Abstract
Background
While stimulant dependent individuals continue to make risky decisions in spite of poor outcomes, much less is known about decision-making characteristics of occasional stimulant users (OSU) at risk for developing stimulant dependence. This study examines whether OSU exhibit inefficient learning and execution of reinforced decision-outcome contingencies.
Methods
OSU (n=161) and stimulant-naïve comparison subjects (CTL; n=48) performed a Paper Scissors Rock task during functional magnetic resonance imaging. Selecting a particular option was associated with a pre-determined probability of winning, which was altered repeatedly to examine neural and behavioral characteristics of reinforced contingencies.
Results
OSU displayed greater anterior insula, inferior frontal gyrus (IFG), and dorsal striatum activation than CTL during late trials when contingencies were familiar (as opposed to being learned) in the presence of comparable behavioral performance in both groups. Follow-up analyses demonstrated that during late trials: (1) OSU with high cannabis use displayed greater activation in these brain regions than CTL, whereas OSU with low cannabis use did not differ from the other two groups; and (2) OSU preferring cocaine exhibited greater anterior insula, IFG, and dorsal striatum activation than CTL and also displayed higher activation in the former two regions than OSU who preferred prescription stimulants.
Conclusions
OSU exhibit inefficient resource allocation during the execution of reinforced contingencies that may be a result of additive effects of cocaine and cannabis use. A critical next step is to establish whether this inefficiency predicts transition to stimulant dependence.
Keywords: stimulants, amphetamine, decision making, reward, dorsal striatum, fMRI
One important goal for addiction research is not only to establish biological markers of substance dependence (1), but more importantly, to identify altered brain systems that precede acquisition of substance dependence as a way of understanding the motivation to use drugs as well as possible pathways to drug dependence. To examine indicators of stimulant use that may be precursors to stimulant addiction, the present study recruited a substantial sample of occasional stimulant users (OSU), at-risk college students who misuse prescription stimulants such as methylphenidate and amphetamine to enhance academic performance and/or use cocaine for non-academic purposes (2–6). A growing literature indicating decision-making impairment in stimulant dependent individuals (7–11) informed the goal of the current investigation: to examine plausible markers of decision making in young adults vulnerable to stimulant dependence.
Successful decision making depends on the ability to efficiently learn from choices that are rewarded versus those that are not, a skill that may be compromised in stimulant using individuals (12). Reduced ability to differentiate advantageous versus disadvantageous options may be due to the fact that stimulant abuse and dependence are associated with heightened responsivity to drug-related rewards (13–15), discounting of delayed monetary rewards in favor of riskier, more immediate payoffs (16, 17), and impaired learning of stimulus-reward associations (18). Thus, the study of the acquisition and progression of reward learning in OSU is relevant to determining whether neural and/or behavioral reward dysfunction is evident prior to chronic, frequent stimulant use apparent in substance dependent individuals. Within the context of reward learning in OSU, brain dysfunction in the presence of intact behavioral performance would be beneficial, as this pattern could be consistent with inefficient or over-recruitment of neural resources involved in risk and reward in order to obtain commensurate behavioral performance as stimulant naïve individuals. Furthermore, it is probable that biological markers of aberrant neural processing in the absence of behavioral differences in the laboratory may still translate into behavioral difficulties when OSU are required to make complex decisions involving uncertain reward in everyday life, choices that may determine transition to stimulant dependence. The present study examined whether OSU demonstrate efficient reward learning when compared to stimulant naïve individuals (CTL) during a Paper Scissors Rock decision-making paradigm known to activate brain regions involved in reinforcement learning: dorsal striatum, inferior frontal gyrus (IFG), and insula (19, 20).
Learning the association between an option and the probability that it results in a rewarding outcome involves assessing the discrepancy between the anticipated versus experienced reward and discerning the amount of risk involved in making that choice, assessments thought to be implemented by dorsal striatum, IFG, and insula (21, 22). The dorsal striatum implements contingencies linking choices with rewarding outcomes (23–26), whereas IFG and insula encode changes in reward variance during reinforcement, with IFG inhibiting the selection of suboptimal choices associated with risk, and the insula representing the affective experience associated with risk avoidance (21). In healthy individuals, dorsal striatum and IFG activations are more robust during early phases of reinforcement, when a contingency between a decision and a reward is initially being learned, as opposed to later phases when reward feedback expectancies are established and behavioral-outcome contingencies become more predictable (26). Similarly, in healthy subjects insula activation is largest during winning decisions made under uncertain conditions as opposed to probable ones (27). In contrast, studies have shown heightened dorsal striatum and IFG activation in cocaine abusers compared to healthy individuals during decision making (28, 29), and increased insula activation in abstinent methamphetamine users during decision making has been shown to predict relapse (30). However, brain and behavior changes during early and late stages of reward learning have yet to be examined in OSU.
