Abstract
In the context of producing enhanced therapeutics for regenerative medicine, our laboratory develops gene-activated matrices (GAMs) using non-viral gene therapy (GT) in combination with collagen-based scaffolds engineered specifically for tissue repair. Non-viral vectors have been referred to as a minority pursuit in GT but considering the concerns associated with viral vectors and as transient gene expression is such a key consideration, further research is clearly warranted for tissue engineering (TE) applications. Mesenchymal stem cells (MSCs) are well regarded for their capability in bone regeneration but as primary cells, they are difficult to transfect. We have recently optimised the non-viral vector, polyethyleneimine (PEI), to achieve high transfection efficiencies in MSCs. Subsequently, a series of PEI-based GAMs were developed using collagen, collagen-glycosaminoglycan and collagen-nanohydroxyapatite (collagen-nHa) scaffolds whereby transgene expression was detected up to 21 d with the collagen-nHa scaffold providing the most prolonged expression. Moreover, all PEI-based GAMs contained a low plasmid DNA dose of 2 µg which is far below doses often required in previous GAMs. Having successfully developed these GAMs, the ephrinB2 gene has recently been incorporated to produce a novel therapeutic GAM for bone repair. Herein, we discuss our recent investigations in the development and application of non-viral GAMs.
Keywords: gene-activated matrices, scaffolds, tissue engineering, regenerative medicine, mesenchymal stem cells, bone repair, non-viral gene delivery vectors, orthopaedic gene therapy
Seeking to revolutionise treatment options, the field of tissue engineering (TE) aspires to ‘create new tissue for the therapeutic reconstruction of the human body’.1 Over the years, many advancements have been made in TE but most success has been in the regeneration of ‘soft’ tissues such as skin,2,3 urethra,4 esophagus5 and trachea.6,7 It is quite striking that in 2013, the implantation of bone grafts harvested from a patient or donor, still remains the gold standard treatment strategy for bone repair. Needless to say therefore, a substantial and unmet need for the design of TE therapeutics for intricate and mechanically strong tissue types such as bone and cartilage still exists.
Researchers in the bone TE field often concentrate their efforts on developing a 3D environment, or scaffold, filled with biological, structural and mechanical cues, in the hope of coaxing cells to lay down the matrix of a desired tissue type.8 A series of collagen-based scaffolds including collagen and composites of collagen-glycosaminoglycan (collagen-GAG),9-11 collagen-hydroxyapatite12,13 and collagen-nanohydroxyapatite (collagen-nHa)14 have been developed for bone repair in our laboratory. The collagen-GAG scaffold was originally created for skin regeneration by Yannas et al.2 and subsequently engineered in Yannas’ laboratory to contain a homogeneous pore structure suitable for bone repair by O’Brien et al. in 2004.15 Since then, a number of further enhanced, bone-targeted, novel collagen-composite scaffolds have been produced by our group including the collagen-nHa scaffold which will be discussed primarily in this commentary.14,16,17 Previous work within our laboratory has demonstrated the ability of a cell free collagen-GAG scaffold and a mesenchymal stem cell (MSC) seeded collagen-GAG scaffold to induce bone formation in a rat cranial defect.18-20 More recently, a collagen-hydroxyapatite composite scaffold has shown good bone healing after 4 weeks resulting in a completely bridged critical defect.12 However, for very large bone defects, an extra agent, i.e., in the form of cells or biomolecules, such as therapeutic proteins or genes, is needed where the scaffold alone is insufficient in providing complete healing. With that in mind, although the cell and biomolecule-free bone graft substitutes developed in our lab have produced commendable results to date, we sought to produce next-generation scaffolds by combining them with a gene therapy (GT) approach to further enhance the therapeutic potential of these constructs.
