Abstract
Revision joint replacement has poorer outcomes that have been associated with poorer mechanical fixation. We investigate a new bone-sparing surgical technique that locally cracks the sclerotic bone rim formed during aseptic loosening. We inserted 16 hydroxyapatite-coated implants bilaterally in the distal femur of eight dogs, using a controlled weight-bearing experimental model that replicates important features of a typical revision setting. At 8 weeks, a control revision procedure and a crack revision procedure were performed on contralateral implants. The crack procedure used a splined tool to perform a systematic local perforation of the sclerotic bone rim of the revision cavity. After 4 weeks, the hydroxyapatite-coated implants were evaluated for mechanical fixation by a push-out test and for tissue distribution by histomorphometry. The cracking revision procedure resulted in significantly improved mechanical fixation, significantly more bone ongrowth and bone volume in the gap, and reduced fibrous tissue compared to the control revision procedure. The study demonstrates that the sclerotic bone rim prevents bone ingrowth and promotes fixation by fibrous tissue. The effect of the cracking technique may be due to improved access to the vascular compartment of the bone. The cracking technique is a simple surgical method that potentially can improve the fixation of revision implants in sclerotic regions important for obtaining the fixation critical for overall implant stability.
Keywords: surgical technique, implant fixation, revision THA, hydroxyapatite, animal model
Approximately one million total hip replacements (THA) are performed worldwide each year. The overall number of revision surgeries is about 15–20% of the number of primary surgeries.1–3 Clinically, it is well established that revision joint replacement implants have shorter longevity, poorer functional outcome, poorer fixation, higher costs, and longer rehabilitation times than primary implants.4–9 Aseptic loosening is the most frequent reason for revision of hip implants.1,10 Aseptic loosening usually results in the formation of a dense sclerotic endosteal bone surface.11 The sclerotic bone typically forms a shell or rim-like structure which may partially or completely cover the revision cavities of the femur and acetabulum (Fig. 1). On the cellular level the sclerotic bone rim is characterized by high and ongoing formative activity and little bone resorption.12 This means that the sclerotic bone rim is more likely to increase in thickness rather than serve as a base for bone growth towards the implant.
Figure 1.
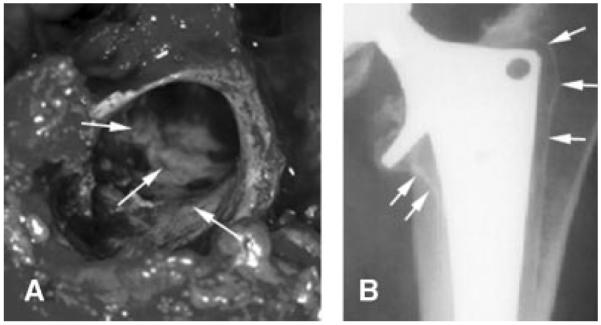
(A) Photograph of the sclerotic bone surface in the proximal femur. (B) Analog preoperative radiograph (anterior–posterior projection) of a loose uncemented femoral component. The sclerotic bone rim is indicated by arrows.
Unlike the more standardized surgical techniques used in primary hip arthroplasty, the techniques used in revision hip arthroplasties are adjusted in each clinical case depending on factors such as the state of bone bed, presence of sclerotic bone, and osteolytic lesions. Reaming of the most distal section of the femur and the acetabulum is most common. However, in many cases, the sclerotic bone of the proximal femur and in some cases the acetabulum must be left intact as reaming procedures or complete removal of the sclerotic bone rim may increase the risk of fracture. The preparation of the bone in these cases is limited to meticulous soft tissue removal, scraping, and lavage.
To address difficulties in maintaining bone stock in the revision setting, and with the decreased osteogenic potential in the revision setting,4,12,13 we have introduced the new bone-sparing surgical technique of cracking the sclerotic bone. The cracking technique that we present is a systematic and controlled cleavage of the sclerotic bone surfaces of the revision cavity, leaving the majority of the sclerotic surface intact. The premise for the technique is that access to the vascular compartment will enhance the biological response of the bone bed and thereby stimulate bone ingrowth to the revision implant.