The present study compared neural and behavioral processing in OSU and CTL during two phases of a decision making task involving rewarding and punishing feedback: (1) in early trials, wherein decision-outcome contingencies were being acquired, and (2) in late trials, wherein decision-outcome contingencies were being executed. It was predicted that OSU would be less efficient than CTL during reinforcement learning. Three specific hypotheses were tested: (1) consistent with prior reinforcement learning studies in healthy individuals, CTL will exhibit greater dorsal striatum, IFG, and insula activation in early than late trials but will demonstrate greater selection of the advantageous response during late than early trials, reflecting successful learning; (2) in early trials, OSU will show comparable behavioral performance and striatum, IFG, and insula activation to CTL due to trial-and-error learning for both groups; and (3) in late trials, OSU will exhibit poorer behavioral performance than CTL concurrent with greater striatum, IFG, and insula activation than CTL due to slower learning of reward contingencies.
Methods
Subjects
The study protocol was approved by the Human Subjects Review Board and carried out in accordance with the Declaration of Helsinki. Over a five-year period, subjects were recruited via flyers mailed to >7000 students at local universities, internet ads, and newspapers. As a result, 1025 individuals underwent detailed phone screens, and 161 (96M, 65F) non-dependent OSU and 48 (21M, 27F) stimulant-naïve CTL were included in the study (67% Caucasian, 14% Asian-American, 11% Other/mixed heritage, 5% Hispanic, 2% Pacific Islander, 1% African-American). Participants were informed that this study was examining brain functioning of people who use stimulants and all subjects gave written informed consent. OSU were defined as having (1) >2 distinct off-prescription uses of cocaine and/or prescription stimulants (amphetamines and/or methylphenidate) in the past 6 months; (2) no evidence for lifetime stimulant dependence; (3) no treatment-seeking for substance-related problems. CTL inclusion criteria were no lifetime stimulant use and no history of substance-related problems.
Lifetime DSM-IV Axis I diagnoses (including attention deficit hyperactivity disorder (ADHD) and substance abuse and dependence) (31) and Axis II antisocial personality disorder (ASPD) were assessed by the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA)(32), a detailed clinical interview including timeline follow-back methods to quantify lifetime drug use based on the number of distinct sessions each drug was used. Diagnoses were based on consensus meetings with a clinician specialized in substance use disorders (MPP). Exclusion criteria for all groups were: (1) ADHD; (2) stimulant dependence; (3) current (and past 6 months) Axis I panic disorder, social phobia, post-traumatic stress disorder, major depressive disorder; (4) lifetime bipolar disorder, schizophrenia, and obsessive compulsive disorder; (5) ASPD and conduct disorder; (6) current positive urine toxicology test (exception: cannabis, which is detectable in urine for up to six weeks after use) and (7) head injuries or loss of consciousness >5 min. Although no participants met criteria for stimulant dependence, some OSU met DSM-IV criteria for current abuse of cocaine (n=1), amphetamine (n=2), and cannabis (n=15) as well as current cannabis abuse and/or dependence (n=20: n=6 with both, and n=14 with dependence only). The interviewer also gathered information on current alcohol and nicotine use patterns, including days and amounts used within a typical week.
During the interview session, subjects also performed the Wechsler Test of Adult Reading (WTAR) (33), a measure of verbal IQ. Of the total sample, 199 were right handed as determined by the Edinburgh handedness inventory (34). Subjects were instructed to abstain from illicit substance use > 72 hours prior to the fMRI session and abstinence was determined by urine toxicology screen. With respect to recent stimulant use, 11.8% OSU endorsed cocaine use within a week of the fMRI session, whereas 10.6% of OSU reported prescription stimulant use during this time. A total of 61.5% of OSU reported using cannabis within the week of the fMRI session and n=57 OSU also tested positive for cannabis at the time of the fMRI session.
Paper Scissors Rock Task
The Paper Scissors Rock task (19, 20) examines how individuals acquire the ability to make decisions associated with advantageous outcomes. This task is based on the well-known Paper-Scissors-Rock game, wherein: (1) rock beats scissors; (2) paper beats rock; (3) scissors beat paper. Subjects were instructed that they were playing against the computer and attempting to maximize points (1 for a win, 0 for a tie, and −1 for a loss). Players were told that they would receive additional payment according to their cumulative point total (each point was worth $1). Unknown to the subject, probability of beating the computer and thus being reinforced (e.g., subject chooses scissors, computer selects paper, scissors beats paper, subject gains 1 point) was predetermined for each response option. A total of 120 trials were presented, consisting of six blocks containing 20 trials each. Within each block, the three possible selections had pre-determined probabilities of having a winning, tying, or losing outcome. The “preferred response” wins on 90% of trials, the “even response” wins 50% of the time, and the “worst response” wins on 10% of trials. Thus, if rock were the preferred response and paper were the worst response in a particular block, then selecting rock would result in a win 90% of the time and selecting paper would result in a win 10% of the time. Unbeknownst to the subject, preferred, even, and worst responses were switched for each of the six blocks presented. Since subjects were instructed to select paper, scissors or rock by pushing the left, middle or right button with the index, middle or ring finger of the right hand, respectively, the statistically optimal corresponding hand position option also changed for each block.