As the name suggests, GT involves the transfer of therapeutic genes, usually in the form of plasmid DNA, for therapeutic purposes. In somatic GT, ‘faulty’ genes are replaced with healthy alleles to reverse a monogenic disease. In the early days, GT was conceived as a ‘last port of call’ treatment for lethal single gene disorders and researchers predicted that somatic GT would become commonplace within a decade.21 However, the emergence of GT as a fully fledged area of medicine has been slow and paved with disappointments and retraction of investment along the way.22-24 In spite of the relatively few success stories and tragically the occurrence of some fatalities, the number of GT trials underway affirms that there is continued interest and belief in the promise of GT.25 Interestingly, Glybera, a product that treats the single gene disease lipoprotein lipase deficiency (LPLD), was the first GT product approved by the European Commission in November 2012 making it the first GT product approved by the regulatory bodies in the Western world.26 This is no doubt a monumental and encouraging outcome for the fields of gene and cell therapy. In addition to treating rare monogenic genetic diseases, GT also holds a lot of promise in the field of TE. Unlike GT for the treatment of single gene diseases where long-term expression and integration of the delivered transgene may be required for effective treatment, there is no need for constitutive expression in GT for TE applications. A sustained but transient expression of the transgene is more beneficial. In applying GT to TE applications, a vector is necessary to deliver the DNA load to a target cell and transient expression of the genes can be achieved using adenoviral or non-viral vectors.27-29
To date, the vast majority of GT research has utilized viral vectors to deliver therapeutic proteins to target cells.30 Orthopedic GT research has been ongoing for approximately two decades but has not progressed to clinical trials for a number of reasons most of which likely hinge on the unsafe perception of the viral vectors used. Fatal clinical trials involving GT and viral vectors for monogenic disorders have occurred which detracts investment and heightens regulatory attention.31,32 On the other hand, although they have been referred to as a minority pursuit in GT in general,22 non-viral vectors might offer a safe and valid alternative and may lead the way to successful clinical translation of orthopedic GT. Considering the concerns associated with using viral vectors and as transient gene expression is such a key consideration, many researchers would agree that non-viral vectors should be the primary choice for GT applications in TE.33,34 Non-viral vectors are designed to essentially mimic the cell-entry abilities of their viral counterparts.35 Non-viral gene transfer methodologies offer several advantages, all of which are independent to individual vectors. They can exhibit low immunogenicity, low toxicity, high transfection efficiency, larger plasmid loads, low production cost and they do not insert into the host genome so insertional mutagenesis does not pose a risk.34 Non-viral vectors also cause a desirable temporary but sustained release of protein from the transfected cell. Simply, therapeutic protein production is temporarily sustained for a limited timeframe after which it subsides. For the aforementioned reasons we opted to develop non-viral gene-activated matrices (GAMs) targeted at bone repair.
Although not new, the idea of a gene delivery vector contained in a biodegradable scaffold is an innovative development in TE.36 GAMs for bone repair were initially conceived as off-the shelf, non-viral, gene-containing scaffolds in the late 1990s.36,37 The scaffold essentially acts as a depot for the gene while simultaneously offering structural support and a matrix for new tissue deposition (Fig. 1). GAMs can instruct cells to follow a certain lineage in vivo by the single application of the GAM to the defect. Once the construct is in place, there is no need for repeat administration and the in vivo response brought about by the release of the therapeutic protein from cells on the GAM governs effective healing. An important concern in TE is the spatiotemporal delivery of therapeutics from the tissue engineered construct.38 Sustained delivery mechanisms stand to increase the therapeutic potential as the proteins are present in the defect for a longer period of time to elicit a therapeutic effect. GAMs offer sustained release of the protein as the transfected cells continually secrete protein over time. The scaffold can be treated as a depot whereby the DNA complex stays adhered to the scaffold and infiltrating cells become transfected as they pass through the GAM (Fig. 1).
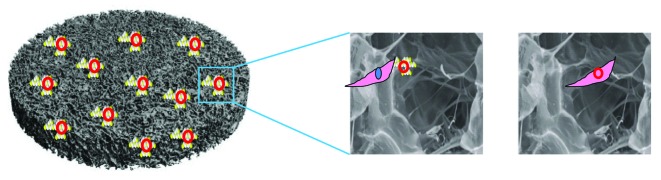
Figure 1. Transfection in gene-activated matrices (GAMs). The scaffold acts as a depot for vector-gene complexes and upon seeding with MSCs, the complexes are taken up and the transgene becomes expressed. The scaffold also retains its key functional role in promoting cell infiltration and as a template for tissue repair.
