Abstract
Objective
Trauma is associated with increased risk for anxiety disorders such as posttraumatic stress disorder (PTSD). To further understand biologic mechanisms of PTSD, we examined the dark-enhanced startle response, a psychophysiological correlate of anxiety, and heart rate variability (HRV) in traumatized individuals with and without PTSD. The associations of these measures with PTSD may be sex-specific because of their associations with the bed nucleus of the stria terminalis, a sexually dimorphic brain structure in the limbic system that is approximately 2.5 times larger in men than in women.
Methods
The study sample (N = 141) was recruited from a highly traumatized civilian population seeking treatment at Grady Memorial Hospital in Atlanta, Georgia. Psychophysiological responses during a dark-enhanced startle paradigm task included startle magnitude, assessed by eyeblink reflex, and measures of high-frequency HRV, during light and dark phases of the startle session.
Results
The startle magnitude was higher during the dark phase than the light phase (mean ± standard error = 98.61 ± 10.68 versus 73.93 ± 8.21 μV, p < .001). PTSD was associated with a greater degree of dark-enhanced startle in women (p = .03) but not in men (p = .38, p interaction = .48). Although HRV measures did not differ between phases, high-frequency HRV was greater in men with PTSD compared with men without PTSD (p = .02).
Conclusions
This study demonstrates that the dark-enhanced paradigm provides novel insights into the psychophysiological responses associated with PTSD in traumatized civilian sample. Sex differences in altered parasympathetic and sympathetic function during anxiety regulation tasks may provide further insight into the neurobiological mechanisms of PTSD.
Keywords: PTSD, acoustic startle response, heart rate, HRV, dark-enhanced startle, sex differences
INTRODUCTION
Posttraumatic stress disorder (PTSD) is an anxiety disorder initiated by an event causing fear, helplessness, and horror (1). The risk of PTSD increases with high rates of exposure to violence and abuse, which is common in low-income urban environments (2–4). One of the hallmark clinical presentations of PTSD is hyperarousal symptoms, which can include an exaggerated startle response (1) and heightened autonomic nervous system (ANS) activity (5,6).
The acoustic startle response is a reflexive contraction of the skeletal musculature that occurs in response to a sudden auditory stimulus (7). This reflex is mediated by a simple three-synapse circuit that is highly conserved phylogenetically (8); thus, animal and human startle studies use similar methodologies. Moreover, the startle response can be a clinically useful tool; a recent meta-analysis of 25 startle studies in PTSD found moderate to large effects with eyeblink electromyography and heart rate and skin conductance responses to startle stimuli (6). Modulation of the acoustic startle response occurs through different neural pathways in the limbic system: the amygdala mediates fear responses to specific cues, and the bed nucleus of the stria terminalis (BNST) mediates responses to contextual cues and nonspecific anxiogenic cues (9). “Light-enhanced” startle, or the elevated response produced when rats are exposed to startling stimuli in the presence of bright light, represents a form of contextual modulation of the acoustic startle reflex (9). It is dependent on the BNST, a sexually dimorphic structure in the limbic system in both rodents (10–12) and humans (13,14). In fact, this brain structure shows sex differences in all vertebrates and is associated with regulation of gonadal hormones, neurotransmitters, and autonomic activity (12). In a postmortem study of human brains, the volume of the BNST was found to be 2.5 times larger in men than in women (13) and develops in adulthood (14).
The human homolog to rodent light-enhanced startle is “dark-enhanced” startle (15) given that darkness is commonly recognized as an aversive context in most human beings (16). The effect of darkness on the acoustic startle response has been previously investigated in Vietnam veterans with and without PTSD (17). The authors of the latter study found that increased startle response in the presence of darkness was specific to combat exposure rather than PTSD. More specifically, all veterans showed elevated startle in the dark as compared with healthy volunteers, but there was no diagnostic group difference between the veterans. On the other hand, studies of adolescents at risk for anxiety disorders have found that elevated dark-enhanced startle is associated with such risk (18).
Altered prefrontal inhibition of limbic areas has been a common neurobiological finding in PTSD (19–21). One of the regulatory targets of the prefrontal cortex is the parasympathetic nervous system (PNS) (22); this connection has been implicated in altered emotional regulation in anxiety disorders (23). The PNS is a component of the ANS; in tandem with the sympathetic nervous system, the two components regulate smooth muscle activity and cardiovascular arousal. Respiratory sinus arrhythmia (RSA), which can be noninvasively measured with spectral analysis of high-frequency heart rate variability (HF-HRV), provides a measure of PNS cardiac control via the vagus nerve (24–27). HF-HRV or RSA is most commonly assessed during resting states, when vagal output should be high (28,29), but it is also sensitive to mental and emotional stressors (27,28,30–33).
