 | 57, −57, 0 | ||||
| Middle occipital gyrus (18) | −33, −96, −6 | 48, −75, −9 | ||||
| Inferior occipital gyrus (18) | −39, −90, −9 | 30, −96, −9 | ||||
| Fusiform gyrus (39) | −39, −66, −18 | 42, −51, −21 | ||||
| Tonsil | −39, −48, −42 | 33, −57, −42 | ||||
| Vermis | 3, −78, −24 | |||||
| Declive | −36, −60, −27 | 36, −60, −27 | ||||
| Cerebellum crus 1 | −30, −60, −33 | 36, −54, −33 | ↓ | |||
| Cerebellum crus 2 | −33, −63, −39 | 42, −48, −39 |
Regions activated for No-Go correct rejects >baseline across groups during placebo. Only variables showing significant experimental effects are shown, thus FHN effect of memantine for the left hemisphere and FHP effect of memantine right hemisphere are not shown
Regions showing differences between groups during placebo. An upward arrow represents greater activity for FHP vs. FHN and a downward arrow represents a reduction in activity for FHP vs. FHN
Regions showing changes in activity for memantine vs. placebo. A downward arrow represents a reduction in activity during memantine challenge vs. placebo, an upward arrow represents an increased in activity during memantine challenge vs. placebo
No-Go false alarms
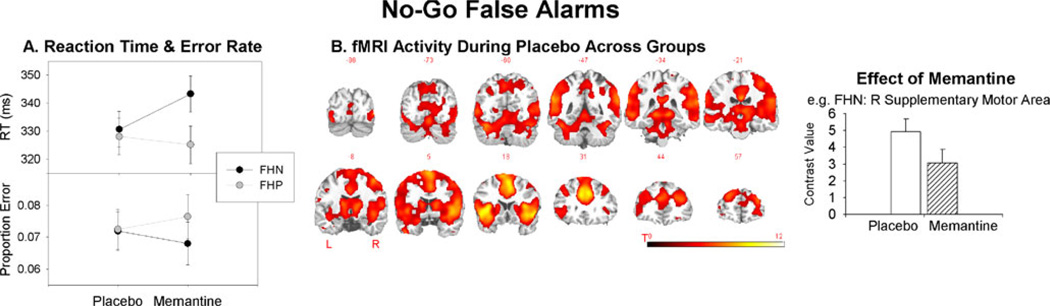
During placebo, there was no FH group difference for No-Go false alarm RTs (F(1,26)=0.11, p=0.74; Fig. 3a). The main effects of dose and group were not significant (both p>0.10). The dose×group interaction (F(1,24)04.53, p=0.04) indicated that there was a difference in the change in RT from placebo to memantine between groups. In analyses of each group separately, the increase in RT for memantine relative to placebo for FHN just failed to reach significance (F(1,13)=2.59,p=0.13), suggesting that FHN showed a trend towards changes in impulsivity following memantine challenge. The reduction in RT for memantine relative to placebo for FHP was not significant (F(1,12)00.17,p=0.69). There were no effects of group, dose, or group × dose interaction for proportion error (all F<1.0).
Fig. 3.
No-Go false alarms. a RT and proportion error for No-Go false alarms for each group. b fMRI activity for No-Go false alarms during placebo averaged across groups (groups did not differ). Following memantine administration, fMRI activity reduced in a broad range of cortical, subcortical, and cerebellar regions relative to placebo in FHN (exemplified by the right graph); for FHP, fMRI activity reduced in a smaller number of (mostly subcortical) regions relative to placebo. As there were many ROIs showing a similar pattern of effects, an example is shown here and full presentation of results across all ROIs is given in the Supplementary material (Supplementary Fig. 2). All error bars show the standard error
fMRI results for No-Go false alarms (No-Go false alarms >baseline) are shown in Fig. 3b and Table 3. No-Go false alarms during placebo activated a similar network as No-Go correct rejects. Activity for No-Go false alarms did not differ between groups during placebo. For the FHN group, memantine challenge decreased activity in the left superior frontal gyrus, middle frontal gyrus, anterior cingulate, posterior cingulate, inferior temporal gyrus, fusiform gyrus, lingual gyrus, and cerebellar vermis; bilateral supplementary motor area; and right precuneus, middle temporal gyrus, right cerebellar culmen, and tonsil relative to placebo. In the FHP group, memantine challenge decreased activity in the left insula and precuneus; bilateral putamen and caudate; and right posterior cingulate and superior temporal gyrus relative to placebo.