Figure 1 illustrates that after an initial fixation lasting 2 s, subjects saw pictures of a hand forming paper, scissors, and rock on the computer screen for 1 s and heard the instruction “one, two, three” over MRI compatible, sound-insulated headphones. At 3 s into the trial, subjects were then presented with a “Go” sign, providing the cue to select paper, scissors, or rock. Subjects had 3.5 s to respond, after which the trial timed out. Upon responding, the outcome was presented, wherein the subject saw the computer’s response, and heard “you win,” “you lose,” or “a tie,” and the updated score was displayed at the top of the screen. Nine null trials were interspersed at the beginning, middle, and end of the task as a temporal jitter and trial duration order was optimized to estimate activation during decision making.
Figure 1.
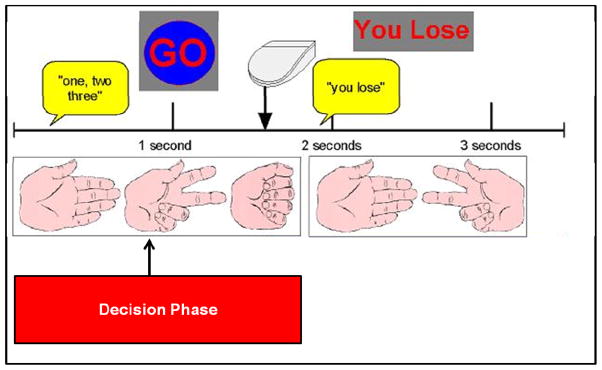
Paper Scissors Rock paradigm.
fMRI Image Acquisition and Analysis
A fMRI run sensitive to blood oxygenation level-dependent (BOLD) contrast was collected in a randomized fast-event related design using a Signa EXCITE (GE Healthcare, Milwaukee, Wisconsin) 3.0 Tesla scanner (T2*-weighted echo planar imaging (EPI) scans, TR=2000 ms, TE=32 ms, FOV=23 mm2, 64×64 matrix, 30 2.6mm axial slices with 1.4 mm gap, flip angle=90°, 290 whole-brain acquisitions). fMRI volume acquisitions were time-locked to task onset. A high-resolution T1-weighted image [spoiled gradient recalled (SPGR), TR=8 ms, TE=3 ms, FOV=25 cm, ~1 mm3 voxels] was obtained for anatomical reference.
Preprocessing
Data were preprocessed with the Analysis of Functional Neuroimages (AFNI) software package (35). GE x-y slices were reconstructed into AFNI BRIK format. The central point of the temporal region with the largest span of fewest voxel-wise outliers was used as the base for registration, adjusting all other time points in dx, dy, dz as well as roll, pitch, and yaw directions to align remaining images to the base image. Automated coregistration of the functional echoplanar image to the anatomical image was performed and a new outlier file was generated to determine if additional time points should be censored based on whether a given time point greatly exceeded the mean number of voxel outliers for the time series.
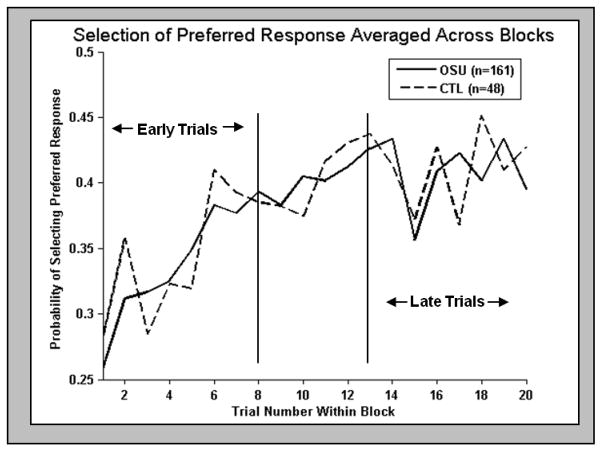
Based on subjects’ learning curves (the frequency of preferred response selection as a function of trial position after each switch; see Figure 2), groups of trials across blocks during the decision phase of the task depicted in Figure 1 (from trial onset until response selection, as opposed to the outcome phase) were separated into early trials, defined as trials 1–8, when contingencies (selection of the preferred response, avoidance of the worst response) were being discerned, and late trials, defined as trials 13–20, when contingencies had been established. Data were inspected to determine successful image alignment and existence of remaining artifacts. Deconvolution was then performed, wherein three motion regressors, a baseline and linear drift regressor, and two decision time regressors (early trials, late trials), were convolved with a modified hemodynamic response function. The baseline for the decision phase consisted of the inter-trial interval, the null trials interspersed between trial blocks, and the outcome phase of each trial. Images were spatially filtered using a Gaussian Spatial Filter (full-width-half-maximum 4 mm) to account for individual anatomical differences. Anatomical images were manually talairached and echoplanar images were transformed into Talairach space.