In terms of utilizing non-viral GAMs for the repair of musculoskeletal tissues other than bone, little work has been performed to date in these areas. Although viral vectors have been used to deliver genes to tendons and ligaments,39 there are no reports on the use of non-viral GAMs. The majority of studies have focused on the repair of articular cartilage possibly due to the ease of access to this tissue. However, due to its relative acellularity, complete avascularity and aneural composition, repairing this complex tissue requires a multistep methodology that necessitates further research to truly reach its full potential. Some studies of note that have described success to date however include a study by Madry et al.40 where FuGENE 6 was used to successfully deliver insulin-like growth factor 1 (IGF1) gene to lapine articular chondrocytes in an alginate gel suspension delivery system in osteochondral defects demonstrating augmented cartilage repair. Chitosan-gelatin scaffolds have also been utilized to deliver naked transforming growth factor β1 (TGF-β1) plasmid demonstrating enhanced cartilage tissue regeneration within the GAM-treated group compared with controls.41 Alginate/chitosan polysaccharide capsules have been used to deliver Sox-9 plasmid DNA to human mesenchymal progenitor cells through the use of the non-viral method nucleofection. In vivo results demonstrated enhanced chondrogenesis within the transfected group compared with untransfected controls.42 A study by Gelse et al.43 compared the use of non-viral liposome transfection to that of adeno-associated virus (AAV) and adenovirus (Ad) when delivering BMP2 to chondral lesions on PGA scaffolds but demonstrated inferior results using the non-viral delivery method compared with that of the viral method in terms of cartilage repair. Most recently, Kayabasi et al.44 have delivered the BMP6 gene to rat MSCs using Lipofectamine on chitosan scaffolds and demonstrated some promising chondrogenic results in vitro. The limited number of non-viral GAM studies performed on the repair of these other musculoskeletal tissues demonstrates that the systems proposed within this review may have the potential to be applied for use in these orthopedic applications and furthermore, they may also have the capacity to be utilized for the regeneration of numerous other tissues.
MSCs are well regarded for their tri-lineage differentiation into bone, cartilage or adipose cells when provided with appropriate cues and their capability in bone regeneration has been extensively documented.45,46 The lack of an ideal vector for MSC transfection is a significant hurdle in the translation of GT and regenerative medicine. In the recent study from our lab published in the Journal of Controlled Release, polyethyleneimine (PEI) was shown to be capable of highly efficient transfection of MSCs.47 PEI has been previously reported as an efficient transfection tool in cell lines48,49 and a small number of stem cells including adipose-derived stem cells and bone marrow-derived MSCs.50,51 However, as primary cells, MSCs are notoriously difficult to transfect and the highest transfection efficiency previously reported is 19%. By analyzing a number of physical parameters, cell viability and transfection efficiency, across a number of PEI-DNA N/P ratios (ratio of amines in PEI to phosphates in DNA), PEI was optimised for MSC transfection in this study. It was demonstrated that MSCs can be transfected with a transfection efficiency of between 30 and 45% depending on the N/P ratio used.