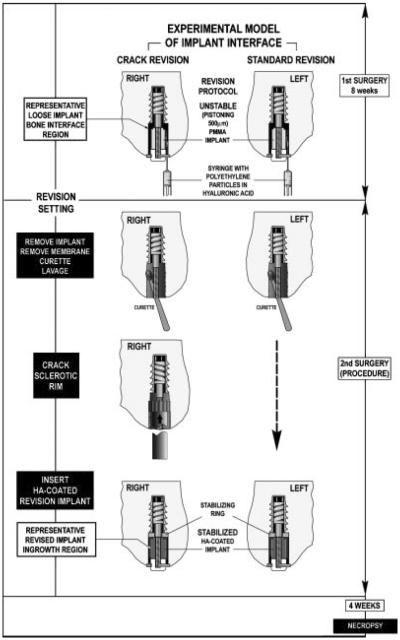
To evaluate this surgical technique we used our established and controlled weight-bearing experimental revision model in the canine.13,14 This model represents the typical bony and soft tissue response occurring in the cancellous region surrounding aseptically loosened implants. It creates an interface of implants with a sclerotic bone rim separating the revision implant from the underlying vascularized cancellous bone bed (Fig. 2).
Figure 2.
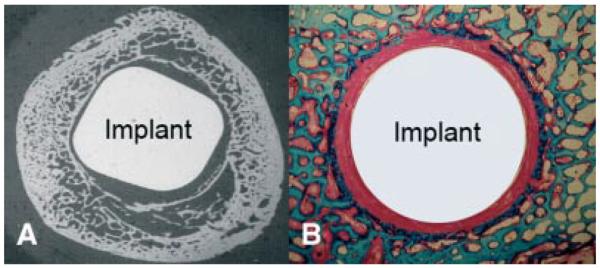
(A) X-ray of the femur with a loose stem and surrounding sclerotic bone rim. (B) Histological section (basic fuchsin and light green) of the experimental revision model of the canine after 8 weeks of implant micromotion. The implant is surrounded by a dense fibrous capsule and a sclerotic bone rim. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
We have previously demonstrated that the cracking technique enhanced the mechanical fixation of titanium alloy revision implants.15 However, no previous studies have investigated the histological tissue response to this technique.
In the present study, we investigate whether the surgical technique of cracking the sclerotic bone will enhance the fixation of implants coated with hydroxyapatite (HA). The HA coating is highly osteoconductive16,17 and experimentally HA-coated implants obtain superior mechanical fixation and osseointegration compared to titanium alloy implants in a controlled revision setting.18 We hypothesize that locally cracking the sclerotic endosteal bone rim increases the mechanical fixation and bony integration of HA coated revision implants, compared with HA-coated revision implants with a control revision procedure. The control revision procedure is limited to fibrous membrane removal, lavage, and scraping the surface with a curette without cracking the sclerotic endosteal rim.
MATERIALS AND METHODS
Following approval by our institution's Animal Care and Use Committee, we included eight skeletally mature female dogs (16 knees) in this study. Average age was 14.2 months; mean weight was 23.0 kg±1.2 kg. We implemented our established revision model in the canine knee to produce a controlled periprosthetic tissue reaction characteristic for loosening implants.14 The revision protocol is based on a micromotion device that represents a clinically loose and pistoning implant (Fig. 3). To engender the tissue reaction surrounding an aseptically loosened implant, a 6.0-mm molded polymethylethacrylate (PMMA) implant axially pistons 0.5 mm concentrically in a hole with a 0.75-mm gap during each gait cycle. The PMMA represents a loose cement mantle. The implant is weight bearing in the medial femoral condyle for 8 weeks in the presence of PE (0.5–50 μm; 0.5×108; 85%<12 μm). The particles consisted of 85% (by concentration) of high-density polyethylene having a mean diameter of 2.09 μm (range 0.2–11 μm), mixed with 15% of an ultrahigh molecular weight polyethylene resin having a mean diameter of 30 μm (range 10–50 μm, spherical; Hoechst Celanese Corporation, Houston, TX). The particles were sterilized and suspended via manual mixing in a viscous 0.27% sterile synthetic hyaluronic acid (LifeCore Biomedical, Minneapolis, MN). The particles were shown to be virtually “endotoxin-free” (0.0002–0.0007 EU/mg) using the high sensitivity version of the Spectrophotometric Limulus Amebocyte Lysate Assay with modifications designed to prevent PE particle aggregation19 and to prevent false positives from B-glucan-like molecules.20
Figure 3.