Figure 2.
Probability of selecting the preferred response as a function of trial position across blocks for occasional stimulant users (OSU) and control subjects (CTL).
Group analysis
Group (OSU, CTL) and decision time (early trials, late trials) were subjected to R linear mixed effects (LME) analysis (36) across all voxels. Subjects were treated as random effects, whereas group and decision time were modeled as fixed effects. Trials were averaged across blocks to increase statistical power. The group by decision time interaction was of interest to examine group differences in discerning versus executing reward contingencies. Threshold adjustment based on Monte-Carlo simulations (AFNI’s Alpha Sim) was applied to guard against identifying false positive activations (considering whole brain voxel size and 4mm smoothness). Alpha Sim identified a minimum cluster volume of 768 μL with a cluster significance of p < .05 corrected for multiple comparisons (voxel-wise probability: p < .05). A limbic mask consisting of neural substrates important for emotional and interoceptive processing was also applied to examine a-priori hypotheses involving insula and dorsal striatum using a minimum cluster volume of 320 and 250 μL, for each region, respectively. For significant clusters, average percent signal change from baseline was extracted.
Secondary group analyses
Analogous LMEs were computed across all voxels to follow-up primary LME results, one for each of the following two subsamples: (1) groups formed on the basis of lifetime cannabis use (n=123): high MJ-OSU (n=43; OSU endorsing ≥ 1000 lifetime cannabis uses), low MJ-OSU (n=35; OSU endorsing < 50 lifetime cannabis uses), and low MJ-CTL (n=45; CTL endorsing ≤ 50 lifetime cannabis uses); and (2) groups formed on the basis of the type of stimulant preferred (n=154): predominantly cocaine users (PCU, n=41: OSU with cocaine comprising ≥ 80% lifetime stimulant use), predominantly prescription users (PPU, n=65: OSU with prescription stimulants comprising ≥ 80% lifetime stimulant use), and CTL (n=48). Findings for a-priori regions (striatum, IFG, insula) are reported.
Behavioral Analysis
Responses were obtained using the first three buttons on a four-button response box recorded during each trial to determine response selection (preferred, even, and worst responses). Group (OSU, CTL) and decision time (early trials, late trials) across blocks were subjected to a LME analysis, with probability of preferred response selection as the dependent variable. Subjects were treated as random effects, whereas group and decision time were modeled as fixed effects. Similar to fMRI analyses, two follow-up LMEs were performed to examine behavioral differences as a function of cannabis and PCU/PPU groups separately.
Results
Subject Characteristics
OSU endorsed greater stimulant and cannabis usage and used alcohol and nicotine more frequently and in larger quantities than CTL (Table 1). OSU also had significantly more Caucasian and less Asian-American participants than CTL (χ2(5)=11.5, p=.04). However, groups did not differ on gender (χ2(1)=3.8, p=.052), age, education, or verbal IQ.
Table 1.
Subject Characteristics as a Function of Group Status (n=209).
| OSU (n = 161) | CTL (n = 48) | Univariate ANOVA | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Demographics | |||||
| Age | 20.8 | 1.5 | 20.9 | 2.1 | F(1, 207) = 0.1, p = .81 |
| Education | 14.3 | 1.2 | 14.5 | 1.5 | F(1, 207) = 0.7, p = .42 |
| Verbal IQ (WTAR) | 108.7 | 7.3 | 109.8 | 7.0 | F(1, 199) = 0.9, p = .34 |
| Alcohol (Typical Drinks/Week) | 20.0 | 14.4 | 4.8 | 3.5 | F(1, 191) = 42.5, p < .001 |
| Alcohol (Typical Days/Week) | 3.2 | 1.6 | 1.5 | 0.7 | F(1, 191) = 38.1, p < .001 |
| Nicotine (Cigarettes/Day) | 2.7 | 4.4 | 0.5 | 2.9 | F(1, 207) = 10.7, p < .001 |
| Nicotine (Typical Days/Week) | 2.6 | 3.2 | 0.2 | 1.1 | F(1, 207) = 26.0, p < .001 |
| Lifetime Drug Use | |||||
| Cocaine | 22.4 | 38.4 | 0.0 | 0.0 | N/A |
| Prescription Stimulant | 22.2 | 51.1 | 0.0 | 0.0 | N/A |
| Cannabis | 914.0 | 1416.7 | 10.3 | 25.9 | F(1, 207) = 19.5, p < .001* |
Note. OSU = occasional stimulant users. CTL = control subjects. ANOVA = Analysis of variance. IQ = Intelligence Quotient. WTAR = Wechsler Test of Adult Reading. DSM-IV = Diagnosis and Statistical Manual of Mental Disorders IV. For a minority of subjects, data were not collected for verbal IQ (n = 8) and weekly alcohol use (n = 16).