HRV has been empirically studied in patients with PTSD with mixed results. Some studies report decreased low-frequency HRV during rest (28), whereas others find decreased RSA (5,34). One study examined HRV during sleep and found that subjects with PTSD had higher low-frequency/HF-HRV ratios during rapid eye movement sleep compared with trauma controls (35). Others have reported no differences during rest but observed increased vagal output in non-PTSD subjects during a stress task (30) or decreased RSA in PTSD subjects during acute stress (31). A study of active duty soldiers undergoing intense training found that lower RSA was associated with superior performance under stress (36). A recent meta-analysis of psychophysiology in PTSD reported several studies with heart rate changes to startle cues (6), but no studies to date have examined heart rate components of PNS and sympathetic nervous system separately in response to startle stimuli. However, one study found that individuals with low HRV showed higher startle magnitude in a threat-of-shock experiment (37). These heart rate measures demonstrate sex differences as well. One study found a greater positive correlation between the prefrontal cortex and the RSA in men than in women (38). The sex disparities seem to extend to PTSD as well; a recent study found that increased heart rate to trauma reminders soon after the trauma exposure predicted PTSD diagnosis 6 months later in women but not in men (39).
The present study examined the effect of an anxiogenic context, darkness, on startle responses and PNS function (HF-HRV) in traumatized subjects with and without PTSD. We hypothesized that subjects with PTSD would show increased startle responses but decreased PNS activity during darkness compared with those without PTSD. Furthermore, given the data on sex differences in the BNST and HRV, we hypothesized that darkness may have different effects in men and women with PTSD.
METHODS
Study Subjects
The study recruited 141 participants as part of a larger study investigating the genetic and environmental factors that contribute to PTSD in a primarily African American, highly traumatized, low socioeconomic status, inner-city population. Participants were recruited from August 2008 to March 2010 from the primary care waiting rooms at Grady Memorial Hospital in Atlanta, Georgia. Exclusion criteria for participation in the study included active psychosis, major medical illnesses including hypertension and other cardiovascular diseases (as assessed by physical examinations conducted by licensed medical professionals), positive urine pregnancy test result, and/or positive urine drug screen result. All subjects were screened for auditory impairment using an audiometer (Model GS1710; Grason-Stadler, Eden Prairie, MN). Before their participation, all participants signed written informed consent that was approved by the Emory University Institutional Review Board.
Psychological Assessment
Modified PTSD Symptom Scale
PTSD was assessed using the modified PTSD Symptom Scale (PSS). This is a psychometrically valid 17-item self-report scale assessing PTSD symptoms over the 2 weeks before rating (2,40,41). The categorical definition of PTSD was determined based on DSM-IV (1) A to E criterion responses to the PSS questionnaire.
Childhood Trauma Questionnaire
The Childhood Trauma Questionnaire (CTQ) is a self-report inventory assessing childhood physical, sexual, and emotional abuse. Studies have established the internal consistency, stability over time, and criterion validity of both the original 70-item CTQ and the current brief version (42,43). The CTQ yields a total score and subscale scores for each of the types of child abuse.
Traumatic Events Interview
The Traumatic Events Interview (TEI) (2) assesses lifetime history of trauma exposure and is a measure of both child abuse and adult trauma. The TEI assesses past experience and frequency of 13 separate types of traumatic events and feelings of terror, horror, and helplessness with such events.
Experimental Design
Figure 1 shows a diagram of the startle session. The startle paradigm began with a 2-minute acclimation period during which no startle probes were delivered, followed by a startle habituation segment and a dark-enhanced startle segment that occurred without interruption. The startle habituation segment consisted of two blocks with four startle probes in each block, for a total of eight probes. Immediately after habituation, participants underwent the dark-enhanced segment consisting of two blocks each with eight startle probes. In each block, four startle probes were delivered in the dark phase, and four were delivered in the light phase. The dark phase refers to total darkness—there was no ambient light, and the startle booth was configured to isolate all external light sources, resulting in absolute darkness. For example, the participants could not see their hand several inches in front of their face. The first startle probe was delivered after 20 seconds of darkness to allow the subject to adjust to the condition. The light and dark phases alternated, with each phase lasting 1 minute. The order of light and dark was counterbalanced across subjects. The lights in the startle booth were controlled by a timer that was synchronized with the presentation of the startle probes. Across the entire experimental session, intertrial intervals ranged from 9 to 22 seconds. The session duration was 8 minutes long (2-minute acclimation, 2-minute habituation, and 4-minute dark-enhanced segment; Fig. 1).