Operative procedure for initiating the revision protocol, and performing the control and crack revision procedures.
Primary Operative Procedure for Insertion of Micromotion Device (Fig. 3)
Bilateral surgery of the medial femoral condyles was performed under general anesthesia, and sterile technique was observed. The animal were given antibiotics Rocephalin (ceftriaxon): 1 g i.v. preoperatively, and 1 g daily i.m. for 3 days or until afebrile. The operative procedure was as follows. Following a medial approach, the weight-bearing area of the medial femoral condyle was identified during flexion through a range of motion. A 2.1-mm guide wire was inserted through the weight-bearing articulating surface into the central portion of the condyle. A deep cavity (6 mm diameter by 10 mm long) and a superficial cavity (7.5 mm diameter by 20 mm long), was created using a cannulated step drill. Drilling was performed a one to two rotations per second to prevent thermal bone trauma. After the superficial 4 mm of the drill hole was tapped, the drill site was irrigated with saline. The micromotion device and a distal centralizing were inserted. The drill hole was filled with PE particles in hyaluronic acid by injection and the PMMA implant was screwed onto the threaded piston. The polyethylene cap attached on the distal portion of the threaded piston. The PE cap protrusion into the joint was at least 0.5 mm to secure full cylinder displacement during loading. Implant displacement and proper functioning was verified intraoperatively. All knees had full range of motion with the implants in place. Soft tissues were closed routinely, and radiographs were obtained to verify implant placement. Two days postoperatively the dogs were allowed unlimited activity. Their hind-limb function was assessed and noted daily, to ensure they were regularly loading their implants.
Revision surgery
At 8 weeks, a second bilateral surgical procedure was performed under general anesthesia and with sterile technique. At this second operation, the crack revision technique was conducted on one side, and the paired control revision technique was conducted on the contralateral side. The animals were euthanized 4 weeks after revision surgeries. Cultures were taken from articular fluid of the knees. Bilateral distal femurs (approximately 12 cm length) were dissected and stored at −20°C until sectioning and testing.
Revision Implants
Plasma sprayed hydroxyapatite coated titanium alloy (Ti-6Al-4V) implants (Biomet Inc., Warsaw, IN) were inserted bilaterally following the respective revision procedures. The cylindrical implants were nominally 6.0 mm in diameter with an overall length of 10 mm, with a threaded core.
Control Revision Procedure
This procedure is intended to represent elements of a basic clinical revision procedure, recognizing that not all surgeons perform revision surgeries alike and that not all revision settings are alike. First, the PMMA implant was removed from the piston. The fibrous membrane was removed and the underlying sclerotic surface was cleaned with a sharp curette and subsequently lavaged. The micromotion device was stabilized to avoid further pistoning. Hence, the revised implant continued to be weight bearing, but was constrained to be stable. The HA-coated implant was screwed onto the piston followed by a PE plug.
Crack Revision Procedure
Following the same procedure to remove the PMMA implant, fibrous membrane, and lavage the sclerotic bone surface, an 8.2 mm (outer diameter) tool with 12 evenly spaced pointed splines (Fig. 4) was axially advanced (with hammer blows) over a guide wire into the revision cavity. The height of the splines was 1.3 mm. The tool did not rotate or ream. This produced a controlled cracking of the sclerotic bone rim (note that the diameter of original drill hole was not increased by this crack procedure). Following this procedure the implantation was carried out as described in the control revision protocol.
Figure 4.