ANOVAs computed using natural log transformed +1 values (due to non-normal distributions) replicated results for raw data.
fMRI Data
Group by decision time analysis
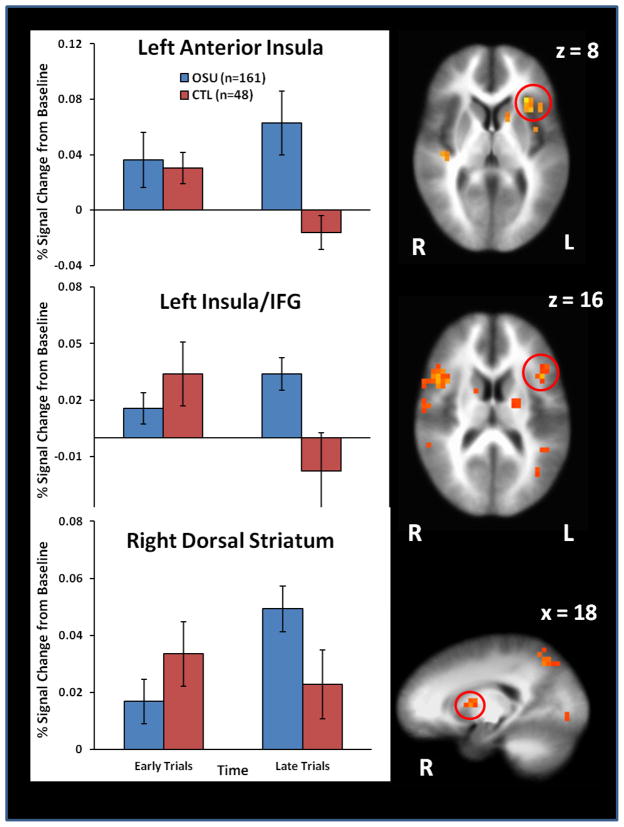
Results (Figure 3) indicated that during late trials, OSU displayed greater anterior insula, IFG, and dorsal striatum activation than CTL (reflected in greater percent signal change from baseline; see Table S1 in Supplement). However, OSU and CTL did not differ in these regions during early trials. In addition, within CTL, insula and IFG activation significantly decreased from early to late trials whereas these regions did not differ between early and late trials within OSU (see Figure 3).
Figure 3.
Stimulant group (OSU, CTL) by decision time (early trials, late trials) interaction results showing that occasional stimulant users (OSU) exhibited greater anterior insula, inferior frontal gyrus (IFG) and dorsal striatum activation than control subjects (CTL) during late trials when reward contingencies were being executed. Error bars reflect standard error.