Upon optimising PEI for MSC transfection, a series of PEI-based GAMs were then developed. It was shown that when merged with collagen, collagen-GAG and collagen-nHa scaffolds, PEI-DNA polyplexes could successfully transfect MSCs. Moreover, all PEI-based GAMs developed contained a low plasmid DNA dose of 2 µg which is far below the higher doses in the order of milligrams which were often required in earlier GAMs.36,50,52 More specifically, it was also found that scaffold composition can affect transgene expression in the GAMs. While all scaffolds proved capable of successful MSC transfection, the collagen-nHa scaffold demonstrated the highest prolonged levels of gene expression (Fig. 2). Temporarily sustained transgene expression was evident in all collagen-based GAMs but the collagen-nHa scaffold resulted in a more prolonged duration of transgene expression over a 14 d period. Similar results were also found in another study from the group by Curtin et al.17 where sustained transgene expression was more prolonged on collagen-nHa scaffolds compared with collagen alone scaffolds. Although an investigation of the exact reasons for this was outside the scope of the study, a number of theories are proposed as to why this occurred. First, it may be attributed to the fact that collagen-nHa scaffolds are stiffer substrates than the collagen alone and the collagen-GAG scaffolds. Enhanced gene expression has previously been observed on stiffer biomaterials53 so it could simply be down to the mechanical strength of the material. It is also possible that it may be attributed to the manner in which the polyplexes are attached to the nHa scaffold. Polyplexes may be bound to the scaffold in one of two possible ways, either by adsorption to the collagen or to the nHa. If the cells preferentially attach to the nHa itself it could explain the higher transfection efficiencies in the nHa-based GAMs. Also, cells respond to their surrounding environment via focal adhesions on the cell surface11,53,54 and mineralised substrates have been shown to stimulate focal adhesion, MSC motility and migration throughout the matrices.55 Therefore, it may be that the collagen-nHa scaffold results in the adherence of more cells than the collagen alone and collagen-GAG scaffolds due to increased focal adhesion if the MSCs preferentially attach to nHa. Taken together, these results support the overall hypothesis that the elevated and prolonged levels of gene expression seen in the collagen-nHa scaffolds is likely attributable to increased cellular attachment and mobility in the GAM, meaning that the cells encounter more PEI polyplexes than cells on mineral-free scaffolds contributing to more transfection.
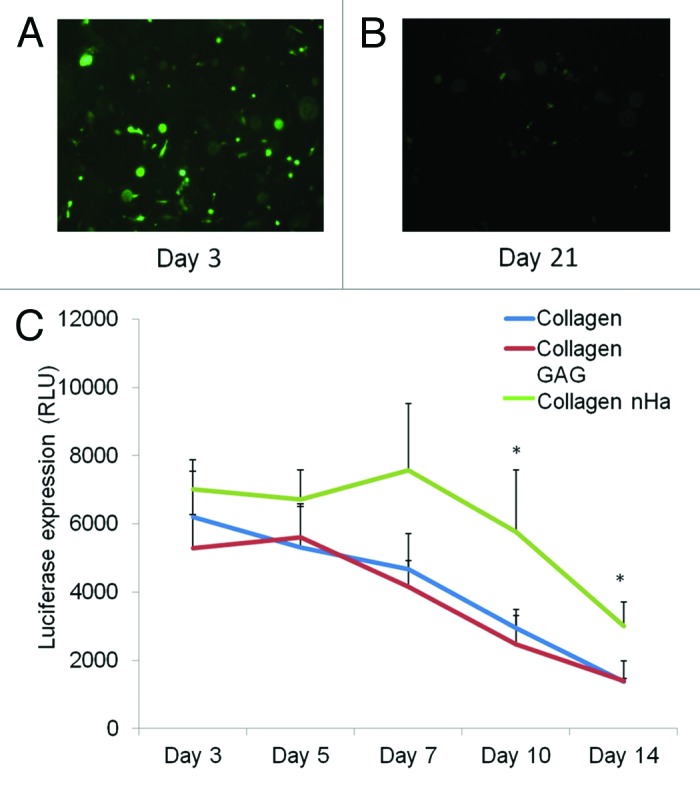
Figure 2. Scaffold composition affects gene expression duration in collagen-based GAMs. The images depict MSC transfection within PEI-GFP collagen-GAG GAMs loaded with 2µg N/P 10 PEI-GFP polyplexes 3 (A) and 21 (B) days after cell seeding on the matrices. PEI-luciferase collagen-based GAMs were fabricated where luciferase expression was shown to be elevated for a prolonged timeframe in the collagen-nHa GAM. Data plotted shows mean standard deviation (n = 3) and p < 0.05.