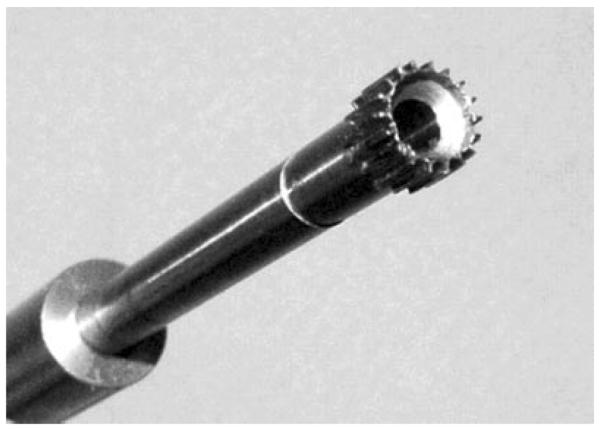
Tool for performing the controlled cracking procedure in the experimental implants. The splines protrude 0.35 mm past the original drill hole, just past the sclerotic bone rim.
Mechanical Push-Out Testing
Transverse sections of 3.0 mm were cut from the distal section of the revision implants on a water-cooled diamond saw (Exact Appartebau, Germany). Mechanical push-out tests were performed blindly on a universal test machine (Model 4302, Instron, UK). A displacement rate of 5 mm/min was used for all tests and load–displacement data were recorded. Data were normalized by surface area of the implant tested and ultimate shear strength (MPa), apparent shear stiffness (MPa/mm) and energy absorption (J/m2) were calculated.
Histomorphometry
The remaining transverse sections of 6.0 mm thickness were prepared for histomorphometry. The specimens were dehydrated in graded ethanol (70–100% containing 0.4% basic fuchsin, embedded in methylmethacrylate and during sectioning counterstained using 2% light green.21 The vertical section technique was used according to stereological principles.22 The embedded specimens with the implant in situ were randomly rotated around the vertical axis and four serial sections of 15–20 μm were obtained from the central portion of the implant using a microtome (KDG-95, 151 MeProTech, Heerhugowaard, Holland). Histomorphometry was performed in a blinded manner using a stereological image-analysis system (C.A.S.T-Grid; Olympus, Denmark). Tissue ongrowth was defined as tissue in direct contact with the implant surface and was determined using a line intercepting technique. Tissue volume percentages were estimated in the original gap (0–750 μm zone adjacent to the implant) by a point-counting technique.
Statistics
STATA Intercooled 8.0 statistical software (STATA, College Station, TX) was used to test differences between pairs. The paired Students' t-test was applied. Results are given as mean and 95% confidence intervals. Significance is considered for two-tailed p < 0.05(*).
RESULTS
All dogs were fully weight bearing by 48 h after surgery. At autopsy, no clinical signs of infection were present. All cultures taken from implantation sites were negative.
The mechanical revision implant fixation was significantly improved by cracking the sclerotic bone rim (Table 1). Approximately twofold increases were observed for ultimate shear strength (p < 0.01), apparent shear stiffness (p < 0.01) and total energy absorption (p < 0.01).
Table 1.
Results from Push-Out Test
| Revision Technique | Shear Strength (MPa) | Energy Abs. (J/m2) | Stiffness (MPa/ mm) |
|---|---|---|---|
| Control revision+HA | 1.6 (1.1–2.1) | 221 (119–323) | 7.3 (4.8–9.7) |
| Cracking+HA | 3.0 (2.3–3.8)* | 471 (319–622)* | 16.0 (10.9–21.2)* |
Data presented as mean and 95% confidence interval.
p<0.01.
The histomorphometrical analysis strongly supports the mechanical test results (Table 2). HA-coated implants inserted with the cracking technique had significantly more bone ongrowth (p < 0.01) compared with the hydroxyapatite-coated control implants (Fig. 5A). The cracking technique also resulted in a significant reduction in fibrous tissue ongrowth (p < 0.01). Seven of eight control implants had more than 50% fibrous tissue ongrowth (Fig. 5 B), while six of the implants with the cracking procedure had no fibrous tissue ongrowth.
Table 2.