Figure 1.
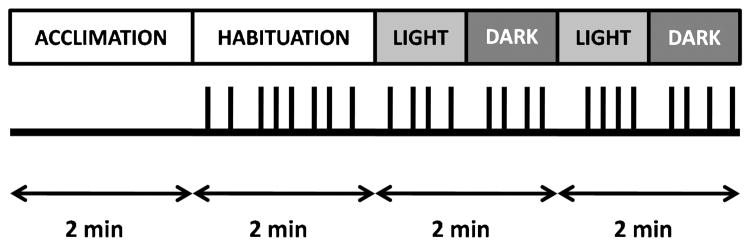
Dark-enhanced startle session diagram. Black lines represent acoustic startle probes (108 dB) delivered during each segment of the session. The order of light and dark phases within each block was counterbalanced across subjects.
Data Acquisition and Reduction
The physiological data were acquired using BIOPAC MP150 for Windows (BIOPAC Systems, Inc, Goleta, CA). The acquired data were filtered, rectified, and smoothed using MindWare software (MindWare Technologies, Ltd, Gahanna, OH) and were exported for statistical analyses. Startle data were collected by recording the eyeblink muscle contraction using the electromyography module of the BIOPAC system. The startle response was recorded with two Ag/AgCl electrodes: one was placed on the orbicularis oculi muscle below the pupil; and the other, 1 cm lateral to the first one. A common ground electrode was placed on the palm. Impedance levels were less than 6 kΩ for each participant. The startle probe was a 108-dB [A] sound pressure level, 40-millisecond burst of broadband noise with zero rise time, delivered binaurally through headphones (TDH-39-P; MAICO, Minneapolis, MN). The maximum amplitude of the eyeblink muscle contraction 20 to 200 milliseconds after presentation of the startle probe was used as a measure of startle magnitude.
Heart rate data were acquired using the electrocardiogram (ECG) and respiration modules of the BIOPAC system. The ECG signal was amplified by a gain of 1000, filtered with a Hamming windowing function, and with a 60-Hz notch filter. ECG was recorded using two disposable Ag/AgCl electrodes pre-coated with electrolyte gel: one was placed on the right side of the upper torso, 1 cm below the clavicle; and the second, on the inside of the left wrist. Respiration was measured using a chest band transducer and was assessed as respiratory rate per minute because this variable has been found to affect HF-HRV (44). HRV was quantified during 1-minute intervals by spectral analysis of the time-sampled interbeat interval series, according to the methods recommended by the Society for Psychophysiological Research Committee on HRV (26). HF-HRV (RSA) was sampled from 0.12 to 0.40 Hz and was transformed by natural log.
Statistical Analyses
Diagnostic groups (PTSD and no PTSD) were based on the PSS assessment using DSM-IV criteria for PTSD and were used as the between-groups factor in all analyses. Sex (men and women) was used as a separate independent factor in analyses.
Demographic and clinical data such as age, PTSD symptoms, and childhood and adult trauma levels were compared between the diagnostic groups and sex using two-way analyses of variance (ANOVAs). Categorical data, such as sex, were analyzed using χ2 analyses with PTSD diagnostic categories. Baseline levels of startle magnitude and HRV during the habituation phase were compared between PTSD and no-PTSD groups using two-way mixed ANOVAs with habituation block (two levels) as the within-subjects factor.
The startle and HRV data during the dark-enhanced segment were analyzed in a four-way mixed ANOVA, with block (two levels) and phase (light and dark) as repeated-measures factors and diagnosis and sex as between-groups factors. The dependent variables were startle magnitude, RSA (HF-HRV), and respiratory rate. Significant interactions were decomposed into respective univariate ANOVAs comparing diagnostic groups within each sex. Finally, to examine the individual contributions of abuse history and psychopathology to the dependent measures, we performed hierarchical regression analyses in which childhood abuse, adult abuse levels, and PTSD diagnosis were added at each step to predict startle magnitude and HRV indices.
In the repeated-measures ANOVAs, we used the Huynh-Feldt statistic to correct for violations of the sphericity assumption. All analyses were performed in SPSS 18.0 for Windows (SPSS, Inc, Chicago, IL) with an α level of 0.05.
RESULTS
Participant Characteristics
One hundred forty-one participants were recruited and completed the study. The study included PTSD (n = 47) and no-PTSD trauma control (n = 94) participants. All participants were African American; 61.7% were women, and the average age was 39.8 years (range = 18–77 years). The diagnostic groups did not differ on any demographic variables (Table 1). Table 2 shows trauma history and levels of PTSD symptoms across diagnostic groups, separately for men and women. Although there was a significant main effect of diagnostic group on these variables, there were no effect of sex and no interaction effect of sex and group (Table 2).