This GAM development study has made a significant contribution to the field of GT and TE. PEI was specifically optimised for MSCs, the target cell in vivo, and combined with a scaffold which was engineered expressly for bone tissue repair to produce a superior construct for TE applications. These GAMs contain a mere 2 µg of polyplex which is a significant reduction on former quantities used thereby decreasing cost and decreasing the quantities of exogenous materials in the injured site. While the GAMs we have developed may have functions in a whole host of applications, the performance of the nHa scaffold is very interesting from a bone repair outlook as nHa has dual benefits in that it prolongs gene expression and has osteoinductive qualities itself.17 Ultimately, the application of this GAM would involve replacing the reporter genes with therapeutic genes. This goal has since been achieved in the interim by incorporating plasmid DNA encoding the ephrinB2 gene.56
Ephrin ligands and their cognate receptors are involved in governing many cellular processes from cell morphology to vasculogenesis and cell migration.57,58 Of most relevance to orthopedic applications, it has been shown that some ephrin ligands and receptors, namely ephrinB2 and EphB4, are involved in bone remodelling.59,60 Bidirectional signaling between an osteoclast expressing ephrinB2 and an osteoblast expressing EphB4 triggers osteoblast differentiation and obstructs osteoclastogenesis.59-61 The field of biomaterials is currently in the midst of a revolutionary change where progressions in the life sciences are of equal importance to the development of novel biomaterials.62 The newly discovered role of ephrinB2 and EphB4 interactions in bone prompted the investigation of the overexpression of the ephrinB2 ligand in a GAM in a follow on paper which was also published in the Journal of Controlled Release in 2013.56
First, in this study, it was shown that ephrinB2 overexpression increases osteogenesis in monolayer human MSCs which was dependent upon an interaction with the EphB4 receptor presented on the surface of adjacent cells. This led to the establishment of a novel PEI-ephrinB2 GAM specifically tailored for bone repair which enhances osteogenesis, and as we know from the literature, also has the potential to inhibit osteoclastogenesis.59 The potential pro-anabolic, anti-catabolic scope of PEI-ephrinB2 GAMs in bone regeneration is outlined in Figure 3. Furthermore, the original paper detailed successful transient overexpression of the transgene in rat MSCs and in the subsequent paper it was verified that the GAMs had translational capabilities when human MSCs were seeded in place of rat MSCs.
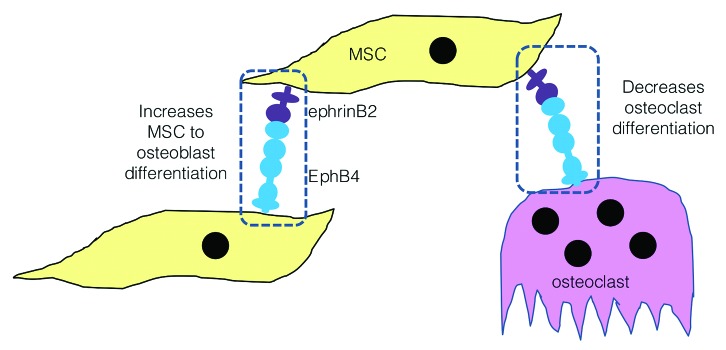
Figure 3. EphrinB2 GAMs have the potential to exert a pro-anabolic, anti-catabolic effect on bone formation. EphrinB2 overexpression stands to enhance bone formation by two mechanisms, first by increasing osteoblast differentiation and second by blocking osteoclast differentiation.
The PEI-ephrinB2 GAM is the first GAM for bone repair which incorporates the ephrinB2 gene and within just 14 d of MSC seeding - a very early timepoint - enhanced osteogenesis was already observed in the GAMs. The finding that ephrinB2-mediated osteogenesis was reliant on EphB4 interaction contributed to the idea that a PEI-ephrinB2 GAM may possess great potential as a select matrix for bone repair. Cytokines such as BMP2 and VEGF can elicit off-site effects and high doses can trigger bone resorption and osteolysis.63 The secondary requirement for EphB4 interaction means that offsite effects such as these are completely minimised and effects are only exercised in EphB4-expressing cells. The osteogenic enhancements witnessed in monolayer culture and mirrored in the PEI-ephrinB2 GAM provides evidence of the great potential of this therapeutic for in vivo translation.