Results from Histomorphometry
| Percentage Tissue Ongrowth |
Percentage Tissue Volume in the Gap (0–750 μm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bone | Fibrous Tissue | Bone Marrow | Cartilage | Bone | Fibrous Tissue | Bone Marrow | Cartilage | |
| HA Control | 27 (16–37) | 65 (43–87)* | 7 (−6–21) | 1 (0–2) | 18 (12–24) | 51 (30–73)*** | 29 (8–49) | 2 (0–4) |
| HA Crack | 41 (33–48)* | 9 (−6–24) | 51 (36–65)** | 0 (0–0) | 22 (19–24) | 10 (−2–34) | 66 (51–81)**** | 2 (−2–6) |
Data is presented as mean and 95% confidence interval.
p=0.001;
p=0.002;
p=0.008;
p=0.01.
Figure 5.
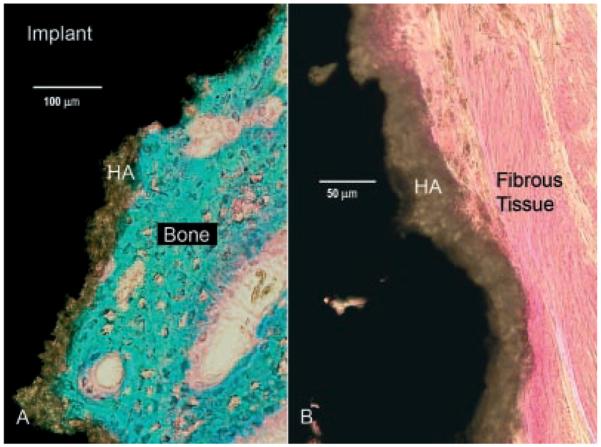
Histological sections (basic fuchsin and light green). Mineralized bone stains green and connective tissue stains red. The hydroxyapatite coating (brown) is the intersecting layer between tissue and the metal implant (black). (A) Bone ongrowth to an implant inserted with the cracking procedure. (B) Control implant with a typical dense fibrous membrane at the interface.
In the surrounding gap no difference was observed for bone volume. Significantly more fibrous tissue was seen in the gap of control implants (p < 0.01). Bone marrow was significantly higher for implants inserted with the cracking technique (p=0.01). In both groups, small percentages of cartilage tissue were seen. Cartilage tissue was mainly seen in the gap and in some cases with signs of endochondral ossification.
DISCUSSION
Loose hip implants are typically surrounded by a sclerotic bone rim, or neocortex.11,23 The sclerotic bone rim functions as a shield between the implant and the marrow space. After revision surgery, the bone rim prevents bone ingrowth and fosters fibrous fixation to cementless implants. The present study investigates the effect of the new surgical technique of cracking in combination with hydroxyapatite coated implants. The experimental revision model uses implant micromotion, wear particles, and load-induced fluid pressure changes to produce the region of a revision cavity analogous to the sclerotic bone shell seen in the human revision setting. The controlled implant motion and simultaneously loading allows the potentially confounding variable of relative implant motion to be identified in a weight-bearing model where pistoning or weight bearing can serve to circulate joint fluid along the implant interface. The tissue response includes fibrous tissue encapsulation of the implant, formation of a dense sclerotic bone rim, high levels of inflammatory cytokines, and reduced TGF-β. As in humans we have also observed a thickened joint capsule and increased joint fluid volume. The revision model does not intend to represent the overall problems of bone loss and loss of structural support that revision implants commonly present with. Instead, this model isolates the sclerotic region of the revision setting that is known to be critical to ensuring secure fixation that leads to long-term implant incorporation. We also recognize that impaction bone allografting is used by many surgeons in cases where reconstruction is needed due to large defects in the bone. Bone impaction in conjunction with this surgical technique is the subject of a future study.
It is well established that initial stability of the revised implant is essential for long-term success of both cementless and cemented revision hip replacements. Although the experimental model in this study focuses on the cancellous bone ingrowth regions of the proximal femur and acetabulum, long revision stems are often used clinically to achieve distal fixation in the femur. However, implants relying solely on distal fixation may still allow relative motion proximally. Distal fixation may also facilitate excessive bone resorption of the proximal femur due to the phenomenon of stress shielding.24 Other implant systems combine the principles of proximal and distal fixation.