Secondary group analyses
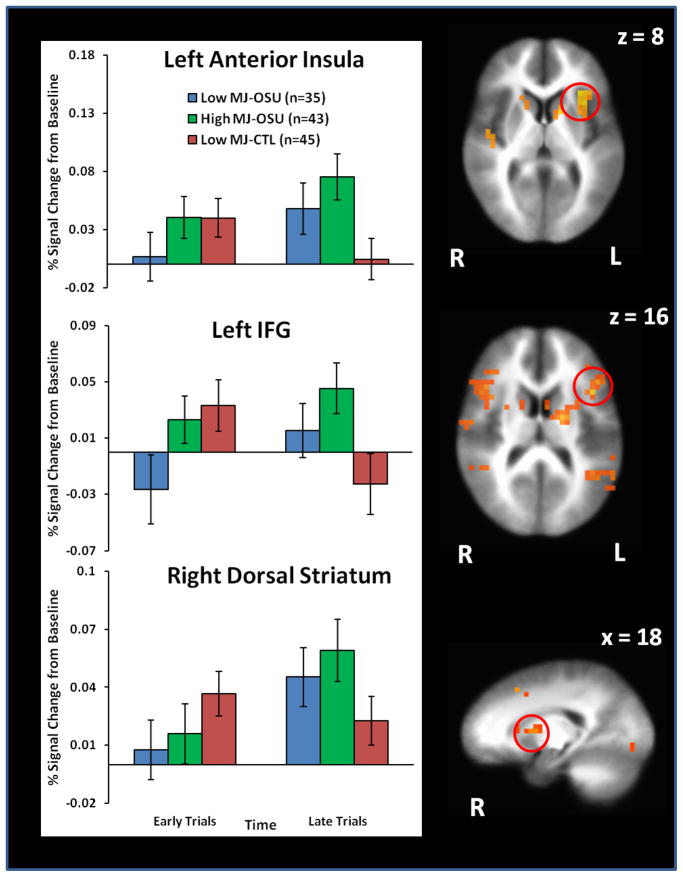
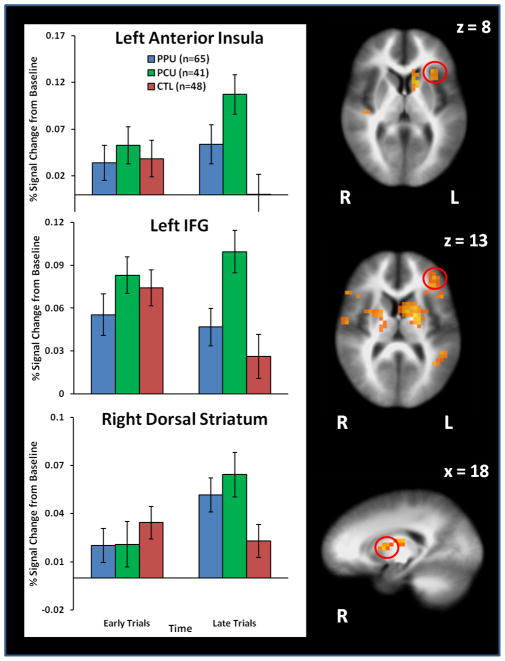
Cannabis LME results (see Figure 4) showed that high MJ-OSU exhibited greater anterior insula, IFG, and dorsal striatum activation than low MJ-CTL during late trials, whereas low MJ-OSU did not differ from either group (see Table S2 in Supplement). High MJ-OSU also endorsed higher lifetime cocaine use than low MJ-OSU (t(76)=3.4, p=.001) but not higher prescription stimulant use (p=.38). PCU/PPU LME results (see Figure 5) indicated that PCU displayed greater anterior insula and IFG activation than PPU and CTL during late trials. PCU also exhibited greater dorsal striatum activation than CTL (see Table S3 in Supplement). In addition, lifetime cannabis use was marginally higher in PCU than PPU (t(104)=1.7, p=.09).
Figure 4.
Cannabis group (Low MJ-OSU, High MJ-OSU, Low MJ-CTL) by decision time (early trials, late trials) interaction results demonstrating that high cannabis occasional stimulant users (High MJ-OSU) exhibited greater anterior insula, inferior frontal gyrus (IFG), and dorsal striatum activation than low cannabis control subjects (Low MJ-CTL) during late trials when reward contingencies were being executed. Low cannabis users (Low MJ-OSU) did not differ from High MJ-OSU or Low MJ-CTL. Error bars reflect standard error.
Figure 5.
Predominantly prescription/predominantly cocaine group (PPU, PCU, CTL) by decision time (early trials, late trials) interaction results depicting that predominantly cocaine users (PCU) exhibited greater anterior insula and inferior frontal gyrus (IFG) activation than predominantly prescription stimulant users (PPU) and control subjects (CTL) during late trials when reward contingencies were being executed. PCU also displayed greater dorsal striatum activation than CTL. Error bars reflect standard error.
Supplementary results
Additional analyses were conducted to examine brain activation as a function of (1) substance use patterns within OSU (Tables S4–S5 in Supplement); (2) early and late trials separately (Table S6 in Supplement); (3) individual decisions of selecting a preferred versus non-preferred option (Table S7 in Supplement); and (4) responses to winning feedback during the task outcome phase (Table S8 in Supplement).
Behavioral Data
Group by decision time analysis
A main effect of decision time indicated that probability of preferred response selection increased from early trials (M=.34, SE=.01) to late trials (M=.41, SE=.01; F(1,343)=38.7, p<.001). No group main effect or group by decision time interaction emerged, however (both p>.70).
Secondary group analyses
A main effect of decision time replicated the behavioral analysis above (cannabis LME: F(1,201)=26.9; PCU/PPU LME: F(1,259)=32.9, both p<.001). No main effects or interactions for cannabis group or PCU/PPU group emerged (all p>.64).