Table 3.
MNI coordinates for the peak of activity in regions activated for No-Go false alarms compared to implicit baseline in FHN and FHP individuals after administration of memantine or placebo
| Region (BA) | Left hemisphere |
FHN—effect of memantine |
FHP—effect of memantine |
Right hemisphere |
FHN—effect of memantine |
FHP—effect of memantine |
|---|---|---|---|---|---|---|
| Superior frontal gyrus (10/9) | −30, 48, 18 | ↓ | 27, 54, 18 | |||
| Supplementary motor area (6/8) | −3, 15, 51 | ↓ | 3, 15, 51 | ↓ | ||
| Middle frontal gyrus (10) | −33, 39, 18 | ↓ | 39, 42, 15 | |||
| Inferior frontal gyrus (47) | −39, 18, −6 | 39, 18, −6 | ||||
| Anterior cingulate (32) | −6, 27, 33 | ↓ | 6, 30, 27 | |||
| Precentral gyrus (6) | −42, −15, 51 | 24, −18, 57 | ||||
| Insula | −42, 9, −9 | ↓ | 39, 15, 0 | |||
| Thalamus | −12, −15, 0 | 12, −6, −3 | ||||
| Putamen | −18, 12, 3 | ↓ | 18, 6, 3 | ↓ | ||
| Caudate | −15, 9, 12 | ↓ | 15, 9, 3 | ↓ | ||
| Subthalamic nucleus | −9, −15, −6 | 12, −15, −6 | ||||
| Parahippocampal gyrus | −9, −33, −3 | 12, −33, −3 | ||||
| Superior parietal lobule (7) | −30, −57, 54 | ↓ | 27, −66, 51 | ↓ | ||
| Precuneus (7) | −6, −51, 51 | ↓ | 15, −66, 54 | ↓ | ||
| Inferior parietal lobule (40) | −63, −42, 21 | 60, −51, 27 | ||||
| Posterior cingulate (30) | −21, −69, 3 | ↓ | 0, −24, 27 | ↓ | ||
| Superior temporal gyrus (42/22/38) | −45, 12, −15, | 45, 12, −15 | ↓ | ↓ | ||
| Middle temporal gyrus (21/39) | −57, −27, −9 | 60, −27, −12 | ||||
| Inferior temporal gyrus (37) | −51, −72−6 | ↓ | 54,−72, −6 | |||
| Fusiform gyrus (37) | −42, −48, −21 | ↓ | 42, −54, −24 | |||
| Lingual gyrus (18) | −12, −72, 0 | ↓ | 18, −66, 0 | |||
| Cuneus (18) | −12, −75, 9 | 18, −69, 6 | ||||
| Culmen | −30, −57, −30 | 30, −57, −30 | ↓ | |||
| Cerebellum crus 1 | −42, −57, −27 | 42, −57, −27 | ||||
| Tonsil | −36, −57, −45 | 36, −57, −42 | ↓ | |||
| Vermis | −3, −69, −9 | ↓ | 3, −9, −15 |
Regions activated for No-Go correct rejects >baseline across groups during placebo. Only variables showing significant experimental effects are shown, thus FHP vs. FHN placebo for both hemispheres is not shown. A downward arrow represents a reduction in activity during memantine challenge vs. placebo, an upward arrow represents an increased in activity during memantine challenge vs. placebo
Discussion
This study evaluated the effects of a single dose of the NMDA glutamate receptor antagonist, memantine, on risk-taking, choice impulsivity, and Go/No-Go performance in individuals with and without a family history of alcoholism. We hypothesized that FHP individuals would show impaired performance on risk-taking and impulsivity measures previously linked to addiction propensity (Meda et al. 2009; Reynolds et al. 2006). We also hypothesized that FHP individuals would show impaired No-Go response inhibition and differential fMRI responses relative to FHN individuals and that the effects of memantine on response inhibition would differ between FH groups. Since (a) FHP individuals manifest reduced sensitivity to the adverse effects—i.e., “intoxication signals”—associated with memantine administration (Petrakis et al. 2004; Krystal et al. 2003a, b), (b) Go/No-Go behavior and fMRI activity are affected by alcohol administration (Anderson et al. 2011), and (c) FHP individuals show differences in Go/No-Go response inhibition fMRI activity (DeVito et al. 2010; Heitzeg et al. 2010; Schweinsburg et al. 2004), a key underlying hypothesis in this study was that FHP individuals would manifest reduced sensitivity to the adverse effects of memantine on intoxication signals: in this case, the Go/No- Go task. The major finding of this study was that memantine had a larger effect on Go/No-Go behavior and neural activation as assessed by fMRI in FHN individuals than in FHP individuals.2 Thus, this study provides the first neural evidence supporting the hypothesis that FHP individuals show reduced “intoxication signals” following administration of an NMDA receptor antagonist, consistent with presumed enhancements in NMDA receptor function in the FHP group.