The preparation of the proximal femur ranges from simple procedures such as removal of soft tissue and lavage to steps of proximal reaming or rasping and if necessary bone allografting. The preparation of the acetabulum usually includes socket reaming if possible, and many surgeons routinely use cups secured with screw fixation. Any of these reaming and rasping procedures involve loss of bone. The cracking technique presented in this study is primarily intended to address clinical situations where other means of removing the sclerotic bone will increase the risk of fracture or otherwise compromise the initial fixation. Therefore, we compared the cracking technique to a basic revision technique limited to removal of the soft tissues and scraping the sclerotic bone surface with a sharp curette. Furthermore, it is important to establish whether this new surgical technique is superior to less invasive procedures. As we previously have established, the cracking technique improves the mechanical fixation of titanium revision implants.15 In this study we investigate whether the cracking technique will improve the fixation of HA-coated implants. HA-coated implants are known to promote a rapid and solid bony integration of both primary and revision experimental implants.18,25 Clinical studies using HA-coated implants in a revision setting have also reported excellent mid- and long-term results.26,27 Therefore, it is of specific interest whether the osteoconductive effects of the HA coating will improve the fixation of the control revision implants and thereby reduce or eliminate the benefits of the cracking technique. In contrast to the previous study using titanium revision implants, this study also investigates the tissue response to the two revision procedures.
The cracking procedure was performed with a custom-fabricated splined instrument (Fig. 4), which was specifically designed for this particular experimental animal model. This tool design was necessary to secure a controlled and reproducible procedure that can be compared to other procedures. Instrumentation for clinical application can be simpler. Until special instruments are available, local perforation of the sclerotic bone rim can be performed with a standard curved osteotome or a cement extraction tool.
In the present study using HA implants, cracking the sclerotic bone rim resulted in a twofold mean increase in shear strength, stiffness, and energy absorption compared to the control revision procedure. In a previous study using titanium revision implants the cracking technique resulted in a similar improvement in mechanical fixation compared to the same control revision procedure as used in the present study.15
We characterized the tissue response to the cracking technique. Specifically, tissue distribution at the interface and in the original circumferential gap of 0.75 mm was quantified. The cracking technique promotes a favourable tissue response. At the interface, bone and bone marrow dominated the interface while fibrous tissue was only found sporadically. In contrast, the control group was dominated by large uninterrupted sections of dense fibrous tissue as seen on Fig. 5B.
In the original gap, the absence of dense fibrous tissue was typical for implants inserted with the cracking technique while half of the gap was filled with fibrous tissue in the control group.
Large amounts of fibrous tissue around the control revision implant may constitute a problem, as a solid osseointegration may be delayed or compromised. It is known that rapid osseointegration is important, as it can prevent early migration of the implant, as documented by Roentgen Stereophotogrammetric Analysis (RSA).28,29 Correspondingly, it is well established that early migration increases the risk of implant loosening.30,31
The mechanical barrier of the sclerotic bone rim prevents passive and active transport of cellular elements and growth factors. The improved revision implant fixation achieved by cracking the sclerotic bone rim is consistent with reestablishment of contact to the vascular compartment, thereby allowing diffusive, convective, and active access to the implant interface. Vascularity is critical, because the ingrowth of blood vessels necessarily precedes the formation of a suitable environment for bone growth. Furthermore, as the cracking procedure only is a disruption of the sclerotic rim, the intact sections can function as a scaffold fostering this bone growth. In many clinical cases of hip revision surgery the proximal femur may be devoid of cancellous bone. We cannot predict from the results of this study whether the cracking technique will be beneficial when the sclerotic bone rim is not supported by an underlying vascularized bone bed. In conclusion, the results of this study indicate that surgeons performing revision hip arthroplasty should be aware that the sclerotic bone rim is a barrier to the vascular bone bed, and its presence dictates unfavorable implant conditions even with the use of osteoconductive coatings such as hydroxyapatite. Our results suggest that the cracking technique, which locally perforates the bone rim, is a simple and efficient way to stimulate bone ingrowth. The cardinal findings were increased bone at the implant interface and an almost absence of fibrous tissue at the implant interface and in the surrounding gap. Further investigations comparing the technique of cracking the sclerotic bone rim to reaming and impaction allografting are forthcoming.