Discussion
This study examined three hypotheses about neural and behavioral processing differences between OSU and CTL during reinforcement-related decision making. Consistent with our first prediction, CTL exhibited greater insula and IFG activation during early trials than late trials along with greater selection of the preferred response in late than early trials, findings replicating prior literature in healthy individuals during reinforcement learning (26). CTL did not show significantly greater dorsal striatum activation in early compared to late trials; however, the average activation differences were in the predicted direction but did not meet the statistical threshold corrected for multiple comparisons. Results also supported our second hypothesis, wherein OSU and CTL showed similar patterns of insula, IFG, and dorsal striatum activation during early trials requiring trial-and-error learning for both groups.
Finally, our third prediction was partially supported in that OSU exhibited greater insula, IFG and dorsal striatum activation than CTL during late trials when contingencies were already familiar and as a result, should require fewer resources to ensure successful performance. In contrast to our hypothesis, however, OSU did not show slower behavioral acquisition of the preferred response than CTL from early to late trials. The absence of group differences in behavioral performance can be construed as an asset of the present study, lending support to the interpretation that OSU deployed greater neural resources than CTL in order to achieve similar performance during reinforcement learning. It is possible that neural markers of inefficient learning will predict future poorer behavioral performance in OSU who transition to stimulant dependence, although longitudinal research is warranted to address this issue. The fact that OSU and CTL exhibited similar behavioral performance is not surprising, given that (1) subjects were high functioning college students attending an academically challenging university; (2) OSU exhibit only very subtle differences in neuropsychological functioning from stimulant naïve individuals (37, 38); and (3) research has shown that methyphenidate does not affect behavioral performance during probability learning in healthy individuals (39, 40), so this may also be the case for OSU. fMRI decision making paradigms may also not offer enough means to detect behavioral differences in a college sample given limited response options.
Overall, findings of the present study suggest that in the presence of similar behavioral performance, OSU as opposed to CTL continued to show activation in insula, IFG, and striatum when contingencies had been established. The continued brain response in the presence of stable and established behavioral contingencies is reminiscent of the residual error model proposed by Redish (41) for cocaine dependent individuals, wherein a persistent residual prediction error drives urges and repetitive behavior, which is thought to be essential for drug addiction. The continued recruitment of resources to maintain and execute learned contingencies in brain regions involved in reward and risk evaluation in OSU supports the notion that these subtle processing differences may actually precede the development of substance dependence. The present findings suggest that inefficient reward processing is not limited to stimulant dependent individuals (9–11) but extends to individuals who have not yet developed problem use and may be a pre-existing marker of the motivation to use stimulants.
However, OSU also used other substances, which may have affected processing differences between groups. Follow-up analyses demonstrated that high cannabis OSU exhibited greater insula, IFG, and dorsal striatum activation during late trials when contingencies were being executed than low cannabis CTL, whereas low cannabis OSU did not differ from high cannabis OSU or CTL. However, OSU endorsing higher cannabis use also endorsed higher cocaine use than low cannabis OSU, findings suggesting that differences between OSU and CTL may be attributable to an additive effect of lifetime cannabis and cocaine use as opposed to prescription stimulant use. These findings are consistent with heightened dorsal striatum and IFG activation previously reported in cannabis users (42, 43) and cocaine users (28, 29) during decision making. Additional support for this assertion arises from analyses wherein OSU who preferred cocaine exhibited greater insula, IFG, and dorsal striatum activation than OSU who preferred prescription stimulants and CTL. Cannabis use was also marginally higher in OSU preferring cocaine than OSU preferring prescription stimulants, data consistent with our assertion that a combination of cocaine and cannabis use may be driving differences between OSU and CTL during decision making. However, the present study cannot answer whether brain activation in OSU with greater cannabis and cocaine use is due to a stronger vulnerability to use substances before substance use was initiated, or simply due to more exposure to substance use.
There are several limitations of this study. First, the present study cannot identify correlates of addiction vulnerability that are distinct from the determinants of occasional use. Research examining reinforcement learning in stimulant naïve young adults with first-degree relatives with stimulant dependence may further distinguish the correlates of genetic vulnerability from those of occasional use. Second, groups were examined cross-sectionally because complete longitudinal outcome information was not yet collected on this sample. Future investigations will examine whether individuals who develop problem use differ from those who terminate use. Third, OSU, although selected on the basis of prescription stimulant and/or cocaine use, also exhibited significant lifetime cannabis use. However, a pure OSU sample is not representative of at-risk individuals; for example, a recent study reported that over 65% of college students using stimulants non-medically also used cannabis within the past year (2). Lastly, since only a three-day abstinence from substance use was required, it is possible that OSU had inadequate drug washout at the time of the fMRI session. Despite these limitations, present results provide evidence that OSU mobilize additional neural resources in regions involved in reward and risk processing when executing reinforced decisions. A critical next step is to investigate whether this neural inefficiency is predictive of future problem use.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by grants from the National Institute on Drug Abuse (Grant Nos. R01-DA016663, P20-DA027834, R01-DA027797, and R01-DA018307 to Martin Paulus).