The discovery that ephrinB2 overexpression enhanced osteogenesis in MSCs was an unusual finding which suggests that ephrin/Eph signaling may be involved within the MSC population itself, independent to previously reported interactions with other cell types. In itself, this implies that ephrinB2-MSCs could be a valuable modification to MSCs utilized in bone repair strategies. Although ephrinB2 has previously been shown to increase angiogenesis in MSCs,64 this study has shown that very high levels of ephrinB2 overexpression can actually trigger osteogenesis. This result prompted the theory that ephrinB2 expression is multi-functional with levels of overexpression critical to the therapeutic outcome. Moreover, ephrinB2 can be hypothesized as a ‘one size fits all gene’ for bone repair in that it has previously been shown to increase angiogenesis,64 to inhibit osteoclastogenesis59 and finally, in this successive paper, to increase osteogenesis in human MSCs.
The collagen-nHa scaffold used in the development of these GAMs has also been used in the development of other GAMs in our research group as described earlier. In a study published in 2012 in Advanced Materials, Curtin et al.17 demonstrated that the nHa particles themselves could be used as vectors for successful MSC transfection when the nano-sized Ha particles were co-precipitated with DNA to produce particles of approximately 100nm which could easily cross the cell membrane via endocytosis.65 A sustained but transient transgene expression profile was noted in these nHa-based GAMs using reporter genes. Furthermore, when the reporter genes were substituted for the osteogenic gene BMP2, significantly enhanced osteogenesis was observed in nHa-BMP2 GAMs upon seeding with MSCs.
Taken together, the work discussed from these publications by our research group provides key support for the use of non-viral vectors in GT-related bone repair indicating that they should not be overlooked in the development of new therapeutics. The combination of gene therapy with scaffolds engineered for tissue regeneration has the capability to provide great potential in orthopedics and other disciplines.
Acknowledgments
The authors acknowledge the European Research Council (ERC grant agreement no 239685) under the EU Seventh Framework Programme (FP7/2007–2013) and a Science Foundation Ireland (SFI) President of Ireland Young Researcher Award (04/Yl1/B531). The collagen used in the scaffolds described in this commentary was provided by Integra Life Sciences, Inc. through a Material Transfer Agreement. Figure 2 was reprinted with permission from ‘Tierney EG, Duffy GP, Hibbitts AJ, Cryan S-A, O'Brien FJ. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. Journal of Controlled Release 2012; 158:304–11’.
Footnotes
Previously published online: www.landesbioscience.com/journals/organogenesis/article/24329
References
- 1.Williams DF. To engineer is to create: the link between engineering and regeneration. Trends Biotechnol. 2006;24:4–8. doi: 10.1016/j.tibtech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86:933–7. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yannas IV, Orgill DP, Burke JF. Template for skin regeneration. Plast Reconstr Surg. 2011;127(Suppl 1):60S–70S. doi: 10.1097/PRS.0b013e318200a44d. [DOI] [PubMed] [Google Scholar]
- 4.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643–50. doi: 10.1089/ten.tea.2010.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 7.Jungebluth P, Bader A, Baiguera S, Möller S, Jaus M, Lim ML, et al. The concept of in vivo airway tissue engineering. Biomaterials. 2012;33:4319–26. doi: 10.1016/j.biomaterials.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Evans ND, Gentleman E, Polak JM. Scaffolds for stem cells. Mater Today. 2006;9:26–33. doi: 10.1016/S1369-7021(06)71740-0. [DOI] [Google Scholar]
- 9.Keogh MB, O’ Brien FJ, Daly JS. A novel collagen scaffold supports human osteogenesis--applications for bone tissue engineering. Cell Tissue Res. 2010;340:169–77. doi: 10.1007/s00441-010-0939-y. [DOI] [PubMed] [Google Scholar]
- 10.Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2009;2:202–9. doi: 10.1016/j.jmbbm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–6. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson JP, Plunkett NA, O’Brien FJ. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur Cell Mater. 2010;20:218–30. doi: 10.22203/ecm.v020a18. [DOI] [PubMed] [Google Scholar]