ACKNOWLEDGMENTS
Financial support was received from NIH (AR4205). Biomet, Inc. provided the PMMA and hydroxyapatite coated implants. We would like to gratefully acknowledge bone pathology expert, Professor Fleming Melsen, MD, DMSc, Aarhus University Hospital (1938–2005), for his many years of excellent and perceptive assistance with histomorphometry. Edward Greenfield and Joscelyn Sebold (Cleveland Clinic Foundation, Cleveland, OH) graciously donated the endotoxin measurements for the polyethylene particles.
REFERENCES
- 1.Havelin LI, Engesaeter LB, Espehaug B, et al. The Norwegian Arthroplasty Register—11 years and 73,000 arthroplasties. Acta Orthop Scand. 2000;71:337–353. doi: 10.1080/000164700317393321. [DOI] [PubMed] [Google Scholar]
- 2.Puolakka TJS, Pajamaki KJJ, Halonen PJ, et al. The Finnish Arthroplasty Register—report of the hip register. Acta Orthop Scand. 2001;72:433–441. doi: 10.1080/000164701753532745. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Mowat F, Ong K, et al. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 4.Dohmae Y, Bechtold JE, Sherman RE, et al. Reduction in cement-bone interface shear-strength between primary and revision arthroplasty. Clin Orthop. 1988;236:214–220. [PubMed] [Google Scholar]
- 5.Dreghorn CR, Hamblen DL. Revision arthroplasty—a high price to pay. BMJ. 1989;298:648–649. doi: 10.1136/bmj.298.6674.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furnes A, Lie SA, Havelin LI, et al. The economic impact of failures in total hip replacement surgery—28,997 cases from the Norwegian Arthroplasty Register, 1987–1993. Acta Orthop Scand. 1996;67:115–121. doi: 10.3109/17453679608994653. [DOI] [PubMed] [Google Scholar]
- 7.Katz RP, Callaghan JJ, Sullivan PM, et al. Results Of cemented femoral revision total hip-arthroplasty using improved cementing techniques. Clin Orthop. 1995;319:178–183. [PubMed] [Google Scholar]
- 8.Lavernia CJ, Drakeford MK, Tsao AK, et al. Revision and primary hip and knee arthroplasty—a cost-analysis. Clin Orthop. 1995;311:136–141. [PubMed] [Google Scholar]
- 9.Robinson AHN, Palmer CR, Villar RN. Is revision as good as primary hip replacement? A comparison of quality of life. J Bone Joint Surg Br. 1999;81:42–45. doi: 10.1302/0301-620x.81b1.8728. [DOI] [PubMed] [Google Scholar]
- 10.Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand. 2000;71:433–439. doi: 10.1080/000164700317381081. [DOI] [PubMed] [Google Scholar]
- 11.Bobyn JD, Jacobs JJ, Tanzer M, et al. The susceptibility of smooth implant surfaces to periimplant fibrosis and migration of polyethylene wear debris. Clin Orthop. 1995;311:21–39. [PubMed] [Google Scholar]
- 12.Vestermark MT, Bechtold JE, Swider P, et al. Mechanical interface conditions affect morphology and cellular activity of sclerotic bone rims forming around experimental loaded implants. J Orthop Res. 2004;22:647–652. doi: 10.1016/j.orthres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Bechtold JE, Mouzin O, Kidder L, et al. A controlled experimental model of revision implants Part II. Implementation with loaded titanium implants and bone graft. Acta Orthop Scand. 2001;72:650–656. doi: 10.1080/000164701317269102. [DOI] [PubMed] [Google Scholar]
- 14.Bechtold JE, Kubic V, Soballe K. A controlled experimental model of revision implants Part I. Development. Acta Orthop Scand. 2001;72:642–649. doi: 10.1080/000164701317269094. [DOI] [PubMed] [Google Scholar]
- 15.Kold S, Bechtold JE, Mouzin O, et al. Fixation of revision implants is improved by a surgical technique to crack the sclerotic bone rim. Clin Orthop. 2005;432:160–166. doi: 10.1097/01.blo.0000149815.78598.ea. [DOI] [PubMed] [Google Scholar]