Go/No-Go performance
Performance and fMRI activity during placebo
The networks activated during No-Go correct rejects and No-Go false alarms during placebo are consistent with those identified in previous studies. No-Go correct rejects and false alarms activated a cortico-thalamic-striatal network commonly activated in tasks of response inhibition (e.g., Aron and Poldrack 2006; Jamadar et al. 2010; Rubia et al. 2001; Stevens et al. 2007).
During placebo, FHN and FHP groups did not differ for Go correct hit RT or No-Go false alarm RT. No-Go false alarms are particularly relevant in the current context given that they are the major index of impulsivity in the Go/No-Go paradigm (Saunders et al. 2008). These results are consistent with the behavioral results of Schweinsburg et al. (2004), Saunders et al. (2008), and Heitzeg et al. (2010). In these studies, Go/No-Go performance did not differ between FHN and FHP individuals, even with large sample sizes (n=230; Saunders et al. 2008). Our fMRI results are also compatible with this pattern of behavioral results: fMRI activity for No-Go false alarms did not differ between FHN and FHP groups during placebo.
For No-Go correct rejects during placebo, the FHP group showed increased activity in the right anterior cingulate and inferior parietal lobule. These results are consistent with our hypothesis that FHP individuals would show differential Go/No-Go-related fMRI activity during placebo in the frontal and parietal regions compared to FHN subjects. The results are also compatible with previous studies of FHN and FHP individuals that showed differential No-Go correct reject activity in FHP relative to FHN individuals in fronto-striatal regions (DeVito et al. 2010; Heitzeg et al. 2010). Heitzeg et al. (2010) found deactivation for No-Go trials in the left caudate in FHN but not FHP participants and attenuated deactivations in the orbitofrontal and medial frontal regions in FHP relative to FHN individuals. Together with the finding that performance did not differ between these groups, they interpreted the attenuated deactivations as reflecting a compensatory mechanism allowing FHP individuals to achieve the same behavioral outcome as FHN individuals. Our results are compatible with this interpretation. Furthermore, Goldstein and Volkow (2002) and others (e.g., Kreek et al. 2005; Dalley et al. 2011) have argued that genetic susceptibility to addiction can be conceptualized as a syndrome of impulsivity, specifically related to impaired response inhibition. These disinhibitory tendencies are likely to result in a shift towards approach and less cautious behaviors. The anterior cingulate and parietal cortex are part of a distributed cortico-striatal network that underlies cognitive control and impulsivity in the brain (Everitt and Robbins 2005). These results are, therefore, consistent with the argument that genetic susceptibility for alcoholism is related to a shift towards more impulsive behavior. These results add to the growing body of literature demonstrating that clinical populations with problems of impulsivity show deficits of response inhibition neural activity (Chamberlain and Sahakian 2007).
Performance and fMRI activity during memantine challenge
FHN During memantine challenge, FHN individuals showed an increase in RT for Go correct hits and a trend towards an increase in RT for No-Go false alarms relative to placebo. Previous studies in primates and humans have shown that NMDA receptor antagonism results in motor hypoactivity (Kato and Kimura 1992) and slowing of perceptual processing, as indexed by N2 and P3a (Watson et al. 2009). The observation that memantine resulted in RT slowing is consistent with these earlier findings.