- 13.Al-Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, et al. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J Biomed Mater Res B Appl Biomater. 2009;90:584–91. doi: 10.1002/jbm.b.31320. [DOI] [PubMed] [Google Scholar]
- 14.Cunniffe GM, Dickson GR, Partap S, Stanton KT, O’Brien FJ. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21:2293–8. doi: 10.1007/s10856-009-3964-1. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077–86. doi: 10.1016/S0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 16.Cunniffe GM, O’Brien FJ, Partap S, Levingstone TJ, Stanton KT, Dickson GR. The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J Biomed Mater Res A. 2010;95:1142–9. doi: 10.1002/jbm.a.32931. [DOI] [PubMed] [Google Scholar]
- 17.Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, et al. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater. 2012;24:749–54. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 18.Lyons FG, Al-Munajjed AA, Kieran SM, Toner ME, Murphy CM, Duffy GP, et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31:9232–43. doi: 10.1016/j.biomaterials.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 19.Alhag M, Farrell E, Toner ME, Claffey N, Lee TC, O’Brien F. Evaluation of early healing events around mesenchymal stem cell-seeded collagen-glycosaminoglycan scaffold. An experimental study in Wistar rats. Oral Maxillofac Surg. 2011;15:31–9. doi: 10.1007/s10006-010-0241-x. [DOI] [PubMed] [Google Scholar]
- 20.Alhag M, Farrell E, Toner M, Lee TC, O’Brien FJ, Claffey N. Evaluation of the ability of collagen-glycosaminoglycan scaffolds with or without mesenchymal stem cells to heal bone defects in Wistar rats. Oral Maxillofac Surg. 2012;16:47–55. doi: 10.1007/s10006-011-0299-0. [DOI] [PubMed] [Google Scholar]
- 21.Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. 2009;157:153–65. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–8. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- 23.Check E. A tragic setback. Nature. 2002;420:116–8. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 24.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–5. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K. Delivery of magic bullets: on the still rocky road to gene therapy. Br J Pharmacol. 2009;157:151–2. doi: 10.1111/j.1476-5381.2009.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ylä-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union. Mol Ther. 2012;20:1831–2. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeney M, van den Beucken JJJP, van der Kraan PM, Jansen JA, Pandit A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165) Biomaterials. 2010;31:2893–902. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 28.Endo M, Kuroda S, Kondo H, Maruoka Y, Ohya K, Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489–97. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 29.Itaka K, Ohba S, Miyata K, Kawaguchi H, Nakamura K, Takato T, et al. Bone regeneration by regulated in vivo gene transfer using biocompatible polyplex nanomicelles. Mol Ther. 2007;15:1655–62. doi: 10.1038/sj.mt.6300218. [DOI] [PubMed] [Google Scholar]
- 30.Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010;12:e18. doi: 10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans CH. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev. 2011;17:437–41. doi: 10.1089/ten.teb.2011.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy--lost in translation? J Cell Physiol. 2012;227:416–20. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheyn D, Mizrahi O, Benjamin S, Gazit Z, Pelled G, Gazit D. Genetically modified cells in regenerative medicine and tissue engineering. Adv Drug Deliv Rev. 2010;62:683–98. doi: 10.1016/j.addr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Santos JL, Pandita D, Rodrigues J, Pêgo AP, Granja PL, Tomás H. Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr Gene Ther. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 35.Midoux P, Pichon C, Yaouanc JJ, Jaffrès PA. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol. 2009;157:166–78. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 37.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan ACA, Ying JY. Nanomaterials for in situ cell delivery and tissue regeneration. Adv Drug Deliv Rev. 2010;62:731–40. doi: 10.1016/j.addr.2010.02.002. [Corrected Proof.] [DOI] [PubMed] [Google Scholar]