Footnotes
Financial Disclosures
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elkashef A, Vocci F. Biological markers of cocaine addiction: implications for medications development. AddictBiol. 2003;8(2):123–39. doi: 10.1080/1355621031000117356. [DOI] [PubMed] [Google Scholar]
- 2.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005 Jan;100(1):96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 3.McCabe SE, Teter CJ. Drug use related problems among nonmedical users of prescription stimulants: a web-based survey of college students from a Midwestern university. Drug Alcohol Depend. 2007 Nov 2;91(1):69–76. doi: 10.1016/j.drugalcdep.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasperski SJ, Vincent KB, Caldeira KM, Garnier-Dykstra LM, O’Grady KE, Arria AM. College students’ use of cocaine: Results from a longitudinal study. Addictive Behaviors. 2011 Apr;36(4):408–11. doi: 10.1016/j.addbeh.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arria AM, Caldeira KM, O’Grady KE, Vincent KB, Fitzelle DB, Johnson EP, et al. Drug exposure opportunities and use patterns among college students: results of a longitudinal prospective cohort study. Subst Abus. 2008;29(4):19–38. doi: 10.1080/08897070802418451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006 Oct;26(10):1501–10. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008 Apr;197(3):421–31. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007 Apr;75(1):45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002 Jan;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 10.Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007 Sep;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007 May;28(5):383–93. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, Perez-Garcia M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychol Rev. 2004 Mar;14(1):1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010 Sep;32(9):748–55. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology. 2008 Sep;45(5):705–13. doi: 10.1111/j.1469-8986.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000 Nov;157(11):1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 16.Verdejo-Garcia A, Perez-Garcia M, Sanchez-Barrera M, Rodriguez-Fernandez A, Gomez-Rio M. Neuroimaging and drug addiction: neuroanatomical correlates of cocaine, opiates, cannabis and ecstasy abuse. Rev Neurol. 2007 Apr 1–15;44(7):432–9. [PubMed] [Google Scholar]
- 17.Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006 Jul;31(7):1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007 Sep;17(3):317–36. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulus MP, Feinstein JS, Tapert SF, Liu TT. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage. 2004 Feb;21(2):733–43. doi: 10.1016/j.neuroimage.2003.09.060. [DOI] [PubMed] [Google Scholar]
- 20.Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005 Apr 1;25(2):607–15. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 21.Li J, McClure SM, King-Casas B, Montague PR. Policy adjustment in a dynamic economic game. PLoS One. 2006;1:e103. doi: 10.1371/journal.pone.0000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008 Mar 12;28(11):2745–52. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004 Apr 16;304(5669):452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 24.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005 Aug;18(4):411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 25.Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, et al. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007 Jul 15;36(4):1253–62. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005 Feb 1;24(3):862–73. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Satterthwaite TD, Green L, Myerson J, Parker J, Ramaratnam M, Buckner RL. Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. Neuroimage. 2007 Sep 1;37(3):1017–31. doi: 10.1016/j.neuroimage.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend. 2007 Mar 16;87(2–3):233–40. doi: 10.1016/j.drugalcdep.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003 Jul;19(3):1085–94. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005 Jul;62(7):761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 31.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington D.C: American Psychological Association; 1994. [Google Scholar]
- 32.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994 Mar;55(2):149–58. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 34.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 35.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC. nlme: Linear and Nonlinear Mixed Effects Models. 2011. [Google Scholar]
- 37.Reske M, Delis DC, Paulus MP. Evidence for subtle verbal fluency deficits in occasional stimulant users: quick to play loose with verbal rules. J Psychiatr Res. 2011 Mar;45(3):361–8. doi: 10.1016/j.jpsychires.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reske M, Eidt CA, Delis DC, Paulus MP. Nondependent stimulant users of cocaine and prescription amphetamines show verbal learning and memory deficits. Biol Psychiatry. 2010 Oct 15;68(8):762–9. doi: 10.1016/j.biopsych.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29(15):4690–6. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodds CM, Muller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. J Neurosci. 2008 Jun 4;28(23):5976–82. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redish AD. Addiction as a computational process gone awry. Science. 2004;306(5703):1944–7. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- 42.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005 Jun;26(2):480–92. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Vaidya JG, Block RI, O’Leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. 2012 Feb;37(3):618–29. doi: 10.1038/npp.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.