The increase in No-Go false alarm RT following mem-antine challenge in FHN was accompanied by a decrease in fMRI activity in the frontal, premotor, and parietal regions. These regions comprise well-known response networks that are highly implicated in the execution of a response. During memantine challenge, there was little change in the fMRI activity for No-Go correct rejects relative to placebo for FHN. To our knowledge, this is the first study to examine the effects of NMDA receptor antagonism in Go/No-Go performance in healthy humans. Oberlin et al. (2010) showed that memantine did not influence impulsivity or vigilance measures in high alcohol preferring mice. Grant et al. (2010) examined the effects of memantine challenge on Stop Signal RT (SSRT) in pathological gamblers. Pathological gamblers had longer SSRT than healthy controls at baseline; following 10 weeks of memantine treatment, SSRT did not differ between groups. However, this study did not examine the effects of memantine treatment on SSRT in the healthy control group. Morgan et al. (2004) examined the effects of ketamine administration on a measure they defined as response inhibition using the Hayling task (Burgess and Shallice 1997). However, the outcome measure was inhibition of a prepotent verbal semantic response, rather than a motor response, as measured here. Our results, therefore, suggest that response inhibition, as opposed to response execution, is not affected by NMDA receptor antagonism with memantine in healthy individuals without a genetic susceptibility to alcoholism.
FHP In contrast to the FHN group, memantine challenge had no effect on RT for Go correct hit or No-Go false alarm trials relative to placebo. For No-Go false alarms, memantine challenge resulted in a reduction in fMRI activity largely in striatal and parietal regions, with no significant effect in frontal regions; this represents a much reduced effect of mem-antine within the cortico-striatal motor networks in FHP vs.
FHN participants. Memantine challenge also reduced fMRI activity in the left anterior cingulate and caudate relative to placebo for No-Go correct rejects. Since NMDA receptor antagonism usually results in motor hypoactivity in primates and humans (Kato and Kimura 1992) and resulted in changes in RT and distributed fMRI activity for No-Go false alarms for FHN, these results are consistent with hypotheses of enhanced NMDA receptor function in these individuals (Krystal et al. 2003a, b; Petrakis et al. 2004; Phelps et al. 2009).
Risk-taking and impulsivity measures
Risk-taking and impulsivity contribute importantly to susceptibility for drug and alcohol addiction (Dalley et al. 2011; Lejuez et al. 2005, 2010). Risk-taking and impulsivity are associated with alcoholism (Lejuez et al. 2007; Petry 2001), individuals with familial risk of alcoholism show increased impulsivity relative to those without such a risk (Cloninger 1987; Ernst et al. 2006; Knop 1985; Petry et al. 2002; Saunders et al. 2008; Sher 1991), and impulsivity is an important intermediary phenotype for addictive disorders including alcoholism (Oberlin and Grahame 2009). Thus, we hypothesized that FHP individuals would show increased risk-taking on the BART and choice impulsivity on the EDT. Contrary to our hypotheses, there were no differences between groups and no effect of memantine vs. placebo on either the BART or the EDT.
Our negative results may be related to the constructs indexed by the tasks used in this study. Impulsivity is a multifactorial construct that has no universally accepted definition and encompasses the diminished ability to wait, a tendency to act without forethought, insensitivity to consequences, and diminished ability to inhibit responses (Reynolds et al. 2006). The EDT assesses one aspect of this construct. The BART and EDT tasks have been shown to factor separately (Meda et al. 2009), thus measuring separate constructs related to self-control. Furthermore, self-report and behavioral measures of impulsivity, even when assessing the same domain, do not consistently relate to one another. For example, behavioral and self-report aspects of choice impulsivity as assessed by delay discounting tendencies did not correlate among adolescent smokers (Krishnan-Sarin et al. 2007). Although our findings indicate that FHP individuals do not show impaired behavioral risk-taking (BART) and delay discounting (EDT), this does not exclude the possibility that they are impaired in other components of impulsiveness. Self-report scales show a more consistent pattern of impulsiveness in FHP individuals. Saunders et al. (2008) showed that FHP individuals showed lower California Psychological Inventory Sociability Scale scores, consistent with self-report measures reported by Cloninger (1987) and Sher (1991). Self-report measures may yield more broad and less specific characterization of impulsivity, encompassing multiple components of impulsivity. Thus, future studies should endeavor to fully characterize components of impulsivity and related constructs in FHP individuals and investigate their relationships to alcohol use risk and other clinically relevant behaviors.