TABLE 1.
Demographic Data for the Sample Divided Between PTSD and No-PTSD Groups
| Demographics | No PTSD (n = 94) | PTSD (n = 47) | p |
|---|---|---|---|
| Sex, n (%) | .71 | ||
| Women | 57 (60.6) | 30 (63.8) | |
| Men | 37 (39.4) | 17 (36.2) | |
| Age, M (SD) | 39.95 (11.62) | 39.86 (12.94) | .97 |
| Education, n (%) | .14 | ||
| <12th grade | 13 (27.7) | 19 (20.2) | |
| High school/GED | 17 (36.1) | 49 (52.1) | |
| Some college/technical school | 15 (31.9) | 18 (19.1) | |
| Technical/college/grad school degree | 2 (4.2) | 8 (8.5) | |
| Employment, n (%) | .11 | ||
| Unemployed | 71 (75.5) | 41 (87.2) | |
| Employed | 23 (24.5) | 6 (12.8) | |
| Income, n (%) | .27 | ||
| $0–$249 | 18 (38.3) | 24 (25.5) | |
| $250–$999 | 22 (46.8) | 47 (50.9) | |
| ≥$1000 | 7 (14.9) | 21 (22.4) |
PTSD = posttraumatic stress disorder; M = mean; SD = standard deviation; GED = General Educational Development.
All participants were African American.
TABLE 2.
Trauma History and PTSD Symptoms Across Diagnostic Groups and Sex
| No PTSD
|
PTSD
|
Effect, p
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
Men
|
Women
|
PTSD | Sex | PTSD × Sex | |||||
| M | SE | M | SE | M | SE | M | SE | ||||
| Trauma history | |||||||||||
| Childhood trauma | 33.5 | 1.2 | 41.0 | 2.4 | 52.5 | 5.3 | 53.8 | 3.7 | <.001 | .16 | .32 |
| Adult trauma | 3.0 | 0.3 | 2.1 | 0.3 | 4.29 | 0.4 | 4.45 | 0.5 | <.001 | .37 | .20 |
| PTSD symptoms | |||||||||||
| Total | 6.5 | 1.1 | 6.7 | 0.9 | 29.9 | 2.3 | 26.3 | 1.6 | <.001 | .24 | .16 |
| Reexperiencing | 1.3 | 0.4 | 1.7 | 0.3 | 8.06 | 1.1 | 6.63 | 0.8 | <.001 | .38 | .11 |
| Avoidance | 2.5 | 0.5 | 2.2 | 0.4 | 11.7 | 0.9 | 10.4 | 0.7 | <.001 | .18 | .41 |
| Hyperarousal | 2.7 | 0.5 | 2.8 | 0.4 | 10.2 | 0.8 | 9.27 | 0.7 | <.001 | .61 | .37 |
PTSD = posttraumatic stress disorder; M = mean; SE = standard error.
The diagnostic groups (PTSD and no PTSD) differ significantly in the degree of traumatization and symptom severity; however, there were no sex differences or interaction effects with sex. Childhood trauma was assessed using the Childhood Trauma Questionnaire, adult trauma was assessed using the Traumatic Events Interview, and PTSD symptoms were assessed using the PTSD Symptom Scale.
Habituation
Startle magnitude decreased significantly from the first habituation block to the second one (F(1,137) = 58.59, p <.001); however, there was no main effect of group and no interaction effect, indicating that both groups had comparable baseline startle and levels of startle habituation. Habituation was not evident in HRV or respiration rate because there were no significant effect of block and no effect of group or group-by-block interaction. Respiration rate was highly stable across experimental phases (Block 1 of habituation: mean ± standard error = 16.21 ± 0.55 breaths per minute; Block 2 of habituation: 16.22 ± 0.53 breaths per minute).
Dark-Enhanced Startle
A four-way mixed model ANOVA with block (1 and 2) and phase (light and dark) as within-subjects factors and group (PTSD and no PTSD) and sex (men and women) as between-groups factors yielded a significant three-way interaction of phase by group by sex on startle magnitude (F(1,136) = 3.78, p = .05) and a significant main effect of block (F(1,136) = 33.52, p <.001). Separate group-by-phase ANOVAs within each sex revealed a significant interaction of group by phase in women (F(1,84) = 5.52, p = .02) but not in men (F(1,52) = 0.52, p = .48). There was a significant increase in startle magnitude during the dark phase compared with the light phase in women (F(1,84) = 27.03, p <.001) and men (F(1,52) = 11.39, p = .001; Table 3).