- 16.Rahbek O, Overgaard S, Jensen TB, et al. Sealing effect of hydroxyapatite coating—a 12-month study in canines. Acta Orthop Scand. 2000;71:563–573. doi: 10.1080/000164700317362181. [DOI] [PubMed] [Google Scholar]
- 17.Soballe K, Overgaard S. The current status of hydroxyapatite coating of prostheses. J Bone Joint Surg Br. 1996;78:689–691. [PubMed] [Google Scholar]
- 18.Soballe K, Mouzin ORG, Kidder LA, et al. The effects of hydroxyapatite coating and bone allograft on fixation of loaded experimental primary and revision implants. Acta Orthop Scand. 2003;74:239–247. doi: 10.1080/00016470310014139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taki N, Tatro JM, Nalepka JL, et al. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–383. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Nalepka JL, Greenfield EM. Adherent endotoxin inhibits spreading and proliferation of osteoblasts. J Bone Miner Res. 2005;20:S359–S359. [Google Scholar]
- 21.Gotfredsen K, Jorgensen EB, Jensen LN. A method for preparing and staining histological sections containing titanium implants for light-microscopy. Stain Tech. 1989;64:121–127. doi: 10.3109/10520298909106984. [DOI] [PubMed] [Google Scholar]
- 22.Overgaard S, Soballe K, Gundersen HJG. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res. 2000;18:313–321. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 23.Mikhail WEM, Weidenhielm LRA, Wretenberg P, et al. Femoral bone regeneration subsequent toimpaction grafting during hip revision—histologic analysis of a human biopsy specimen. J Arthroplasty. 1999;14:849–853. doi: 10.1016/s0883-5403(99)90036-0. [DOI] [PubMed] [Google Scholar]
- 24.Oh I, Harris WH. Proximal strain distribution in loaded femur—in vitro comparison of distributions in intact femur and after insertion of different hip-replacement femoral components. J Bone Joint Surg Am. 1978;60:75–85. [PubMed] [Google Scholar]
- 25.Soballe K, Hansen ES, Rasmussen HB, et al. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg Br. 1993;75:270–278. doi: 10.1302/0301-620X.75B2.8444949. [DOI] [PubMed] [Google Scholar]
- 26.Reikeras O, Gunderson RB. Excellent results with femoral revision surgery using an extensively hydroxyapatite-coated stem—59 patients followed for10–16 years. Acta Orthop. 2006;77:98–103. doi: 10.1080/17453670610045759. [DOI] [PubMed] [Google Scholar]
- 27.Trikha SP, Singh S, Raynham OW, et al. Hydroxyapatite-ceramic-coated femoral stems in revision hip surgery. J Bone Joint Surg Br. 2005;87:1055–1060. doi: 10.1302/0301-620X.87B8.16053. [DOI] [PubMed] [Google Scholar]
- 28.Snorrason F, Karrholm J. Primary migration of fully-threaded acetabularprostheses—a roentgen stereophotogrammetric analysis. J Bone Joint Surg Br. 1990;72:647–652. doi: 10.1302/0301-620X.72B4.2199453. [DOI] [PubMed] [Google Scholar]
- 29.Soballe K, Toksvig-Larsen S, Gelineck J, et al. Migration of hydroxyapatite-coated femoral prostheses—a roentgen stereophotogrammetric study. J Bone Joint Surg Br. 1993;75:681–687. doi: 10.1302/0301-620X.75B5.8397213. [DOI] [PubMed] [Google Scholar]
- 30.Karrholm J, Borssen B, Lowenhielm G, et al. Does early micromotion of femoral stem prostheses matter—4–7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br. 1994;76:912–917. [PubMed] [Google Scholar]
- 31.Karrholm J, Herberts P, Hultmark P, et al. Radio-stereometry of hip prostheses—review of methodology and clinical results. Clin Orthop. 1997;344:94–110. [PubMed] [Google Scholar]