Limitations and future directions
To our knowledge, this is the first study to investigate the effects of acute NMDA receptor antagonism on risk-taking, choice impulsivity, and Go/No-Go performance and associated BOLD activation in FHP and FHN groups. There exist several limitations. Firstly, our design was not optimized to study activity associated with Go correct hits. It would be meaningful to compare No-Go correct rejects to a Go correct hits baseline, thereby increasing the specificity of the results to inhibition over execution. Secondly, to measure the BOLD correlates of response inhibition, we compared No-Go correct rejects to implicit baseline. Although this offers some advantages over subtractive contrasts in interpretation (i.e., changes in activity in subtractive contrasts can be attributed to an increase/decrease in BOLD in one condition compared to another, or a change in both), there is also a disadvantage in attributing the results specifically to an inhibitory process. To address this, we conducted a supplementary analysis of No-Go correct rejects > No-Go false alarms (successful vs. unsuccessful inhibition; see the Supplementary material). These results support our conclusion that our No-Go correct rejects >baseline contrast indexed inhibition. Thirdly, memantine shows limited selectivity to the NMDA receptor, and at high concentrations, can influence other neurotransmitter systems (Johnson and Kotermanski 2006). Since at the dose used in the current study memantine affects primarily NMDA receptors, we believe that the results are due to NMDA receptor antagonism; however, future studies using other NMDA receptor antagonists such as ketamine should be conducted. Lastly, our study was limited to exploring effects of FH group and memantine in well-described response inhibition network regions that were robustly engaged across FH groups during placebo. This approach may have diminished our ability to detect the effects of FH group and memantine within regions where groups differed substantially during placebo. This should be explored in future work using samples that provide sufficient statistical power to conclusively address that issue.
Conclusions, implications, and future directions
In sum, although we found no differences between groups or effect of memantine on several behavioral risk-taking or choice impulsivity measures, we did show that memantine had an attenuated effect in FHP vs. FHN on both behavior and associated brain activation patterns during Go/No-Go performance. Our results, in some respects, point to a double-edged sword with regards to the understanding of patterns of alcohol use and response. Together with previous results, our study suggests that FHP individuals may not detect their growing level of intoxication compared to FHN. This is compatible with prior data on drinking behavior in FHP (Lipscomb and Nathan 1980; Lansky et al. 1978). However, our results also indicate that FHN individuals are more vulnerable to impair-mentin Go/No-Go performance evenbylow levels ofNMDA receptor blockade—suggesting that they may be more likely to have problems with driving and other critical aspects of behavioral control at modest levels of intoxication.
In summary, the current results are compatible with the hypothesis that NMDA receptor function is abnormal in FHP, such that memantine has an attenuated effect on Go and No-Go false alarm RT and fMRI activity in FHP relative to FHN individuals. Petrakis et al. (2004) showed that FHP individuals showed attenuated perceptual alterations and dysphoric mood following ketamine challenge relative to FHN individuals. This reduced response to ketamine and memantine in FHP individuals is compatible with the argument that NMDA receptor function is enhanced in these individuals. Findings indicate that (a) alcohol is a potent NMDA receptor antagonist (Tsai et al. 1995), (b) chronic intake of alcohol results in the upregulation of NMDA receptors (e.g., Krystal et al 2003a, b), (c) FHP individuals show reduced sensitivity to the sedative, dysphoric, and cognitive effects of acute alcohol intoxication relative to FHN individuals (reviewed in Krystal et al. 2003a, b), and (d) FHP individuals show reduced sensitivity to ketamine (Petrakis et al. 2004) and memantine during response execution (current study). Together, these results suggest that deficits of impulsivity and NMDA receptor function may underlie the genetic susceptibility towards alcoholism in FHP.