TABLE 3.
Startle and HRV Data Across Experimental Conditions and Diagnostic Groups for Men and Women
| No PTSD
|
PTSD
|
Effect, p
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block 1
|
Block 2
|
Block 1
|
Block 2
|
||||||||||||||||
| Light
|
Dark
|
Light
|
Dark
|
Light
|
Dark
|
Light
|
Dark
|
PTSD
|
Phase
|
PTSD × Phase
|
|||||||||
| M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | M | SE | ||||
| Men | |||||||||||||||||||
| Startle magnitude, μV | 103.4 | 21.9 | 137.3 | 27.1 | 89.1 | 20.5 | 120.5 | 29.3 | 70.5 | 32.3 | 106.1 | 40.0 | 56.7 | 30.2 | 63.5 | 43.3 | .38 | .001 | .48 |
| RSA (lnHF-HRV) | 5.1 | 0.3 | 5.0 | 0.3 | 4.9 | 0.3 | 5.1 | 0.3 | 6.4 | 0.5 | 6.0 | 0.4 | 6.4 | 0.4 | 6.2 | 0.4 | .015 | .31 | .18 |
| Respiration rate, breaths per min | 15.8 | 1.0 | 16.7 | 1.0 | 15.6 | 1.0 | 17.0 | 0.9 | 15.7 | 1.4 | 17.6 | 1.5 | 18.4 | 1.5 | 18.8 | 1.4 | .25 | .07 | .96 |
| Women | |||||||||||||||||||
| Startle magnitude, μV | 66.3 | 10.5 | 87.1 | 12.6 | 62.8 | 9.8 | 69.9 | 12.0 | 75.7 | 14.3 | 127.5 | 17.3 | 63.8 | 13.4 | 85.7 | 16.4 | .35 | <.001 | .021 |
| RSA (lnHF-HRV) | 5.9 | 0.3 | 5.7 | 0.2 | 5.9 | 0.3 | 5.8 | 0.3 | 5.1 | 0.4 | 5.0 | 0.3 | 5.3 | 0.4 | 5.3 | 0.3 | .13 | .52 | .43 |
| Respiration rate, breaths per min | 16.2 | 0.8 | 15.2 | 0.9 | 15.2 | 0.9 | 15.8 | 0.8 | 16.3 | 1.1 | 15.2 | 1.1 | 15.2 | 1.1 | 16.2 | 1.0 | .91 | .73 | .93 |
HRV = heart rate variability; PTSD = posttraumatic stress disorder; M = mean; SE = standard error; RSA = respiratory sinus arrhythmia; HF = high frequency.
Startle magnitude was greater in the dark phase; in women, there was a significant phase–by–diagnostic group interaction. RSA was higher in men with PTSD but did not vary with experimental phase. Respiration rate did not vary across diagnostic groups or phase for either sex.
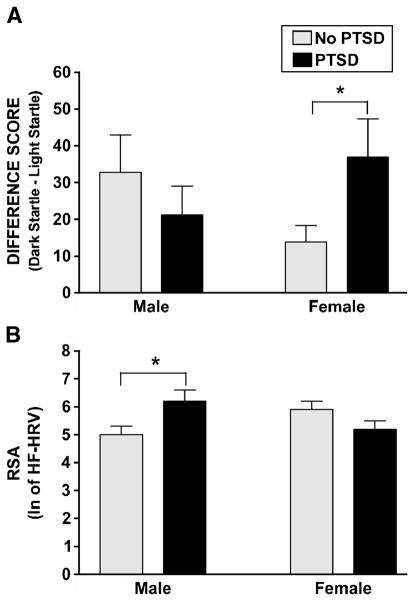
A comparison of difference scores between the dark and light phases (dark minus light) between women with PTSD and women without PTSD showed that those with PTSD had a significantly greater degree of dark-enhanced startle response compared with those without PTSD (F(1,86) = 5.68, p = .02; Fig. 2A). For men, no differences between participants with versus without PTSD were found (F(1,52) = 0.79, p = .38). We repeated this analysis with age as a covariate to account of the effects of age on startle. An analysis of covariance of dark-enhanced startle showed the same results mentioned previously: women with PTSD had significantly higher levels than women without PTSD (F(1,86) = 5.74, p = .02). A hierarchical regression entering childhood abuse, adult trauma, and PTSD diagnosis at each step indicated that only PTSD contributed significantly to dark-enhanced startle, with 5.6% of the variance accounted for by PTSD alone (Fchange(1,81) = 4.87, p = .03; Table 4A).