- 39.Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, et al. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J Bone Joint Surg Am. 2002;84-A:1123–31. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–9. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 41.Guo T, Zhao J, Chang J, Ding Z, Hong H, Chen J, et al. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2006;27:1095–103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Babister JC, Tare RS, Green DW, Inglis S, Mann S, Oreffo RO. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using “bead-in-bead” polysaccharide capsules. Biomaterials. 2008;29:58–65. doi: 10.1016/j.biomaterials.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Gelse K, Mühle C, Franke O, Park J, Jehle M, Durst K, et al. Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum. 2008;58:475–88. doi: 10.1002/art.23124. [DOI] [PubMed] [Google Scholar]
- 44.Kayabaşi GK, Tiğli Aydin RS, Gümüşderelioğlu M. In vitro chondrogenesis by BMP6 gene therapy. J Biomed Mater Res A. 2013;101:1353–61. doi: 10.1002/jbm.a.34430. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–52. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 46.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 47.Tierney EG, Duffy GP, Hibbitts AJ, Cryan S-A, O’Brien FJ. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J Control Release. 2012;158:304–11. doi: 10.1016/j.jconrel.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen YT, Kim HK, Kwon JS, Kim YS, Yoon TR, Ahn Y, et al. Efficient transfer of reporter gene-loaded nanoparticles to bone marrow stromal cells (D1) by reverse transfection. J Nanosci Nanotechnol. 2010;10:3170–4. doi: 10.1166/jnn.2010.2236. [DOI] [PubMed] [Google Scholar]
- 49.Tripathi SK, Goyal R, Kumar P, Gupta KC. Linear polyethylenimine-graft-chitosan copolymers as efficient DNA/siRNA delivery vectors in vitro and in vivo. Nanomedicine. 2012;8:337–45. doi: 10.1016/j.nano.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Huang YC, Connell M, Park Y, Mooney DJ, Rice KG. Fabrication and in vitro testing of polymeric delivery system for condensed DNA. J Biomed Mater Res A. 2003;67:1384–92. doi: 10.1002/jbm.a.20036. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Li W, Ou L, Flick E, Mark P, Nesselmann C, et al. Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. J Cell Mol Med. 2011;15:1989–98. doi: 10.1111/j.1582-4934.2010.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang YC, Riddle K, Rice KG, Mooney DJ. Long-term in vivo gene expression via delivery of PEI-DNA condensates from porous polymer scaffolds. Hum Gene Ther. 2005;16:609–17. doi: 10.1089/hum.2005.16.609. [DOI] [PubMed] [Google Scholar]
- 53.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–4. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 54.Rao R. R., He J, Leach JK. Biomineralized composite substrates increase gene expression with nonviral delivery. J Biomed Mater Res A. 2010;94A:344–54. doi: 10.1002/jbm.a.32690. [DOI] [PubMed] [Google Scholar]
- 55.Grochowsky JC, Alaways LW, Siskey R, Most E, Kurtz SM. Digital photogrammetry for quantitative wear analysis of retrieved TKA components. J Biomed Mater Res B Appl Biomater. 2006;79:263–7. doi: 10.1002/jbm.b.30537. [DOI] [PubMed] [Google Scholar]
- 56.Tierney EG, McSorley K, Hastings CL, Cryan S-A, O’Brien T, Murphy MJ, et al. High levels of ephrinB2 over-expression increases the osteogenic differentiation of human mesenchymal stem cells and promotes enhanced cell mediated mineralisation in a polyethyleneimine-ephrinB2 gene-activated matrix. J Control Release. 2013;165:173–82. doi: 10.1016/j.jconrel.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–80. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–9. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JMW, Crimeen-Irwin B, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–81. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 61.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–32. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu NY, Schindeler A, Tagil M, Ruys AJ, Little DG. Use of BMPs and bisphosphonates in improving bone fracture healing. Front Biosci (Elite Ed) 2012;4:2647–53. doi: 10.2741/E572. [Elite Ed] [DOI] [PubMed] [Google Scholar]
- 64.Duffy GP, D’Arcy S, Ahsan T, Nerem RM, O’Brien T, Barry F. Mesenchymal stem cells overexpressing ephrin-b2 rapidly adopt an early endothelial phenotype with simultaneous reduction of osteogenic potential. Tissue Eng Part A. 2010;16:2755–68. doi: 10.1089/ten.tea.2009.0623. [DOI] [PubMed] [Google Scholar]
- 65.Orrantia E, Chang PL. Intracellular distribution of DNA internalized through calcium phosphate precipitation. Exp Cell Res. 1990;190:170–4. doi: 10.1016/0014-4827(90)90181-9. [DOI] [PubMed] [Google Scholar]