Supplementary Material
Acknowledgments
Acknowledgments/disclosures This work was funded by 1 P50 AA12870-05 (J Krystal PI; G. Pearlson Project 4). E.E. DeVito was supported by T32 AA015496 (Petrakis, PI) and K12 DA031050. Dr. Potenza was supported in part by RL1 AA017539. J.H. Krystal has served as a paid scientific consultant to Merz Pharmaceuticals, Forest Pharmaceuticals, and Lundbeck, Inc., companies that distribute memantine.
Footnotes
Formal tests of group×dose interaction yielded results that did not survive correction for multiple comparisons. These are shown in the Supplementary material.
Given that the effects of memantine were restricted to RT and not error rate, it is plausible that the effects were due to a general slowing rather than an effect specific to response inhibition. We prefer our explanation for two reasons. (1) There was a differential effect of memantine on RT between FHN and FHP for No-Go false alarms and Go correct hits. Also note that, as outlined in the “Methods” and “Limitations and future directions” sections, the current design is not optimized to study No-Go errors. Thus, the error data should be interpreted with caution. (2) More importantly, in an auditory oddball EEG paradigm, Narayanan et al. (in preparation) show that (a) FHP showed decreased alpha and theta activity relative to FHN—this is consistent with cognitive deficits and abnormal inhibitory control in FHPs; (b) following memantine administration, alpha activity was normalized in FHP—i.e., approached FHN levels during placebo; and for FHN, alpha activity was reduced following memantine; (c) following memantine, theta power decreased in both groups, but more markedly for FHN than FHP. These results are more compatible with an effect of memantine on inhibitory behavior rather than a simple target detection effect.
Electronic supplementary material The online version of this article (doi:10.1007/s00213-011-2628-2) contains supplementary material, which is available to authorized users.
Contributor Information
S. Jamadar, Email: sjamadar@harthosp.org, Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT 06106, USA.
E. E. DeVito, Department of Psychiatry, Yale University, New Haven, CT, USA
R. E. Jiantonio, Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT 06106, USA
S. A. Meda, Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT 06106, USA Center of Human Genetics and Research, Vanderbilt University, Nashville, TN, USA.
M. C. Stevens, Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT 06106, USA Department of Psychiatry, Yale University, New Haven, CT, USA.
M. N. Potenza, Department of Psychiatry, Yale University, New Haven, CT, USA Department of Neurobiology, Yale University, New Haven, CT, USA; Child Study Center, Yale University, New Haven, CT, USA.
J. H. Krystal, Department of Psychiatry, Yale University, New Haven, CT, USA Clinical Neuroscience Division, VA National Center for PTSD, VA Connecticut Healthcare System, West Haven, CT, USA; Psychiatry Services, Yale-New Haven Hospital, New Haven, CT, USA.
G. D. Pearlson, Olin Neuropsychiatry Research Center, Institute of Living, Hartford, CT 06106, USA Department of Psychiatry, Yale University, New Haven, CT, USA; Department of Neurobiology, Yale University, New Haven, CT, USA.
References
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:1–10. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, et al. Individuals family history positive for alcoholism show functional magnetic resonances imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacol. 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Burgess P, Shallice T. The Hayling and Brixton tests. Thames Valley est Company Bury St Edmunds; 1997. [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impul-sivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Collins ED, Vosberg SK, Ward AS, Haney M, Foltin RW. The effects of acute pretreatment with high-dose memantine on the cardiovascular and behavioral effects of cocaine in humans. ExpClin Psychopharmacol. 2007;15:228–237. doi: 10.1037/1064-1297.15.3.228. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Ridenour T, Ben-Abdallah A, Spitznagel EL. The specificity of family history of alcohol and drug abuse in cocaine abusers. Am J Addict. 2002;11:85–94. doi: 10.1080/10550490290087866. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity and top-down control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Meda SA, Potenza MN, Krystal JH, Pearlson GD. The effects of memantine on the neural correlates of response inhibition with a family history of alcoholism; Poster presented at the Research Society of Alcoholism.2010. [Google Scholar]
- Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eschel N, London ED, Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addition: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB. The DSM series and experience with DSM-IV. Psy-chopathol. 2002;35:67–71. doi: 10.1159/000065121. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Odlaug BL, Potenza MN, Kim SW. Memantine shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling: a pilot study. Psychopharmacol. 2010;212:603–612. doi: 10.1007/s00213-010-1994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zucker RA, Zubieta J-K. Striatal dysfunction marks preexisting risk and medial prefrontal dysfunction is related to problem drinking in children of alcoholics. Biol Psychiatry. 2010;68:287–295. doi: 10.1016/j.biopsych.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamadar S, Hughes ME, Fulham WR, Michie PT, Karayanidis F. The spatial and temporal dynamics of anticipatory preparation and response inhibition in task switching. NeuroImage. 2010;51:432–449. doi: 10.1016/j.neuroimage.2010.01.090. [DOI] [PubMed] [Google Scholar]
- Jarvis B, Figgitt DP. Memantine. Drugs Aging. 2003;20:465–476. doi: 10.2165/00002512-200320060-00005. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ. Event related fMRI. Hum Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Kato M, Kimura M. Effects of reversible blockade of basal ganglia on a voluntary arm movement. J Neurophysiol. 1992;68:1516–1534. doi: 10.1152/jn.1992.68.5.1516. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiol. 2000;37:216–223. [PubMed] [Google Scholar]
- Knop J. Premorbid assessment of young men at high risk for alcoholism. Recent Dev Alcohol. 1985;3:53–64. doi: 10.1007/978-1-4615-7715-7_5. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, et al. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D’Souza DC. NMDA receptor antagonism and the ethanol intoxication signal. From alcoholism risk to pharmacotherapy. Ann NY Acad Sci. 2003a;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003b;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Nappi SK, Trevisan L, Pittman B, D’Souza DC. Characterization of the interactive effects of glycine and D-cycloserine in men: further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharm. 2011;36:701–710. doi: 10.1038/npp.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansky D, Nathan PE, Ersner-Hershfield SM, Lipscomb TR. Blood alcohol level discrimination: pre-training monitoring accuracy of alcoholics and nonalcoholics. Addict Behav. 1978;3:209–214. doi: 10.1016/0306-4603(78)90021-7. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez C, Aklin W, Bornovalova M, Moolchan E. Differences in risk-taking propensity across inner-city adolescent ever- and never-smokers. Nicotine Tob Res. 2005;7:71–79. doi: 10.1080/14622200412331328484. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the youth version of the Balloon Analogue Risk Task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. J Clin Child Adolesc Psychol. 2007;36:106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems and disorders. Alcohol Clin Exp Res. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb TR, Nathan PE. Effect of family history of alcoholism, drinking pattern and tolerance on blood ethanol level discrimination. Arch Gen Psychiatry. 1980;37:571–576. doi: 10.1001/archpsyc.1980.01780180085010. [DOI] [PubMed] [Google Scholar]
- Loskutova LV, Kostjunina NV. Effects of memantine on latent inhibition of active avoidance in Wistar rats. Bull Exp Biol Med. 2009;147:691–694. doi: 10.1007/s10517-009-0605-0. [DOI] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Potenza MD, Pittman B, Gueorguieva R, Andrews MM, Thomas AD, Muska C, Hylton JL, Pearlson GD. Investigating the behavioral constructs of impulsivity domains using principal component analysis. Behav Pharmacol. 2009;20:390–399. doi: 10.1097/FBP.0b013e32833113a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacol. 2004;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Grahame NJ. High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol Clin Exp Res. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Bristow RE, Heighton ME, Grahame NJ. Pharma-cologic dissociation between impulsivity and alcohol drinking in high alcohol preferring mice. Alcohol Clin Exp Res. 2010;34:1363–1375. doi: 10.1111/j.1530-0277.2010.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Maji HK, et al. Family history of alcohol dependence and initial antide-pressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Process. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, et al. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Sorocco KH, Lovallo WR. Impulsive errors on a go/no-go reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, et al. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann NY Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Psychological characteristics of children of alcoholics. Overview of research methods and findings. Recent Dev Alcohol. 1991;9:301–326. [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- van Wageningen H, Jorgensen HA, Specht K, Hugdahl K. Evidence for glutamatergic neurotransmission in cognitive control in an auditory attention task. Neurosci Letters. 2009;454:171–175. doi: 10.1016/j.neulet.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.