Figure 2.
The figure shows diagnostic group and sex with mean dark-enhanced startle (difference score between dark and light phases) (A) and RSA (HF-HRV) (B). PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia; HF-HRV = high-frequency heart rate variability.
TABLE 4.
Statistics for the Hierarchical Regression Analyses Examining the Contributions of Trauma History and PTSD on Dark-Enhanced Startle in Women and RSA (HF-HRV) in Men
| Model | R2 | R2 Change | F Change | p | |
|---|---|---|---|---|---|
| A. Dark-enhanced startle in women | Childhood trauma | 0.005 | 0.005 | 0.44 | .51 |
| Childhood and adult trauma | 0.006 | 0.001 | 0.04 | .84 | |
| Childhood and adult trauma and PTSD | 0.062 | 0.056 | 4.87 | .03 | |
| B. RSA (HF-HRV) in men | Childhood trauma | 0.000 | 0.000 | 0.01 | .93 |
| Childhood and adult trauma | 0.001 | 0.001 | 0.05 | .82 | |
| Childhood and adult trauma and PTSD | 0.150 | 0.149 | 8.58 | .005 |
PTSD = posttraumatic stress disorder; RSA = respiratory sinus arrhythmia; HF-HRV = high-frequency heart rate variability.
Heart Rate Variability
An ANOVA with block (1 and 2) and phase (light and dark) as within-subjects factors and group (PTSD and no PTSD) and sex (men and women) as between-groups factors yielded a significant two-way interaction of group by sex on RSA (HF-HRV) (F(1,136) = 8.01, p = .005) but no significant main effect of phase or other significant main effects or interaction effects.
Comparing diagnostic groups within each sex showed that men with PTSD had higher RSA compared with men without PTSD (F(1,52) = 6.28, p = .02; Table 3). Given the absence of phase and block effects, we collapsed the HRV data across these variables. Figure 2B shows HRV data for diagnostic group and sex. We repeated this analysis with age as a covariate to account of the effects of age on autonomic function. An analysis of covariance of RSA showed the same results mentioned previously: men with PTSD had significantly higher levels than men without PTSD (F(1,54) = 6.09, p = .02). Regression analyses of childhood abuse, adult abuse, and PTSD diagnosis entered at each step indicate that PTSD alone accounted for 14.9% of the variance in increased RSA in men, indicating a significant effect of the disorder after controlling for trauma history (Fchange(1,49) = 8.58, p = .005; Table 4B).
Respiration Rate
An ANOVA of respiration rate as the dependent variable, block (1 and 2) and phase (light and dark) as within-subjects factors, and group (PTSD and no PTSD) and sex (men and women) as between-groups factors showed no significant main effects of phase, sex, or block and no significant interaction effects (Table 3). However, there was a trend for respiration rate to be higher during the dark phase in men. To see whether this tendency may have accounted for the relationship between PTSD and RSA in men, we repeated the regression analysis mentioned previously and added respiration rate during the dark phase as a last step in the model. This additional analysis did not change the original results because PTSD still accounted for a significant amount of variance in RSA (13.7%; Fchange(1,45) = 7.18, p = .01), whereas respiration rate only accounted for 0.1%.
DISCUSSION
The present study found that PTSD was associated with greater dark-enhanced startle in women and higher RSA in men. Regression analyses indicated that dark-enhanced startle in women and increased RSA in men were related to the disorder itself and were not accounted for by the degree of either childhood or adult trauma. Respiration rate was not affected by experimental context, sex, or diagnosis and did not account for the group differences in RSA.
Dark-enhanced startle is a measure of nonspecific anxiety and is dependent on the BNST. This nucleus of the limbic system is one of the brain areas that are sexually dimorphic in animals (10) and humans (13). In rodents, light-enhanced startle is related to gonadal hormones and seems to be specifically associated with estrogen (45). The small number of studies that have examined this paradigm has focused exclusively on men (Vietnam veterans) (17) or adolescents of both sexes (18). In men, darkness increased startle in both PTSD and non-PTSD groups; however, a multigenerational study of risk for anxiety disorders found that women offspring at risk had the highest levels of startle in the dark (18). Given the higher prevalence of anxiety disorders in women than in men (46), dark-enhanced startle may be a specific measure of PTSD in women.
This is the first study to investigate the effects of darkness on HF-HRV or RSA. Previous studies from the available literature have primarily examined HRV in PTSD during rest conditions (5,28,29); however, a few have investigated HRV during emotionally challenging conditions. For example, RSA was studied during stressful mental tasks (e.g., arithmetic (30,31)) and during recollection of traumatic events (28,39). Interestingly, these studies have reported different effects of the stress condition on patients with PTSD and controls. Keary and colleagues (31) found that RSA had greater reductions during the challenge task relative to rest in patients with PTSD compared with the controls. On the other hand, Cohen and colleagues (28) found that the decrease in RSA in the trauma recall phase relative to rest was more pronounced in the controls than in patients with PTSD, and Sahar and colleagues (30) found that RSA increased during the stressor in the controls but not in subjects with PTSD. In contrast, Morgan and colleagues (36) found that decreased basal vagal function (RSA) was associated with superior performance under stress. Sex differences may account for this discrepancy in the literature: the first study used only women (31), and the latter two studies only included men (30,36).
Given the heterogeneity of psychophysiological findings in PTSD and the preponderance of such research in men due to the emphasis on combat-related PTSD (6), our study offers a novel contribution to the literature. We found significant interactions of psychophysiology and sex, in as much as PTSD was associated with increased HRV in men but not in women. This seems to be inconsistent with HRV studies that have found that patients with PTSD show decreased parasympathetic function (29). On the other hand, if men who have low vagal function perform well during dangerous training tasks (indicating a superior ability to regulate emotions (36)), it is possible that the opposite propensity, that is, high vagal function, is associated with poor emotional regulation under stressful circumstances and presents a risk factor for PTSD. These study populations, such as Special Forces soldiers and traumatized civilians with PTSD, may represent opposite ends of the resiliency versus vulnerability spectrum, and PNS function may represent a potential biomarker for delineating them.
The nature of the present study sample provides both strengths and limitations. Our participants were selected from the primary care patient pool at Grady Memorial Hospital in Atlanta, which serves a majority African American, low socioeconomic status, highly traumatized population. Because of this unique subject pool, these results may not generalize to other populations. However, this population is uniquely and significantly more susceptible to trauma-related stress disorders (3) and subsequent health disparities, and more investigation on the psychological outcomes of similarly vulnerable populations is needed. Another caveat is the use of the PSS, CTQ, and TEI to assess diagnosis and trauma history. These are based on the self-report of the participant and may be susceptible to reporting biases. However, all of these instruments have been used in the Grady Memorial Hospital population and validated with more in-depth interviews (3,47–48).
A significant strength of the present study is statistically controlling for the degree of trauma exposure. Although other studies have also used trauma-exposed control groups (30,35), there seems to be a linear “dose-response” association between trauma load and PTSD symptoms (47), such that no-PTSD controls may have significantly lower levels of trauma than individuals who meet DSM criteria for PTSD. Because any level of trauma history may affect PNS flexibility and cardiovascular health, we controlled for trauma history by statistically regressing childhood and adult trauma levels in addition to using a traumatized control group. Indeed, we found that the startle response and PNS activity seemed to be independent of trauma history. Given the large range of age in our sample, we also accounted for the effects of age on startle reflexes and autonomic function by replicating the main sex-specific findings using age as a covariate.
In summary, these findings indicate that dark-enhanced startle may be related to PTSD in women, whereas alterations in vagal function may be associated with PTSD in men. Regulation of emotional states such as anxiety has been associated with prefrontal cortex inhibition of the nuclei in the limbic system (21) and ANS activity (38), and sex differences have been observed in both pathways (13,38). Taken together, these data suggest that psychophysiological markers could be used as sex-specific risk factors for PTSD; moreover, these markers indicate that there may be common risk factors for anxiety disorders and cardiovascular disease.
Acknowledgments
This work was primarily supported by the National Institute of Mental Health and the National Institutes of Health Grant MH071537 (K.J.R.). Support was also received from the Emory and Grady Memorial Hospital General Clinical Research Center; the National Centers for Research Resources (M01RR00039 and P20RR16435), National Institutes of Health; the Burroughs Wellcome Fund (K.J.R.); the National Institute on Drug Abuse; and the Howard Hughes Medical Institute.
We thank the staff of the Grady Trauma Project, especially Allen Graham, Daniel Crain, and Justine Phifer, for their excellent technical support.
Glossary
- PTSD
posttraumatic stress disorder
- HRV
heart rate variability
- HF
high frequency
- BNST
bed nucleus of the stria terminalis
- PNS
parasympathetic nervous system
- RSA
respiratory sinus arrhythmia
- PSS
PTSD Symptom Scale
- CTQ
Childhood Trauma Questionnaire
- TEI
Traumatic Events Interview
- ECG
electrocardiogram
Footnotes
The authors report no conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4. Washington, DC: American Psychiatric Association; 1994. pp. 424–9. [Google Scholar]
- 2.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56:212–5. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie CF, Bradley RG, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31:505–14. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alim TN, Graves E, Mellman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. J Natl Med Assoc. 2006;98:1630–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozaric-Kovacic D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. Int J Psychophysiol. 2009;71:264–8. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–46. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- 7.Landis C, Hunt W. The Startle Pattern. New York, NY: Farrar & Rinehart; 1939. [Google Scholar]
- 8.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19:461–73. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 11.Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985;5:40–7. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- 14.Chung WCJ, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–33. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grillon C, Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. Int J Psychophysiol. 1998;28:223–31. doi: 10.1016/s0167-8760(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 16.Muhlberger A, Weiser MJ, Pauli P. Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biol Psychol. 2008;77:47–52. doi: 10.1016/j.biopsycho.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Grillon C, Morgan CA, Davis M, Southwick SM. Effect of darkness on acoustic startle in Vietnam veterans with post-traumatic stress disorder. Am J Psychiatry. 1998;155:812–7. doi: 10.1176/ajp.155.6.812. [DOI] [PubMed] [Google Scholar]
- 18.Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, Nomura Y, Leite P, Weissman MM. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry. 2005;57:953–60. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2007;167:151–69. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 20.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–8. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol Psychol. 1998;47:243–63. doi: 10.1016/s0301-0511(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 24.Cohen H, Benjamin J. Power spectrum analysis and cardiovascular morbidity in anxiety disorders. Auton Neurosci. 2006;128:1–8. doi: 10.1016/j.autneu.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Porges SW. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: a commentary on contemporary heart rate variability research. Biol Psychol. 2007;74:301–7. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berntson GG, Bigger JTJR, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 27.Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–96. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 28.Cohen H, Benjamin J, Geva AB, Matar MA, Kaplan Z, Kotler M. Autonomic dysregulation in panic disorder and in post-traumatic stress disorder: application of power spectrum analysis of heart rate variability at rest and in response to recollection of trauma or panic attacks. Psychiatry Res. 2000;96:1–13. doi: 10.1016/s0165-1781(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 29.Hopper JW, Spinazzola J, Simpson WB, van der Kolk BA. Preliminary evidence of parasympathetic influence on basal heart rate in posttraumatic stress disorder. J Psychosom Res. 2006;60:83–90. doi: 10.1016/j.jpsychores.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol Psychiatry. 2001;49:637–43. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- 31.Keary TA, Hughes JW, Palmieri PA. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol. 2009;73:257–64. doi: 10.1016/j.ijpsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004;55:284–90. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- 33.Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–22. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 34.Blechert JP, Michael TP, Grossman PP, Lajtman MM, Wilhelm FHP. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med. 2007;69:935–43. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- 35.Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry. 2004;55:953–6. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Morgan CA, III, Deane EA, George S, Vladimir C, Steven S. Relation between cardiac vagal tone and performance in male military personnel exposed to high stress: three prospective studies. Psychophysiology. 2007;44:120–7. doi: 10.1111/j.1469-8986.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 37.Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. Int J Psychophysiol. 2009;71:109–17. doi: 10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, III, Thayer JF, Kirschbaum C, Tranel D. Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology. 2010;35:56–66. doi: 10.1016/j.psyneuen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleim B, Wilhelm FH, Glucksman E, Ehlers A. Sex differences in heart rate responses to script-driven imagery soon after trauma and risk of posttraumatic stress disorder. Psychosom Med. 2010;72:917–24. doi: 10.1097/PSY.0b013e3181f8894b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falsetti S, Resnick H, Resick P, Kilpatrick D. The modified PTSD Symptom Scale: a brief self-report measure of posttraumatic stress disorder. Behav Ther. 1993;16:161–2. [Google Scholar]
- 41.Foa EB, Tolin DF. Comparison of the PTSD Symptom Scale–interview version and the clinician-administered PTSD scale. J Trauma Stress. 2000;13:181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 43.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 44.Grossman P, Wilhelm FH, Spoerle M. Respiratory sinus arrhythmia, cardiac vagal control, and daily activity. Am J Physiol Heart Circ Physiol. 2004;287:H728–34. doi: 10.1152/ajpheart.00825.2003. [DOI] [PubMed] [Google Scholar]
- 45.Toufexis D, Davis C, Hammond A, Davis M. Sex differences in hormonal modulation of anxiety measured with light-enhanced startle: possible role for arginine vasopressin in the male. J Neurosci. 2005;25:9010–6. doi: 10.1523/JNEUROSCI.0127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 47.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]